Significance
Essentiality is a phenotypic trait of genes frequently used to identify new potential drug targets. However, genes function within the context of complex and extensively interconnected physiologic networks, making it difficult to discern the specific mechanisms responsible for their essentiality. Here, we apply a combination of chemogenetic and metabolomic approaches to resolve the specific biochemical mechanism associated with the bactericidal essentiality of the metabolic enzyme, isocitrate lyase (ICL), for survival of Mycobacterium tuberculosis (Mtb), a chronic intracellular pathogen, on fatty acids, a major carbon source thought to be encountered in vivo. These studies not only provide a systems level insight into the essentiality of Mtb’s ICL but also reveal a previously unrecognized link between propionate metabolism and membrane bioenergetics.
Keywords: metabolic essentiality, membrane bioenergetics, metabolic homeostasis
Abstract
Few mutations attenuate Mycobacterium tuberculosis (Mtb) more profoundly than deletion of its isocitrate lyases (ICLs). However, the basis for this attenuation remains incompletely defined. Mtb’s ICLs are catalytically bifunctional isocitrate and methylisocitrate lyases required for growth on even and odd chain fatty acids. Here, we report that Mtb’s ICLs are essential for survival on both acetate and propionate because of its methylisocitrate lyase (MCL) activity. Lack of MCL activity converts Mtb’s methylcitrate cycle into a “dead end” pathway that sequesters tricarboxylic acid (TCA) cycle intermediates into methylcitrate cycle intermediates, depletes gluconeogenic precursors, and results in defects of membrane potential and intrabacterial pH. Activation of an alternative vitamin B12-dependent pathway of propionate metabolism led to selective corrections of TCA cycle activity, membrane potential, and intrabacterial pH that specifically restored survival, but not growth, of ICL-deficient Mtb metabolizing acetate or propionate. These results thus resolve the biochemical basis of essentiality for Mtb’s ICLs and survival on fatty acids.
Metabolic enzymes fuel the biochemical activities of all cells. However, our understanding of the pathways and physiologic functions they serve is incomplete. Enzymes catalyze reactions whose identities, rates, and directions are often difficult to determine and, where known, function within the context of complex and extensively interconnected physiologic networks (1). Thus, although recent genetic and biochemical approaches have made it possible to determine the essentiality of a given enzyme, our understanding of the biochemical mechanisms underlying their essentiality remains incomplete.
Unlike the case for most microbial pathogens, humans serve as both host and reservoir for Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB). Within humans, Mtb resides within the biochemically stringent environment of the macrophage phagosome. Growing evidence has separately implicated Mtb’s metabolic enzymes as a key determinant of its pathogenicity. Mtb’s metabolic network thus appears to have evolved to serve interdependent physiologic and pathogenic roles (2, 3).
Isocitrate lyase (ICL) is a metabolic enzyme used by bacteria to sustain growth on even-chain fatty acids through an anaplerotic pathway called the glyoxylate shunt. Mtb encodes two paralogous ICLs, each of which also functions as a methylisocitrate lyase (MCL), and serves a parallel pathway involved in the metabolism of propionyl CoA generated by β-oxidation of odd-chain fatty acids, called the methylcitrate cycle. Mtb lacking both ICLs were shown to be incapable of either establishing or maintaining infection in a mouse model of pulmonary TB (4, 5). Although loss of ICL activity is expected to result in the inability to incorporate carbon from fatty acids (a form of starvation), simultaneous loss of MCL activity is predicted to result in the potentially toxic accumulation of propionyl-CoA metabolites (a form of intoxication). Here, we applied 13C-based metabolomic tracing to elucidate the intrabacterial metabolic fates of an even (acetate) and odd (propionate) chain fatty acid in ICL-deficient Mtb and identify a unifying biochemical mechanism that explains its vulnerability to both even- and odd-chain fatty acids.
Results
Bactericidal Activity of Acetate and Propionate Against ICL-Deficient Mtb.
Previous studies reported that, consistent with predicted defects in the glyoxylate shunt and methylcitrate cycles, ICL-deficient Mtb were specifically unable to grow when cultured in media containing the model fatty acid metabolites, acetate or propionate (4–6). We found that these growth defects were associated with a 1–2 log10 reduction in viability over a 72-h incubation. These results thus indicate that both acetate and propionate are bactericidal to ICL-deficient Mtb (Fig. S1 A and B).
13C Metabolomic Profiling of ICL-Deficient Mtb Metabolizing Permissive and Nonpermissive Carbon Sources.
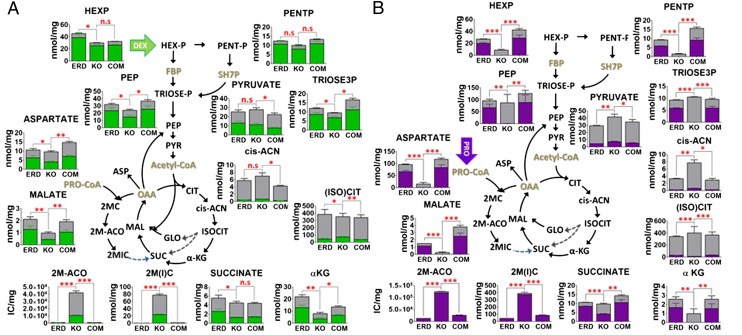
To elucidate the biochemical basis of this toxicity, we conducted metabolomic tracing studies of wild-type and ICL-deficient Mtb following transfer from a growth-permissive medium, containing dextrose, to one containing uniformly 13C-labeled ([U-13C]) dextrose, acetate, or propionate; after which, we sampled cells before a measurable loss of viability. Focusing first on intermediates of the glyoxylate shunt and methycitrate cycles, we observed specific, time-dependent accumulations of (iso)citrate, aconitate, methyl(iso)citrate, and methylaconitate with reciprocal reductions in the levels of downstream tricarboxylic acid (TCA) cycle intermediates in ICL-deficient, but not wild-type or genetically complemented, Mtb strains, as predicted. Contrary to expectation, however, these changes were observed when cultured on dextrose and on acetate or propionate (Fig. 1 A and B and Fig. S2; colored segments of each bar). These results thus indicate that both the glyoxylate shunt and methylcitrate cycle are operative in wild-type Mtb, when metabolizing either glycolytic or gluconeogenic carbon sources.
Fig. 1.
Metabolomic profiles of ICL-deficient Mtb when cultured in media containing different carbon sources. Intrabacterial pool sizes and isotopic labeling of metabolic intermediates of glycolysis/gluconeogenesis, TCA cycle, glyoxylate shunt, and methylcitrate cycle in wild-type (Erdman), ICL1/2-deficient (KO), and ICL-reconstituted (COM) Mtb strains incubated in [U-13C]-containing media for 24 h. Total bar heights indicate the intrabacterial concentration, whereas the colored area of each bar denotes the extent of 13C labeling achieved following transfer to [U-13C] dextrose (green) (A) and [U-13C] propionate (purple)-containing media (B) under the condition indicated. Schematic diagram when transferring to [U-13C] acetate (red)-containing media is shown in Fig. S2. ICL encodes both isocitrate lyase (gray dotted line) and methylisocitrate lyase (blue dotted line). All values are average of three independent experiments, each of which consisted in biological triplicates ± SE. Intrabacterial pool sizes of metabolites analyzed were described as nmol/mg except for 2 methyl cis-aconitate, whose relative abundance is instead reported as ion counts per milligram, due to lack of a commercially available standard. ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05; and ns, not significant by ANOVA. αKG, α-ketoglutarate; 2MC, 2 methylcitrate; 2M-ACO, 2 methyl cis-aconitate; 2MIC, 2 methylisocitrate; Asp, aspartate; cis-ACN, cis-aconitate; FBP, fructose 1,6-bisphosphate; FUM, fumarate; GLO, glyoxylate; Hex-P, hexose phosphate; IC, ion counts; MAL, malate; Pent-P, pentose 5-phosphate; PEP, phosphoenolpyruvate; PYR, pyruvate; SH7P, sedoheptulose 7-phosphate; Triose-P, triose 3-phosphate (glyceraldehyde 3-phosphate and dihydroxyacetone phosphate).
ICL-deficient Mtb incubated with [U-13C] acetate or propionate also exhibited specific reductions in the labeling of gluconeogenic and pentose phosphate pathway intermediates (Fig. 1B and Fig. S2). Lacking a glyoxylate shunt, ICL-deficient Mtb is expected to be unable to assimilate additional carbon biomass when metabolizing acetate because of the oxidative loss of two CO2 molecules by the downstream TCA cycle enzymes. Absence of MCL activity, in contrast, is predicted to result in the accumulation of methylisocitrate as a metabolic dead end product arising from the stoichiometric consumption of oxaloacetate by propionyl-CoA, which we observed as the near exclusive accumulation of methyl(iso)citrate molecules derived from the en bloc incorporation of [U-13C]-labeled propionate and secondary accumulation of nonmetabolized propionyl-CoA (Fig. S3 A and B). Studies in Salmonella enterica further reported that accumulation of 2-methylcitrate (2-MC) could inhibit the gluconeogenic enzyme, fructose 1,6-bisphosphatase (FBPase) (7). Using a purified recombinant form of Mtb’s sole annotated FBPase, GlpX, we found that 2-MC acted as a noncompetitive inhibitor (Fig. S4 A–C). These results thus suggest that ICL-deficient Mtb is unable to metabolize both even- and odd-chain fatty acids because of the dead-end depletion of TCA cycle intermediates by a constitutively active, but broken, methylcitrate cycle; secondary inhibition of gluconeogenesis arising from the depletion of the gluconeogenic substrates, oxaloacetate and malate, and active inhibition of the gluconeogenic enzyme FBPase by the accumulated methylcitrate cycle intermediate, 2-MC (Fig. 1B and Fig. S2).
Chemical-Phenotypic Profiling of Even- and Odd-Chain Fatty Acid Toxicity Against ICL-Deficient Mtb.
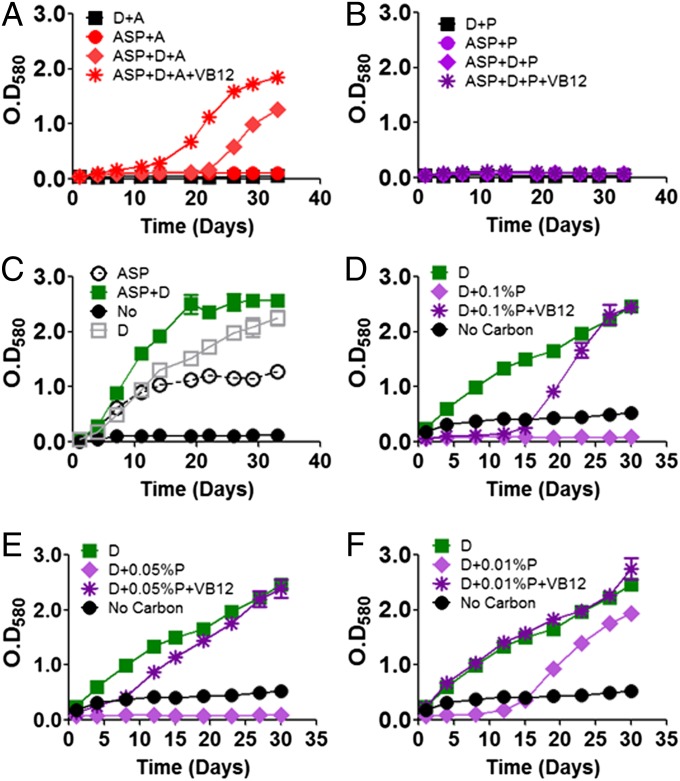
We next sought to resolve the relative impact of the foregoing deficiencies in TCA cycle activity and gluconeogenesis on the vulnerability of ICL-deficient Mtb to fatty acids. To do so, we first tested the extent to which the addition of exogenous oxaloacetate (supplied as aspartic acid) and dextrose could correct the observed defects in TCA cycle and gluconeogenic activity, respectively. As shown in Fig. 2 A–C, neither aspartate nor dextrose alone was sufficient to restore growth of ICL-deficient Mtb cultured in acetate or propionate. A combination of both, however, led to a partial restoration of growth in acetate-, but not propionate-, containing media (Fig. 2 A and B). Although this restoration followed a prolonged lag and was only partial with respect to final biomass, we found that this phenotype was stable to repeated passage in media supplemented with dextrose and aspartate, and not attributable to a mutation conferring resistance to acetate. Given these findings, we tested whether the propionate toxicity observed in Fig. 2B might instead be overcome by reducing the level of propionate used in the culture medium. Accordingly, we found that the addition of 0.2% dextrose to the culture medium could restore growth of ICL-deficient Mtb when propionate levels were reduced 20-fold to 0.01% with a prolonged lag phase similar to that described for acetate above (Fig. 2 D–F).
Fig. 2.
Chemical-phenotypic characterization of ICL-deficient Mtb growth. (A) Growth phenotypes of ICL KO (reported by OD580) cultured on media containing 0.2% acetate supplemented with the combination of 0.2% dextrose (precursor of GL pathway intermediates), 20 mM aspartic acid (precursor of TCA cycle intermediates), and 10 μg/mL VB12 (activator of alternate methylmalonyl-CoA pathway for propionate metabolism). (B) Same as A except using 0.2% propionate as the carbon source instead of acetate. (C) Same as A using 0.2% dextrose, a growth permissive carbon source. (D–F) Effect of 10 μg/mL VB12 and/or 0.2% dextrose on ICL-deficient Mtb when cultured on varying concentrations of propionate as indicated (D, 0.1%; E, 0.05%; F, 0.01% propionate, respectively). All values are the average of three independent experiments, each of which consisted in biological triplicates, ± SE.
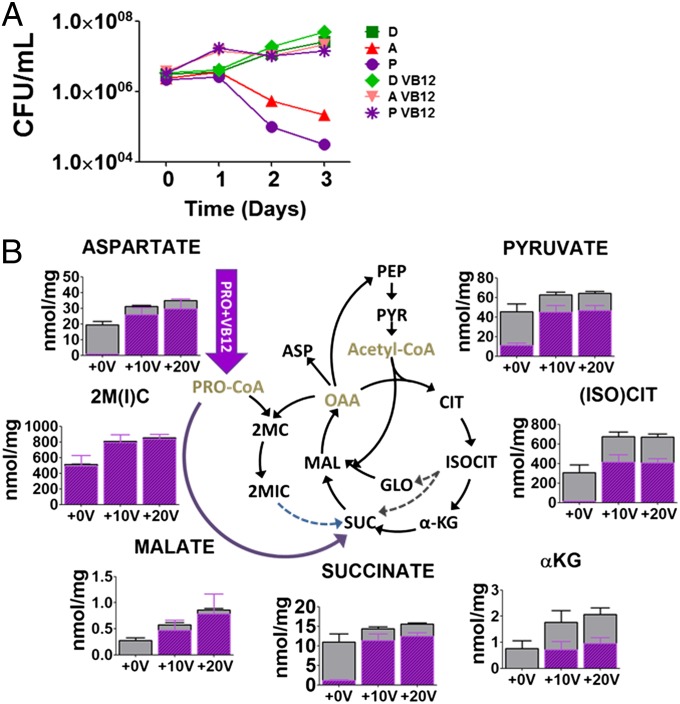
Work by Savvi et al. (8) showed that Mtb also encodes an intact methylmalonyl-CoA pathway which, in the presence of vitamin B12 (VB12), is capable of metabolizing propionate in the absence of a functional methylcitrate cycle. We found that the addition of VB12 was sufficient to selectively protect ICL-deficient Mtb from the bactericidal effects of acetate and propionate, and that this attenuation was accompanied by a dose-dependent restoration of TCA cycle activity and propionyl-CoA levels (Figs. 2 D–F and 3 A and B and Figs. S3B and S5 A and B). Consistent with the specific role of VB12 as a cofactor for the terminal enzyme of the methlymalonyl-CoA pathway, methylmalonyl-CoA mutase (which catalyzes the interconversion of methylmalonyl-CoA and succinyl-CoA), we discovered that ICL-deficient Mtb cultured on propionate accumulated a second CoA species matching that of methylmalonyl-CoA, whose accumulation is predicted to arise from the carboxylation of excess propionyl-CoA by propionyl-CoA carboxylase (the first enzyme of the methylmalonyl-CoA pathway), and whose abundance decreased in response to VB12. Levels of acetyl-CoA, in contrast, were unchanged (Fig. S3B). We further found that VB12 could also augment the growth-promoting effects of dextrose and/or aspartate on ICL-deficient Mtb metabolizing acetate, as reported by its ability to reduce and/or eliminate the lag phase of growth observed in Fig. 2A, and support growth on propionate concentrations up to fivefold higher (0.05%) than those achievable in the absence of VB12 (Fig. 2 D–F), respectively. These results thus identify the lack of methylisocitrate lyase activity as the primary metabolic defect responsible for the vulnerability of ICL-deficient Mtb to acetate and propionate alike.
Fig. 3.
Chemical rescue of ICL-deficient Mtb from propionate mediated killing by VB12. (A) Effect of VB12 on CFU-based viability of ICL-deficient Mtb cultured in m7H9 containing indicated carbon sources, each provided at 0.2%. A, acetate; D, dextrose; P, propionate. (B) Metabolic effect of VB12 on TCA cycle intermediates of ICL-deficient Mtb cultured on propionate. Total bar heights indicate the intrabacterial concentration, whereas the purple colored areas of each bar denotes the extent of 13C labeling achieved following transfer to [U-13C] propionate containing media. αKG, α-ketoglutarate; 2MC, 2 methylcitrate; 2M-ACO, 2 methyl cis-aconitate; 2MIC, 2 methylisocitrate; Asp, aspartate; cis-ACN, cis-aconitate; FBP, fructose 1,6-bisphosphate; FUM, fumarate; GLO, glyoxylate; Hex-P, hexose phosphate; IC, ion counts; MAL, malate; Pent-P, pentose 5-phosphate; PEP, phosphoenolpyruvate; PYR, pyruvate; SH7P, sedoheptulose 7-phosphate; Triose-P, triose 3-phosphate (glyceraldehyde 3-phosphate and dihydroxyacetone phosphate). +0V; no VB12, +10V; 10 μg/mL of vitamin VB12, +20V; 20 μg/mL of VB12 added to the media.
Chemical-Phenotypic Characterization of Fatty Acid-Mediated Killing of ICL-Deficient Mtb.
Defects in propionate metabolism have been associated with a broad range of secondary effects arising from the accumulation of propionyl-CoA and/or propionate-derived metabolites. These secondary effects include the inhibition of enzymes involved in branched chain amino acid turnover, and those of the pyruvate dehydrogenase complex and electron transport chain and, ultimately, result in a cellular acidosis (9–12). Membrane potential and cytosolic pH homeostasis are essential for the survival of all cells and potentially more so for those residing in acidic niches, including the phagosomes of immune-activated macrophages (13, 14). Given the selective protection afforded by VB12 against fatty acid-induced killing (Fig. 3A), we sought to elucidate the specific metabolic derangements associated with the bactericidal effects of acetate and propionate on ICL-deficient Mtb.
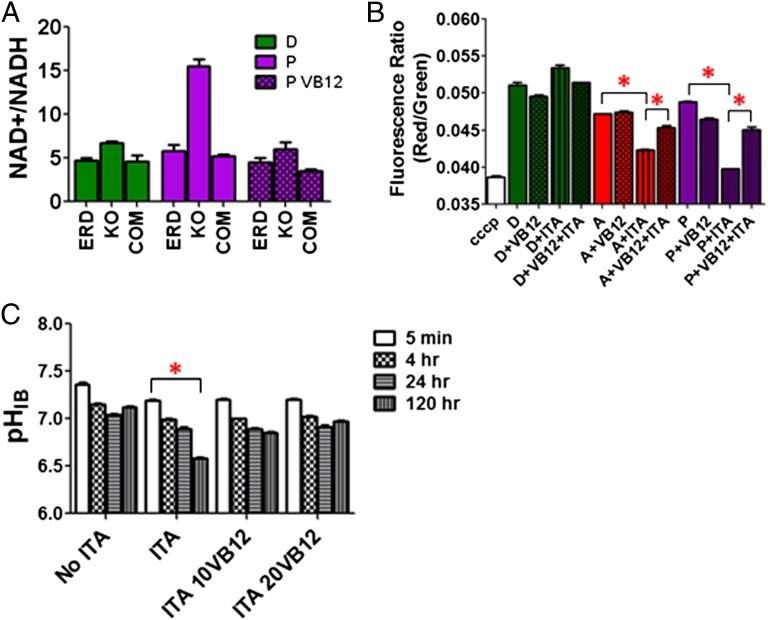
As described above, we observed that ICL-deficient Mtb underwent a progressive depletion of TCA cycle intermediates and accumulation of propionyl-CoA when metabolizing fatty acid substrates (Fig. 1B and Figs. S2 and S3B). We therefore hypothesized that these changes might impair Mtb’s respiratory activity due to a slowdown of TCA cycle activity, resulting in secondary impacts on homeostasis of membrane potential and intrabacterial pH. Accordingly, we first discovered changes in both the NAD/NADH ratios and membrane potential of ICL-deficient Mtb that followed transfer onto acetate- or propionate-containing media, but preceded any measurable loss of viability (Fig. 3A and Fig. S6 A and B).
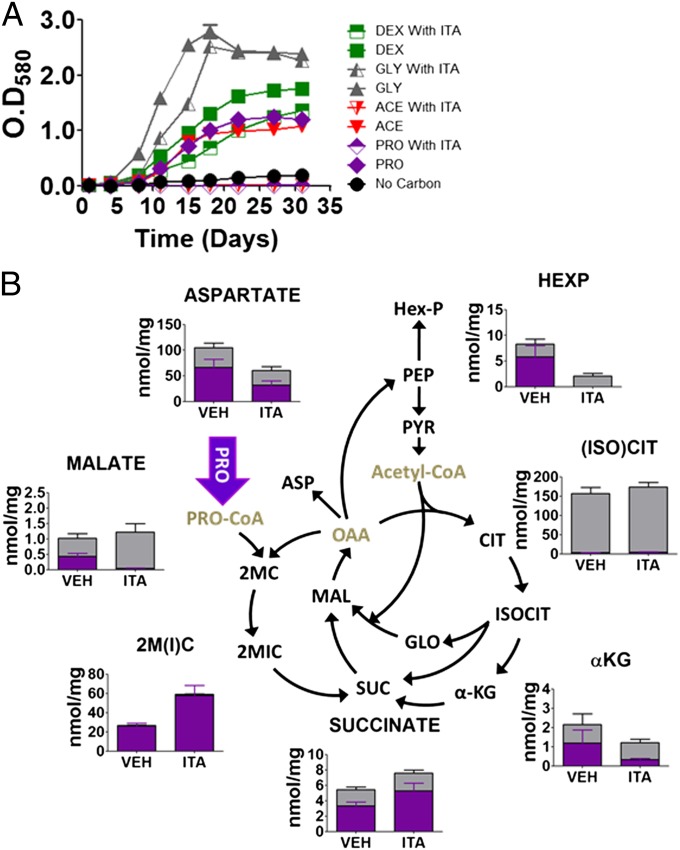
Using a previously described ratiometric pH-sensitive GFP adapted for use with Mtb, we next discovered changes in the intrabacterial pH of ICL-deficient Mtb that followed the foregoing changes in NAD/NADH ratios and membrane potential (15). We were unable to stably express this GFP reporter in ICL-deficient Mtb, but overcame this limitation by using wild-type Mtb and itaconic acid, a specific and recently validated chemical inhibitor of Mtb’s ICLs in intact bacteria (16). We found that, as predicted, itaconic acid specifically inhibited growth of wild-type Mtb on acetate and propionate, but not dextrose, in an ICL-dependent manner, and elicited metabolomic changes similar to those observed with ICL-deficient Mtb (Figs. 1 A and B and 4 A and B and Figs. S2 and S7 A–C).
Fig. 4.
Chemical-phenotypic characterization of the ICL-specific inhibitor, itaconic acid, on intact wild-type Mtb. (A) Growth phenotypes of wild-type Mtb H37Rv in the presence and absence of 2 mM itaconic acid when cultured in m7H9 media containing 0.2% carbon sources as indicated. ACE, acetate; DEX, dextrose; GLY, glycerol; ITA, itaconic acid; PRO, propionate. (B) Intrabacterial pool sizes and isotopic labeling of select intermediates of Mtb central carbon metabolic pathway when cultured in media containing [U-13C]propionate for 24 h. Total bar heights indicate the intrabacterial concentration, whereas purple colored area of each bar denotes the extent of 13C labeling achieved following transfer to [U-13C] carbon source. αKG, α-ketoglutarate; 2MC, 2 methylcitrate; 2M-ACO, 2 methyl cis-aconitate; 2MIC, 2 methylisocitrate; Asp, aspartate; cis-ACN, cis-aconitate; FBP, fructose 1,6-bisphosphate; FUM, fumarate; GLO, glyoxylate; Hex-P, hexose phosphate; IC, ion counts; MAL, malate; Pent-P, pentose 5-phosphate; PEP, phosphoenolpyruvate; PYR, pyruvate; SH7P, sedoheptulose 7-phosphate; Triose-P, triose 3-phosphate (glyceraldehyde 3-phosphate and dihydroxyacetone phosphate). All values are average of three independent experiments, each of which consisted in biological triplicates, ± SE.
Using wild-type Mtb expressing this pH-sensitive GFP cultured on propionate, we found that: (i) ICL inhibition by itaconic acid resulted in a specific decrease in Mtb’s intrabacterial pH from ∼7.3 to 6.4 that was not observed with acetate but accompanied a decrease in viability of 36-fold in 3 d, a magnitude similar to that of previously reported mutants with similar quantitative defects in pH homeostasis; and (ii) both pH and viability defects could be corrected with the addition of extracellular VB12 (Fig. 5 B and C and Fig. S7A). As predicted, we observed no such changes in ICL-deficient Mtb cultured on dextrose (Fig. 5 A and B and Fig. S8). These results thus demonstrate that the bactericidal activity of propionate against ICL-deficient Mtb is mediated, in part, by linked decreases in TCA cycle and respiratory activity that result in an inability to maintain an energized membrane and intrabacterial pH.
Fig. 5.
Biochemical characterization of ICL-deficient Mtb cultured on various carbon sources and chemical rescue by VB12. Determination of intrabacterial NAD/NADH ratio of the three Mtb strains; Erdman wild-type (Erdman), ICL1/2-deficient (KO), and ICL-reconstituted (COM) Mtb strains (A) and membrane potential of wild type Mtb H37Rv when cultured on media containing combinational supplements such as varying carbons (A, acetate; D, dextrose; P, propionate), itaconic acid (ITA), and VB12 (B) at 24 h after treatment. Cultures grown on media containing 5 μM of the protonophore carbonyl-cyanide 3-chlorophenylhydrazone (cccp), a membrane depolarization agent, were used as a positive control. (C) Determination of intrabacterial pH of Mtb H37Rv cultured in the presence of propionate with/without 2 mM itaconic acid and either 10 or 20 μg/mL VB12 (10 or 20 VB12) in a time-dependent manner. All values are average of three independent experiments, each of which consisted in biological triplicates, ± SE. *P < 0.001 by ANOVA.
Discussion
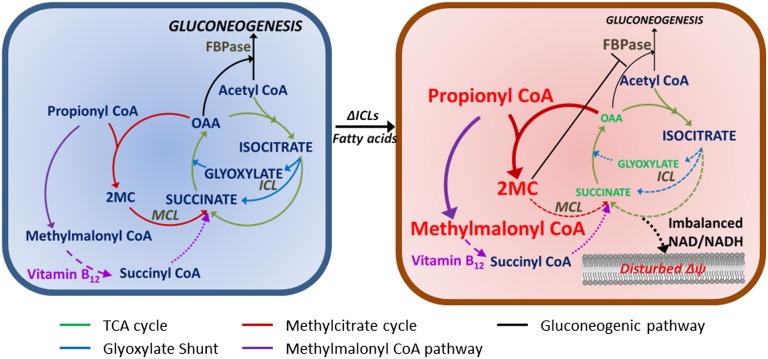
Despite the widely recognized essentiality of intermediary metabolism, the underlying biochemical mechanisms that define this essentiality remain incompletely defined. Our studies help to close this gap by elucidating the specific metabolic functions responsible for the essentiality of Mtb’s ICLs during growth on fatty acids. We specifically found that although Mtb’s ICLs are essential for growth on even-chain fatty acids, this essentiality is not solely attributable to their role in the glyoxylate shunt, a pathway canonically associated with assimilation and anaplerosis of carbon into TCA cycle intermediates, as ICL-deficient Mtb were found to succumb, rather than persist, on acetate as might be predicted. Instead, we showed that Mtb’s ICLs were required for metabolism of both even- and odd-chain fatty acids through the methylcitrate cycle and that loss of its MCL activity resulted in a common state of propionate-based vulnerability. Moreover, we showed that this vulnerability, although associated with the loss of a single enzymatic activity, was due to a multiplicity of metabolic defects that fundamentally stemmed from the conversion of the methylcitrate cycle into a dead end pathway. These defects consisted in the: (i) constitutive depletion of oxaloacetate from the TCA cycle and gluconeogenic pathways; (ii) secondary accumulation of 2-MC, resulting in a progressive noncompetitive inhibition of the gluconeogenic enzyme fructose 1,6-bisphosphatase; and (iii) tertiary accumulation of propionyl-CoA and methylmalonyl-CoA (arising from the conversion of excess propionyl-CoA in the ICL-deficient Mtb by propionyl-CoA carboxylase), following titration of cellular oxaloacetate pools, resulting in alterations of its NAD/NADH pools, membrane potential and, in the case of propionate, intrabacterial pH (Fig. 6). That activation of the VB12-dependent methylmalonyl-CoA pathway of propionate metabolism was able to selectively restore viability, but not growth, of ICL-deficient Mtb incubated on fatty acid substrates specifically identified defects in TCA cycle-dependent respiratory activity (reported by NAD/NADH ratios), membrane potential, and intrabacterial pH as the mechanistic basis of the bactericidal toxicity of both acetate and propionate; whereas the ability of further supplementation with dextrose and oxaloacetate (provided as aspartic acid) to restore growth identified carbon assimilation through the TCA cycle and gluconeogenesis as growth-limiting deficiencies in ICL-deficient Mtb. Moreover, these studies establish that the essentiality of Mtb’s ICLs for metabolism of fatty acids is due to a complex and previously unrecognized set of interconnected metabolic roles, whose deficiency results in a metabolic phenotype distinct from even those of other enzymes in the same pathway such as the previously characterized methylcitrate synthase (6).
Fig. 6.
Metabolic consequences of ICL-deficient Mtb when metabolizing fatty acid carbon sources. Metabolic state of central carbon metabolism in wild-type Mtb is shown in Left. Genetic or chemical inactivation of ICLs in Mtb leads to metabolic perturbation when cultured in media containing fatty acids (Right). As shown in Right, inactivation of ICLs converts the methylcitrate cycle (red line) into a dead-end pathway. Fatty acid-mediated metabolic perturbation in ICL-deficient Mtb is primarily due to (i) constitutive depletion of the TCA cycle and gluconeogenic pathway intermediates; (ii) secondary accumulation of 2-MC, allosterically inhibiting the gluconeogenic enzyme, fructose 1,6-bisphosphatase (FBPase); and (iii) tertiary accumulation of propionyl-CoA and/or methylmalonyl-CoA, following broad secondary impact on metabolic homeostasis including NAD/NADH, membrane potential (Δψ), and intrabacterial pH, which are essential factors for cell viability. TCA cycle (green line); glyoxylate shunt (blue line); methylcitrate cycle (red line); VB12-dependent methylmalonyl-CoA pathway (purple line); gluconeogenic pathway (black line). Mtb ICLs encode a bifunctional enzyme: isocitrate lyase activity (blue colored dotted line) and methylisocitrate lyase activity (red colored dotted line).
Multiple lines of microbiologic, genetic, and biochemical evidence have implicated fatty acids as the predominant carbon substrates used by Mtb in the host (5, 17–20). This evidence derives chiefly from measures of the essentiality and/or activity of metabolic enzymes and pathways associated with fatty acid metabolism. However, such views have largely neglected the possibility that such pathways may serve both endogenously generated and exogenous substrates, as demonstrated by the constitutive activity of Mtb’s methylcitrate cycle activity on both fatty acid and carbohydrate substrates and its shared essentiality for metabolism of both acetate and propionate. Thus, although ICL-deficient Mtb is among the most severely attenuated mutant strains tested in a mouse model of TB, it is possible that this attenuation may reflect the loss of multiple, and yet incompletely defined, functions such as those described here and a recently reported role in hypoxia unrelated to fatty acid catabolism (21).
The essentiality of metabolic enzymes is often defined in relation to the biochemical toxicity or essentiality of their corresponding substrates and products. However, such views have largely failed to elucidate the specific downstream pathways that mediate these effects. Enzymes function within the context of extensively interconnected pathways and networks that have evolved to be both robust and efficient. This study reveals a previously unrecognized link between Mtb’s ICLs and membrane bioenergetics that exemplifies the potential for metabolomics studies to resolve the specific biochemical pathways associated with a given phenotype. From a therapeutic perspective, concerted efforts to identify potent and specific inhibitors of Mtb’s ICL have failed to yield any pharmacologically tractable lead compounds. The biochemical resolution of ICL’s essentiality for metabolism of fatty acids reported here offers a conceptually fresh window into unique potential drug targets of the methylcitrate cycle whose chemical inhibition might mimic that of ICL deficiency and prove pharmacologically more robust.
Materials and Methods
Mtb Filter Culture and Metabolite Extraction.
Mtb Erdman wild type, icl knock-out (ICL KO), and the complemented strain (COM) were cultured in a biosafety level 3 facility at 37 °C in Middlebrook 7H9 broth (m7H9) or on 7H10 agar (m7H10) (Difco) supplemented with 0.2% dextrose, acetate, or propionate; 0.04% Tyloxapol (broth only); 0.5 g/L BSA; and 0.085% NaCl. Mtb-laden filters used for metabolomic profiles were generated as described (22, 23) and incubated at 37 °C for 5 d to reach the midlogarithimic phase of growth. Mtb-laden filters were then transferred onto chemically identical media containing fresh m7H10 containing 12C or [U-13C] carbon source and metabolites were harvested after 24 h. Mtb-laden filters were metabolically quenched by plunging filters into a mixture of acetonitrile/methanol/H2O (40:40:20) precooled to −40 °C; metabolites were extracted by mechanical lysis with 0.1-mm Zirconia beads in a Precellys tissue homogenizer (Bertin Technologies, France) for 3 min (6,500 rpm) twice under continuous cooling at or below 2 °C. Lysates were clarified by centrifugation and then filtered across a 0.22-μm filter. Residual protein content of metabolite extracts was determined to normalize samples to cell biomass (BCA protein assay kit; Thermo Scientific). Itaconic acid and VB12 (cyanocobalamin) were purchased from Sigma-Aldrich and used at 2 mM and either 10 or 20 µg/mL final concentration, respectively. All data obtained by metabolomics were the average of at least two independent experiments, each of which consisted in triplicate samples for each condition tested.
Short-Chain Acyl-CoA Ester Extraction.
Mtb-laden filters were used for extraction of propionyl, methylmalonyl/succinyl, and acetyl-CoA as described (24). Mtb cells were harvested with ice-cold PBS and were metabolically quenched by plunging cells into chloroform/methanol (2:1), followed by homogenization by adding H2O to give a total chloroform/methanol/H2O ratio of 2.6:1.3:1. Two-phase separation was achieved by centrifugation at 3,200 × g for 30 min. The upper phase containing short-chain acyl-CoA esters was transferred to new tubes and interface cell debris extracted at least three times with 400 mM ammonium acetate in 80% (vol/vol) methanol. The three extracts and upper phase from chloroform/methanol/H2O were combined and lyophilized. For LC-MS, the dry pellet was dissolved in 0.3 mL of 150 mM ammonium acetate.
Metabolomics with LC-MS.
LC-MS–based metabolomics analysis was as described (21).
Cell Viability Test.
Mtb viability was determined by using liquid cultures manipulated under experimentally identical conditions as filter-grown cultures used for metabolomic profiling, which we had demonstrated to be microbiologically similar (25). Colony forming units (CFU) were determined by plating on m7H9 with Bacto Agar because of the apparent toxicity of malachite green to ICL-deficient Mtb with supplements; 0.2% glycerol, 0.2% dextrose, 0.5 g/L BSA, and 0.085% NaCl (26).
Measurement of Membrane Potential and NAD/NADH Ratio.
Mtb membrane potential was measured as described (21, 27–29). Details are in SI Materials and Methods.
Determination of Intrabacterial pH.
We used Mtb expressing a reported, pH-sensitive ratiometric green fluorescent protein (Mtb-pHGFP) (15, 28, 30). Mtb-pHGFP cultures were grown to midlog phase (OD580 ∼ 0.6), resuspended, and diluted to an OD580 ∼ 0.4 in m7H9 containing 0.2% propionate either with or without 2 mM itaconic acid treatment at various time points. Fluorescence was measured by using a SpectraMax M5 spectrofluorimeter at excitation 395 nm, emission 510 nm (reading 1) and at excitation 475 nm, emission 510 nm (reading 2). Intrabacterial pH was inferred from the ratio of reading 1 to reading 2 by reference to a calibration curve performed on lysates of Mtb-pHGFP as described (15).
Isotopologue Data Analysis Using Isotope-Labeled Carbon Sources.
Details are in SI Materials and Methods.
Generation of Recombinant Mtb FBPase and Development of in Vitro Enzyme Assay.
Details are in SI Materials and Methods.
Statistical Analysis.
Analyses were performed by the ANOVA test. A P value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank John McKinney for the generous gift of the Erdman and icl mutant stains used herein; Carl Nathan, Dirk Schnappinger, and Sabine Ehrt for critical discussions; and Zhe Wang for analytical support. This work was supported by the Bill and Melinda Gates Foundation Grand Challenges Exploration and TB Drug Accelerator programs, and a Burroughs Wellcome Fund Career Award in the Biomedical Sciences (to K.Y.R.), and the Stony Wold Herbert Fund (H.E.). K.Y.R. also received support from the William Randolph Hearst Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400390111/-/DCSupplemental.
References
- 1.Stryer L, Berg J, Tymoczko J, editors. Biochemistry. San Francisco: W. H. Freeman; 2002. [Google Scholar]
- 2.Rhee KY, et al. Central carbon metabolism in Mycobacterium tuberculosis: An unexpected frontier. Trends Microbiol. 2011;19(7):307–314. doi: 10.1016/j.tim.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell DG, Barry CE, 3rd, Flynn JL. Tuberculosis: What we don’t know can, and does, hurt us. Science. 2010;328(5980):852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gould TA, van de Langemheen H, Muñoz-Elías EJ, McKinney JD, Sacchettini JC. Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Mol Microbiol. 2006;61(4):940–947. doi: 10.1111/j.1365-2958.2006.05297.x. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz-Elías EJ, McKinney JD. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med. 2005;11(6):638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muñoz-Elías EJ, Upton AM, Cherian J, McKinney JD. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol. 2006;60(5):1109–1122. doi: 10.1111/j.1365-2958.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- 7.Rocco CJ, Escalante-Semerena JC. In Salmonella enterica, 2-methylcitrate blocks gluconeogenesis. J Bacteriol. 2010;192(3):771–778. doi: 10.1128/JB.01301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savvi S, et al. Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: Implications for propionate metabolism during growth on fatty acids. J Bacteriol. 2008;190(11):3886–3895. doi: 10.1128/JB.01767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brock M, Buckel W. On the mechanism of action of the antifungal agent propionate. Eur J Biochem. 2004;271(15):3227–3241. doi: 10.1111/j.1432-1033.2004.04255.x. [DOI] [PubMed] [Google Scholar]
- 10.Schwab MA, et al. Secondary mitochondrial dysfunction in propionic aciduria: A pathogenic role for endogenous mitochondrial toxins. Biochem J. 2006;398(1):107–112. doi: 10.1042/BJ20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee W, VanderVen BC, Fahey RJ, Russell DG. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J Biol Chem. 2013;288(10):6788–6800. doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin JE, et al. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem Biol. 2012;19(2):218–227. doi: 10.1016/j.chembiol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster JW. Escherichia coli acid resistance: Tales of an amateur acidophile. Nat Rev Microbiol. 2004;2(11):898–907. doi: 10.1038/nrmicro1021. [DOI] [PubMed] [Google Scholar]
- 14.Vandal OH, Nathan CF, Ehrt S. Acid resistance in Mycobacterium tuberculosis. J Bacteriol. 2009;191(15):4714–4721. doi: 10.1128/JB.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med. 2008;14(8):849–854. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michelucci A, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013;110(19):7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumenthal A, Trujillo C, Ehrt S, Schnappinger D. Simultaneous analysis of multiple Mycobacterium tuberculosis knockdown mutants in vitro and in vivo. PLoS ONE. 2010;5(12):e15667. doi: 10.1371/journal.pone.0015667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell Microbiol. 2009;11(8):1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry CE, 3rd, et al. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolaj-Robin O, et al. Biochemical and biophysical characterization of succinate: Quinone reductase from Thermus thermophilus. Biochim Biophys Acta. 2011;1807(1):68–79. doi: 10.1016/j.bbabio.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Eoh H, Rhee KY. Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2013;110(16):6554–6559. doi: 10.1073/pnas.1219375110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bollo S, et al. The effect of 5-substitution on the electrochemical behavior and antitubercular activity of PA-824. Bioorg Med Chem Lett. 2011;21(2):812–817. doi: 10.1016/j.bmcl.2010.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Carvalho LP, et al. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem Biol. 2010;17(10):1122–1131. doi: 10.1016/j.chembiol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Rosendal J, Knudsen J. A fast and versatile method for extraction and quantitation of long-chain acyl-CoA esters from tissue: Content of individual long-chain acyl-CoA esters in various tissues from fed rat. Anal Biochem. 1992;207(1):63–67. doi: 10.1016/0003-2697(92)90500-7. [DOI] [PubMed] [Google Scholar]
- 25.Manjunatha UH, et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103(2):431–436. doi: 10.1073/pnas.0508392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelman E, McKinney JD, Dhar N. Malachite green interferes with postantibiotic recovery of mycobacteria. Antimicrob Agents Chemother. 2012;56(7):3610–3614. doi: 10.1128/AAC.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Carvalho LP, Darby CM, Rhee KY, Nathan C. Nitazoxanide disrupts membrane potential and intrabacterial pH homeostasis of Mycobacterium tuberculosis. ACS medicinal chemistry letters. 2011;2(11):849–854. doi: 10.1021/ml200157f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mak PA, et al. A high-throughput screen to identify inhibitors of ATP homeostasis in non-replicating Mycobacterium tuberculosis. ACS Chem Biol. 2012;7(7):1190–1197. doi: 10.1021/cb2004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2008;105(33):11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCracken ST, Kaiser M, Boshoff HI, Boyd PD, Copp BR. Synthesis and antimalarial and antituberculosis activities of a series of natural and unnatural 4-methoxy-6-styryl-pyran-2-ones, dihydro analogues and photo-dimers. Bioorg Med Chem. 2012;20(4):1482–1493. doi: 10.1016/j.bmc.2011.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.