Abstract
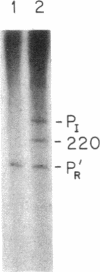
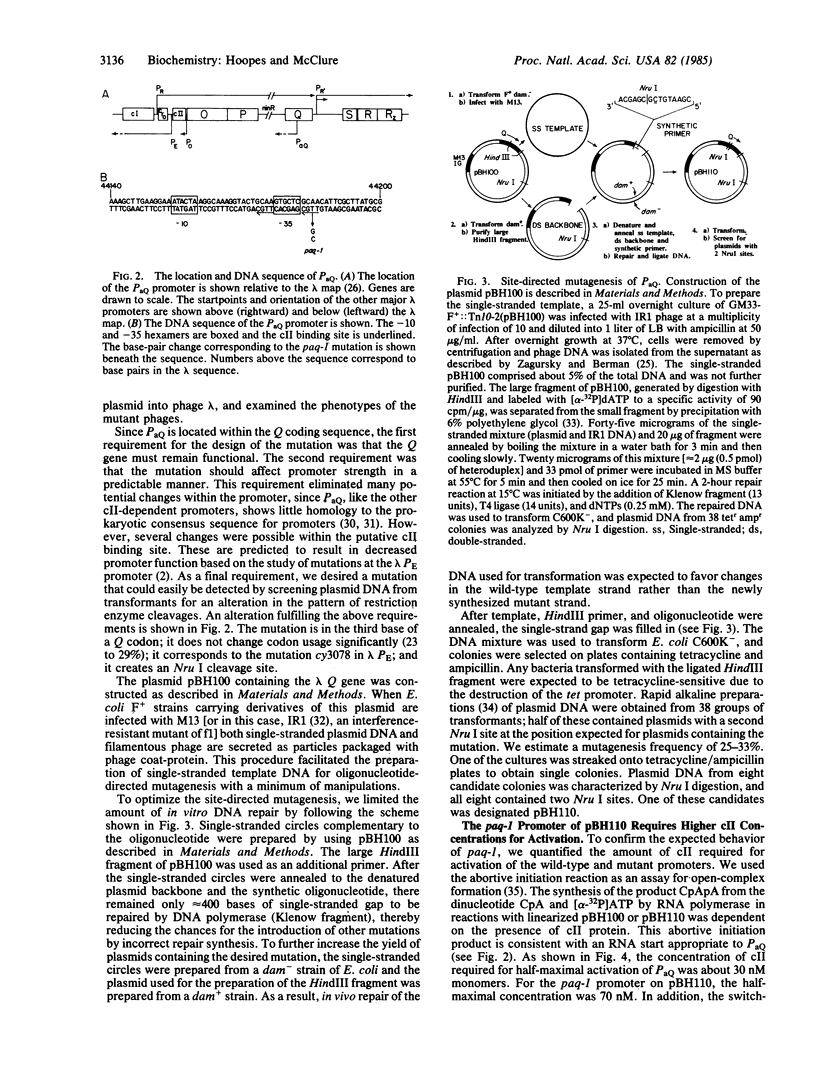
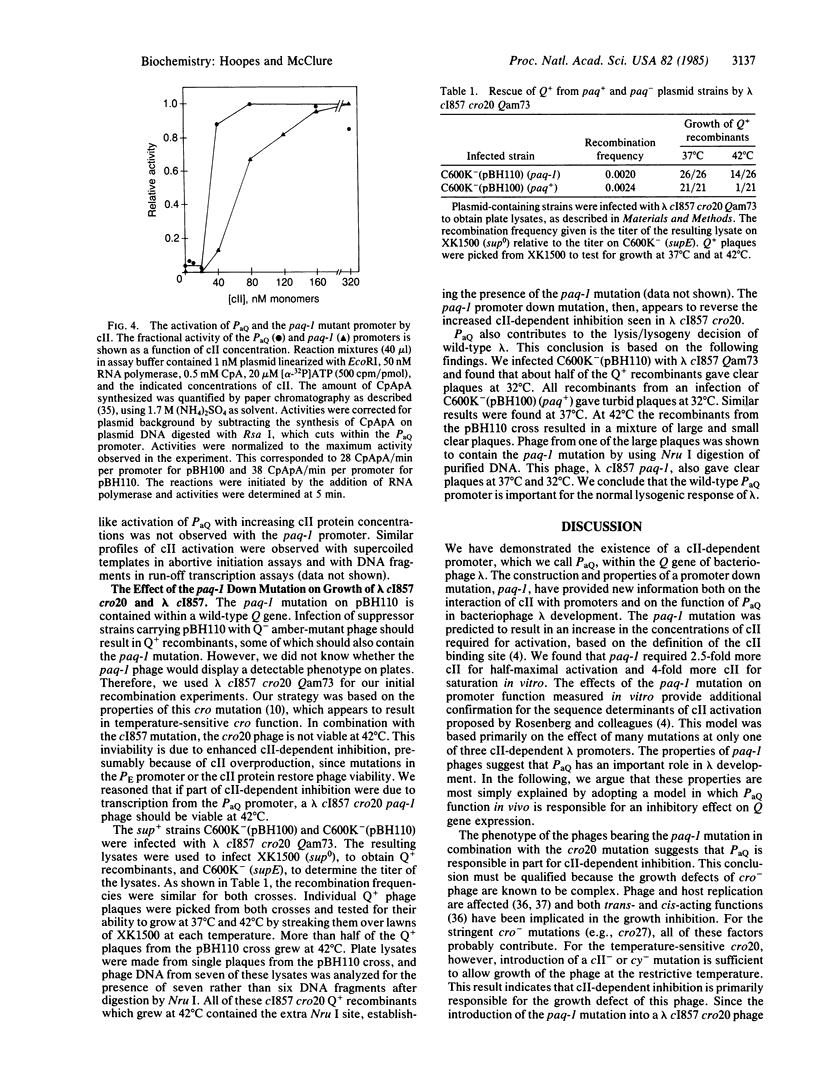
We have found a cII-dependent promoter, PaQ, within the Q gene of bacteriophage lambda. Transcription experiments and abortive initiation assays performed in vitro showed that the promoter strength and the cII affinity of PaQ were comparable to the other cII-dependent lambda promoters, PE and PI. The location and leftward direction of PaQ suggests a possible role in the delay of lambda late-gene expression by cII protein, a phenomenon that has been called cII-dependent inhibition. We have constructed a promoter down mutation, paq-1, by changing a single base pair in the putative cII binding site of the promoter by oligonucleotide site-directed mutagenesis. The paq-1 mutant promoter required about 4-fold higher cII concentrations for maximal activation compared to the wild-type PaQ. We tested the hypothesis that PaQ is responsible in part for the delay of lambda late-gene expression by recombining the paq-1 mutation into a phage showing severe cII-dependent inhibition. We found that the paq-1 mutation relieved the cII-dependent growth defect of this phage. The paq-1 mutation (in combination with lambda cI857) resulted in a clear-plaque phenotype at the permissive temperature of 32 degrees C. The role of the PaQ-initiated antisense transcript in the control of lambda development is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Butler B., Echols H. Regulation of bacteriophage lambda development by gene N: properties of a mutation that bypasses N control of late protein synthesis. Virology. 1970 Feb;40(2):212–222. doi: 10.1016/0042-6822(70)90396-x. [DOI] [PubMed] [Google Scholar]
- Cech C. L., McClure W. R. Characterization of ribonucleic acid polymerase-T7 promoter binary complexes. Biochemistry. 1980 May 27;19(11):2440–2447. doi: 10.1021/bi00552a023. [DOI] [PubMed] [Google Scholar]
- Coleman J., Green P. J., Inouye M. The use of RNAs complementary to specific mRNAs to regulate the expression of individual bacterial genes. Cell. 1984 Jun;37(2):429–436. doi: 10.1016/0092-8674(84)90373-8. [DOI] [PubMed] [Google Scholar]
- Court D., Green L., Echols H. Positive and negative regulation by the cII and cIII gene products of bacteriophage lambda. Virology. 1975 Feb;63(2):484–491. doi: 10.1016/0042-6822(75)90321-9. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. Interference resistant mutants of phage f1. Virology. 1982 Oct 15;122(1):222–226. doi: 10.1016/0042-6822(82)90395-6. [DOI] [PubMed] [Google Scholar]
- Folkmanis A., Maltzman W., Mellon P., Skalka A., Echols H. The essential role of the cro gene in lytic development by bacteriophage lambda. Virology. 1977 Sep;81(2):352–362. doi: 10.1016/0042-6822(77)90151-9. [DOI] [PubMed] [Google Scholar]
- Georgiou M., Georgopoulos C. P., Eisen H. An analysis of the Tro phenotype of bacteriophage lambda. Virology. 1979 Apr 15;94(1):38–54. doi: 10.1016/0042-6822(79)90436-7. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I., Hagen D. The lysis-lysogeny decision of phage lambda: explicit programming and responsiveness. Annu Rev Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Wulff D. L., Rosenberg M. Bacteriophage lambda protein cII binds promoters on the opposite face of the DNA helix from RNA polymerase. Nature. 1983 Aug 25;304(5928):703–708. doi: 10.1038/304703a0. [DOI] [PubMed] [Google Scholar]
- Ho Y., Lewis M., Rosenberg M. Purification and properties of a transcriptional activator. The cII protein of phage lambda. J Biol Chem. 1982 Aug 10;257(15):9128–9134. [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Construction of a plasmid that overproduces the large proteolytic fragment (Klenow fragment) of DNA polymerase I of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1830–1834. doi: 10.1073/pnas.80.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B. J. Isolation and characterization of mutations in the cIII gene of bacteriophage lambda which increase the efficiency of lysogenization of Escherichia coli K-12. Virology. 1979 Jan 30;92(2):518–531. doi: 10.1016/0042-6822(79)90154-5. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Schleif R. Size fractionation of double-stranded DNA by precipitation with polyethylene glycol. Nucleic Acids Res. 1975 Mar;2(3):383–389. doi: 10.1093/nar/2.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe P. A., Hager D. A., Burgess R. R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979 Apr 3;18(7):1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Biological function for 6-methyladenine residues in the DNA of Escherichia coli K12. J Mol Biol. 1974 May 15;85(2):309–322. doi: 10.1016/0022-2836(74)90366-0. [DOI] [PubMed] [Google Scholar]
- McClure W. R., Cech C. L., Johnston D. E. A steady state assay for the RNA polymerase initiation reaction. J Biol Chem. 1978 Dec 25;253(24):8941–8948. [PubMed] [Google Scholar]
- McMacken R., Mantei N., Butler B., Joyner A., Echols H. Effect of mutations in the c2 and c3 genes of bacteriophage lambda on macromolecular synthesis in infected cells. J Mol Biol. 1970 May 14;49(3):639–655. doi: 10.1016/0022-2836(70)90288-3. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A. B., Gottesman S., Gottesman M. Regulation of bacteriophage lambda int gene expression. J Mol Biol. 1982 Jul 5;158(3):327–346. doi: 10.1016/0022-2836(82)90201-7. [DOI] [PubMed] [Google Scholar]
- Oppenheim A., Belfort M., Katzir N., Kass N., Oppenheim A. B. Interaction of cII, cIII, and cro gene products in the regulation of early and late functions of phage lambda. Virology. 1977 Jun 15;79(2):426–436. doi: 10.1016/0042-6822(77)90368-3. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Transcription termination and late control in phage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3300–3304. doi: 10.1073/pnas.72.9.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Schmeissner U., Court D., Shimatake H., Rosenberg M. Promoter for the establishment of repressor synthesis in bacteriophage lambda. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3191–3195. doi: 10.1073/pnas.77.6.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M. C., Gussin G. N. Differential effects of mutations on discrete steps in transcription initiation at the lambda PRE promoter. Cell. 1983 Oct;34(3):941–949. doi: 10.1016/0092-8674(83)90551-2. [DOI] [PubMed] [Google Scholar]
- Shih M. C., Gussin G. N. Kinetic analysis of mutations affecting the cII activation site at the PRE promoter of bacteriophage lambda. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6432–6436. doi: 10.1073/pnas.81.20.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M. C., Gussin G. N. Role of cII protein in stimulating transcription initiation at the lambda PRE promoter. Enhanced formation and stabilization of open complexes. J Mol Biol. 1984 Feb 5;172(4):489–506. doi: 10.1016/s0022-2836(84)80019-4. [DOI] [PubMed] [Google Scholar]
- Simatake H., Rosenberg M. Purified lambda regulatory protein cII positively activates promoters for lysogenic development. Nature. 1981 Jul 9;292(5819):128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Hedgpeth J. Oligo(A) not coded by DNA generating 3'-terminal heterogeneity in a lambda phage RNA. J Biol Chem. 1975 Jun 25;250(12):4818–4821. [PubMed] [Google Scholar]
- Zagursky R. J., Berman M. L. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. doi: 10.1016/0378-1119(84)90139-2. [DOI] [PubMed] [Google Scholar]