Significance
Type 1 Gaucher disease (GD1) is a rare autosomal recessive disorder caused by inherited mutations in the glucocerebrosidase (GBA1) gene. This disease results in a marked accumulation of glycosphingolipid substrates, causing visceromegaly, cytopenia, and osteopenia. Here, we have rescued this clinical phenotype in GD1 mice by genetically deleting Gba2, a gene encoding a downstream extralysosomal enzyme, GBA2. We also report that sphingosine production in GD1 patients may contribute to the low-turnover bone loss. Our data suggest that GBA2 and sphingosine are potential targets for pharmacological intervention in GD1 patients.
Keywords: macrophage, knockout mice, S1P
Abstract
The inherited deficiency of the lysosomal glucocerebrosidase (GBA) due to mutations in the GBA gene results in Gaucher disease (GD). A vast majority of patients present with nonneuronopathic, type 1 GD (GD1). GBA deficiency causes the accumulation of two key sphingolipids, glucosylceramide (GL-1) and glucosylsphingosine (LysoGL-1), classically noted within the lysosomes of mononuclear phagocytes. How metabolites of GL-1 or LysoGL-1 produced by extralysosomal glucocerebrosidase GBA2 contribute to the GD1 pathophysiology is not known. We recently recapitulated hepatosplenomegaly, cytopenia, hypercytokinemia, and the bone-formation defect of human GD1 through conditional deletion of Gba in Mx1–Cre+:GD1 mice. Here we show that the deletion of Gba2 significantly rescues the GD1 clinical phenotype, despite enhanced elevations in GL-1 and LysoGL-1. Most notably, the reduced bone volume and bone formation rate are normalized. These results suggest that metabolism of GL-1 or LysoGL-1 into downstream bioactive lipids is a major contributor to the bone-formation defect. Direct testing revealed a strong inhibition of osteoblast viability by nanomolar concentrations of sphingosine, but not of ceramide. These findings are consistent with toxicity of high circulating sphingosine levels in GD1 patients, which decline upon enzyme-replacement therapy; serum ceramide levels remain unchanged. Together, complementary results from mice and humans affected with GD1 not only pinpoint sphingosine as being an osteoblast toxin, but also set forth Gba2 as a viable therapeutic target for the development of inhibitors to ameliorate certain disabling consequences of GD1.
Gaucher disease (GD) is the most common lysosomal storage disorder, with a frequency as high as 1 in 850 live births in Ashkenazi Jews. Inherited deficiency of lysosomal glucocerebrosidase (GBA) arising from biallelic mutations in the GBA gene underlies the clinical phenotype of GD (1–3). As a consequence, the glucosphingolipids glucosylceramide (GL-1) and glucosylsphingosine (LysoGL-1) accumulate conspicuously within the lysosomes of mononuclear phagocytes (1–4). These lipids spill over into circulation with modest elevation in serum GL-1 levels, but with dramatic increases in LysoGL-1 due to its high solubility (5). It is not understood, however, how the metabolites of GL-1 and LysoGL-1 produced by extralysosomal glucocerebrosidase (GBA2) contribute to GD pathophysiology.
Nonneuronopathic type 1 GD (GD1) manifests classically with striking hepatosplenomegaly, profound cytopenia, and a complex pattern of severe skeletal disease. However, some GD1 patients can display broader phenotypes, including neurodegeneration, autoimmune diathesis, lymphoproliferative neoplasms, and osteopenia (1, 3). Furthermore, there is unexplained phenotypic variability among patients harboring identical GBA mutations, between affected sibling pairs, and even between identical twins (6). Enzyme-replacement therapy (ERT) with macrophage-targeted, recombinant glucocerebrosidase ameliorates certain, but not all of, the manifestations (1). Thus, efforts to understand the basis of phenotypic diversity, unravel disease mechanism, and develop therapies have prompted the generation of mouse models that recapitulate human GD1 (7–9). To this end, we have developed a GD1 mouse, Mx1–Cre+:GD1, in which the Gba gene is deleted in hematopoietic and mesenchymal lineage cells (4, 10, 11). This mouse displays hepatosplenomegaly, cytopenia, osteopenia, Th1/Th2 hypercytokinemia, and an array of defects in early T-cell maturation, B-cell recruitment, and antigen presentation (4, 10).
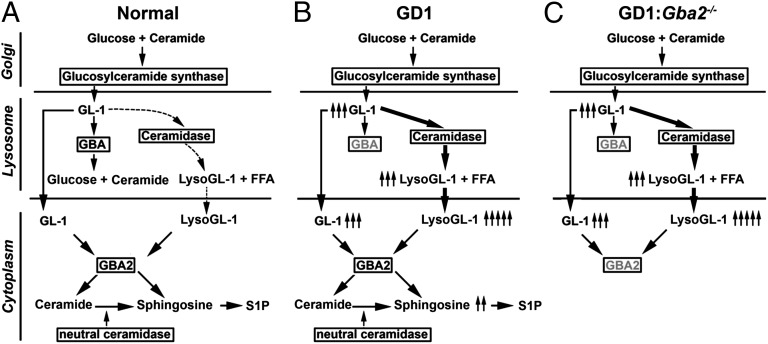
Glucosylceramide synthase in Golgi complex converts ceramide to GL-1, which is used in the production of more complex glycosphingolipids. As membranes turn over in the lysosome, lysosomal glycosidases sequentially cleave off the sugar residues from nonreducing end of glycosphingolipids, and GL-1 is eventually hydrolyzed to ceramide and glucose by lysosomal GBA. In addition, acid ceramidase exhibits expanded enzymatic activity to deacylate GL-1 to LysoGL-1, which is then converted to sphingosine by GBA2 (Fig. 1A) (12–14). GBA deficiency in GD1 thus results in the accumulation of GL-1 and LysoGL-1 in lysosomes and subsequently in the cytosol (Fig. 1B) (15). Hence, cytosolic GL-1 and LysoGL-1 potentially become substrates for the extralysosomal neutral GBA, encoded by GBA2, which should result in the production of ceramide and sphingosine, respectively (16, 17).
Fig. 1.
Aberration of sphingolipid pathways in Gba deficiency. (A) In normal mice, the lipid substrate glucosylceramide (GL-1) is converted by the lysosomal acid glucocerebrosidase Gba to ceramide and by ceramidase to glucosylsphingosine (LysoGL-1). (B) In Gba deficiency, the accumulating GL-1 and LysoGL-1 spill over into the cytoplasm, where the extralysosomal neutral glucocerebrosidase Gba2 converts them to ceramide and sphingosine, respectively. (C) We predict that if the defects in GD are due to products of GL-1 and LysoGL-1—namely, sphingosine or ceramide—the Gba−/− phenotype will be rescued in vivo by the deletion of Gba2.
We speculate that if ceramide and/or sphingosine are the active disease-causing agents in GD1, then deleting Gba2 in Mx1–Cre+:GD1 mice will rescue aspects of the GD1 phenotype, despite enhanced elevations of GL-1 and LysoGL-1 (Fig. 1C). We report visceral, hematologic, skeletal, and partial cytokine rescue of the GD1 phenotype in compound mutant mice. We also show that sphingosine, but not ceramide, inhibits osteoblast survival and that GD1 patients display elevated sphingosine levels that are reduced upon imiglucerase therapy. The data identify sphingosine as one molecular mediator of GD1 pathophysiology and suggest that the extralysosomal GBA2 could potentially be targeted to ameliorate certain disabling manifestations of GD1.
Results
We hypothesized that Gba2-encoded extralysosomal glucocerebrosidase plays a pivotal role in GD pathophysiology. Having generated a Mx1–Cre+:GD1 mouse that recapitulates human GD1, we sought to delete Gba2 in this mouse to generate a compound Mx1–Cre+:GD1:Gba2−/− mouse with a goal to rescue the GD1 clinical phenotype. To generate this mouse, we crossed Gba floxed mice—either wild-type Gbafl/fl or Gbafl/−—with Gba2+/− mice. The mice were finally bred with Mx1–Cre+ mice to yield Mx1–Cre+:GD1:Gba2−/− mice. We have previously shown that Mx1–Cre+:Gbafl/fl and Mx1–Cre+:Gbafl/− mice have an identical clinical phenotype (4). Hence, these mutants were pooled for the rescue studies.
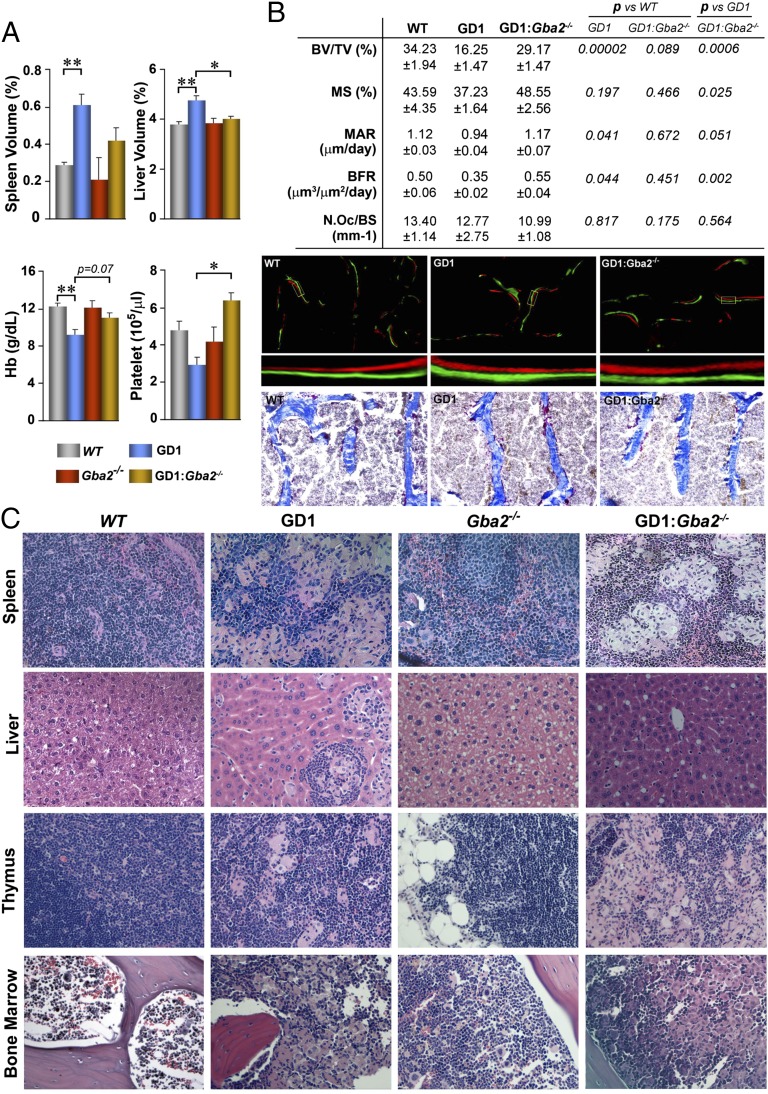
Significant hepatosplenomegaly and cytopenia were noted in Mx1–Cre+:GD1 mice, but not in the global Gba2−/− mice. These phenotypic features were rescued in Mx1–Cre+:GD1:Gba2−/− mice (Fig. 2A). In addition, there was a reversal of the bone mass phenotype. Notably, fractional bone volume (BV/TV), as well as bone formation parameters in calcein/xylelol orange-labeled sections—notably, mineralizing surface (MS), mineral apposition rate (MAR), and bone formation rate (BFR)—were all increased in Mx1–Cre+:GD1:Gba2−/− mice compared with Mx1–Cre+:GD1 mice and were not different from wild-type mice (Fig. 2B). In contrast, osteoclast numbers in tartrate-resistant alkaline phosphatase (TRAP)-labeled sections remained unchanged in all three groups (Fig. 2B). Despite clinical rescue, Gaucher cells identified in Mx1–Cre+:GD1 mice persisted in spleen, thymus, and bone marrow of the double mutant, but were not seen in any tissue of Gba2−/− mice (Fig. 2C).
Fig. 2.
Gba2 deletion in GD1 mice rescues the visceral, hematologic, and skeletal phenotype. (A) Comparison of organ volume, as well as hemoglobin (Hb) and platelet count in wild-type (WT; n = 15), Mx1–Cre+:GD1 (GD1; n = 15), Gba2−/−, and Mx1–Cre+:GD1:Gba2−/− (GD1:Gba2−/−, n = 15) mice. *P ≤ 0.05; **P ≤ 0.01. (B, Top) Dynamic histomorphometry showing rescue of the GD1 skeletal phenotype in compound mutants, as assessed by measurements of bone-volume fraction (BV/TV), mineralizing surface (MS), mineral apposition rate (MAR), bone formation rate (BFR), and osteoclast number/bone surface (N.Oc/BS). (Middle and Bottom) Also shown are representative photomicrographs (magnification: x100) of calcein/xylelol orange-labeled bone (Middle; with digital magnification of the box), as well as TRAP-stained sections (Bottom; Materials and Methods). (C) Hematoxylin/eosin staining (magnification: x200) showing the persistence of focal collections of classical foamy Gaucher cells in spleen, liver, thymus, and bone marrow of GD1 and GD1:Gba2−/− mice; these were not seen in WT or Gba2−/− mice.
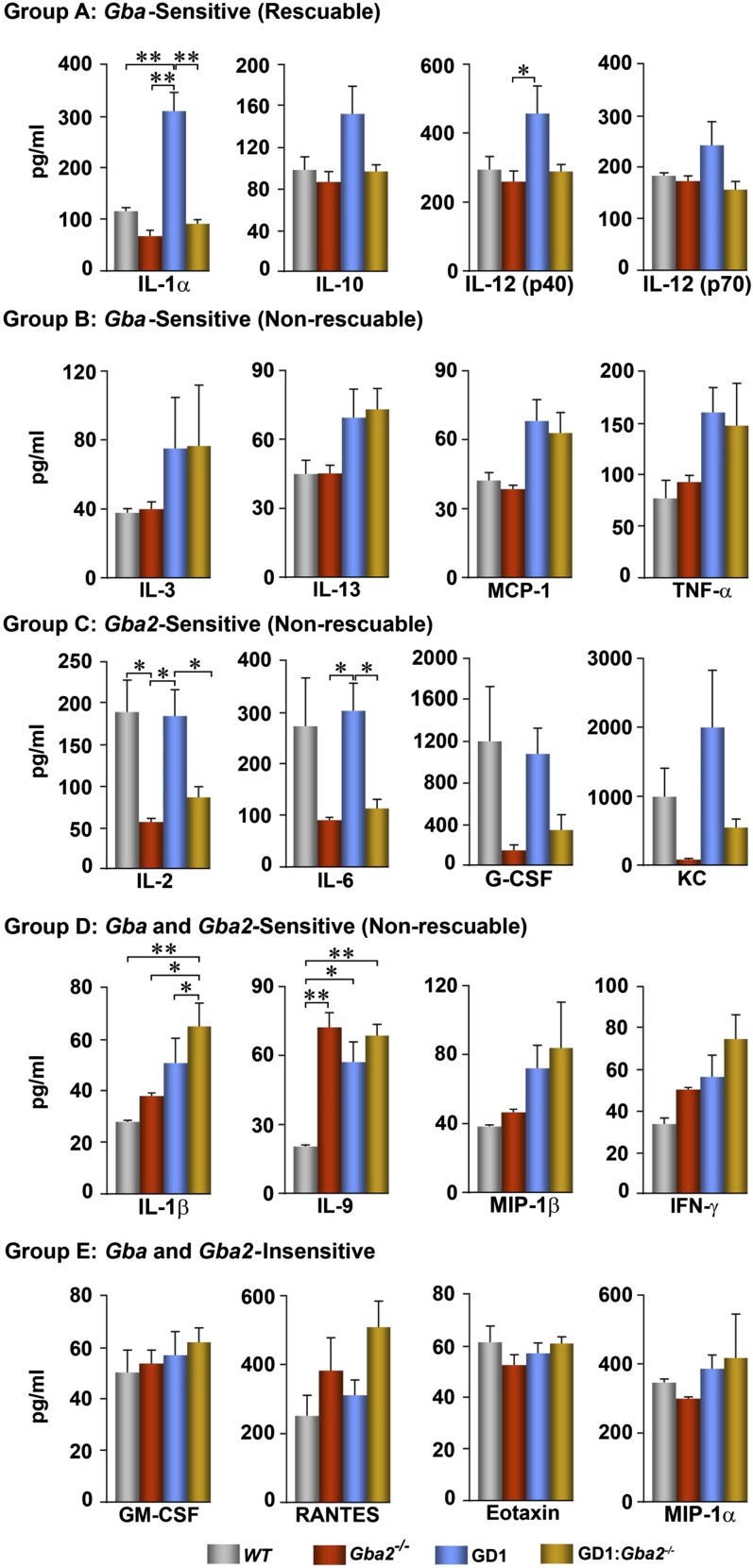
We have shown previously that Mx1–Cre+:GD1 mice exhibit Th1 and Th2 hypercytokinemia and display an array of immune cell defects (4, 10). The immune cell defects included T-cell maturation, B-cell recruitment, and antigen presentation defects (10). Here, we identify five groups of cytokines, groups A–E, which are differently regulated by sphingolipids (Fig. 3). The group A cytokines IL-1α, -10, and -12 were increased significantly in Mx1–Cre+:GD1 mice and reduced to basal levels in the double mutants. That all of these cytokines were B-cell–derived and were sensitive to Gba2 deletion underscores the candidacy of extralysosomal Gba2 as a target to control at least some aspects of B-cell involvement in GD1. Group B cytokines, including IL-3, -13, monocyte chemotatic protein-1 (MCP-1), and TNF-α, were increased in Mx1–Cre+:GD1 mice, but were not sensitive to Gba2 deletion either alone or in combination with Gba deletion. Group C cytokines that included IL-2, -6, granulocyte colony-stimulating factor (G-CSF), and keratinocyte-derived chemokine (KC) were all elevated in Mx1–Cre+:GD1 mice, but suppressed in global Gba2−/− mice, compared with controls. Reduction of these Th1 cytokines in GD1 by targeting Gba2 may provide additional therapeutic benefit in attenuating the accompanying systemic inflammation. Group D cytokines comprised IL-1β, -9, macrophage inflammatory protein-1-beta (MIP-1β), and IFN-γ, which were elevated in response to both Gba and Gba2 deletion, but were not rescueable. The patterns of lack of rescue of certain cytokines in the double mutant may be due to the continued elevation of GL-1 and LysoGL-1 (Fig. 4A), which might itself drive this hypercytokinemia. This GL1/LysoGL1 elevation is consistent with the persistence of Gaucher cells in the double mutant (Fig. 2C), raising the question whether clinical and microscopic rescue involves distinct mechanisms. In contrast, the liver in the double mutant showed a reduction in GL-1 content (Fig. S1) and Gaucher cell burden (Fig. 2C), which might arise from enhanced biliary GL-1 excretion, although the increase was not statistically significant (Fig. S1). Group E contained GM-CSF, regulated upon activation normal T-cell expressed and presumably secreted (RANTES), Eotaxin, and MIP-1α and was insensitive to modulation by Gba or Gba2 deficiency alone or in combination.
Fig. 3.
Different patterns of rescue of hypercytokinemia upon Gba2 deletion. Th1 and Th2 cytokines were measured in wild-type (WT; n = 7), Mx1–Cre+:GD1 (GD1; n = 7), Gba2−/− (n = 7), and GD1:Gba2−/− (n = 7) mice, and, based on responses, were assigned to one of five groups, A–E, as noted. *P ≤ 0.05; **P ≤ 0.01.
Fig. 4.
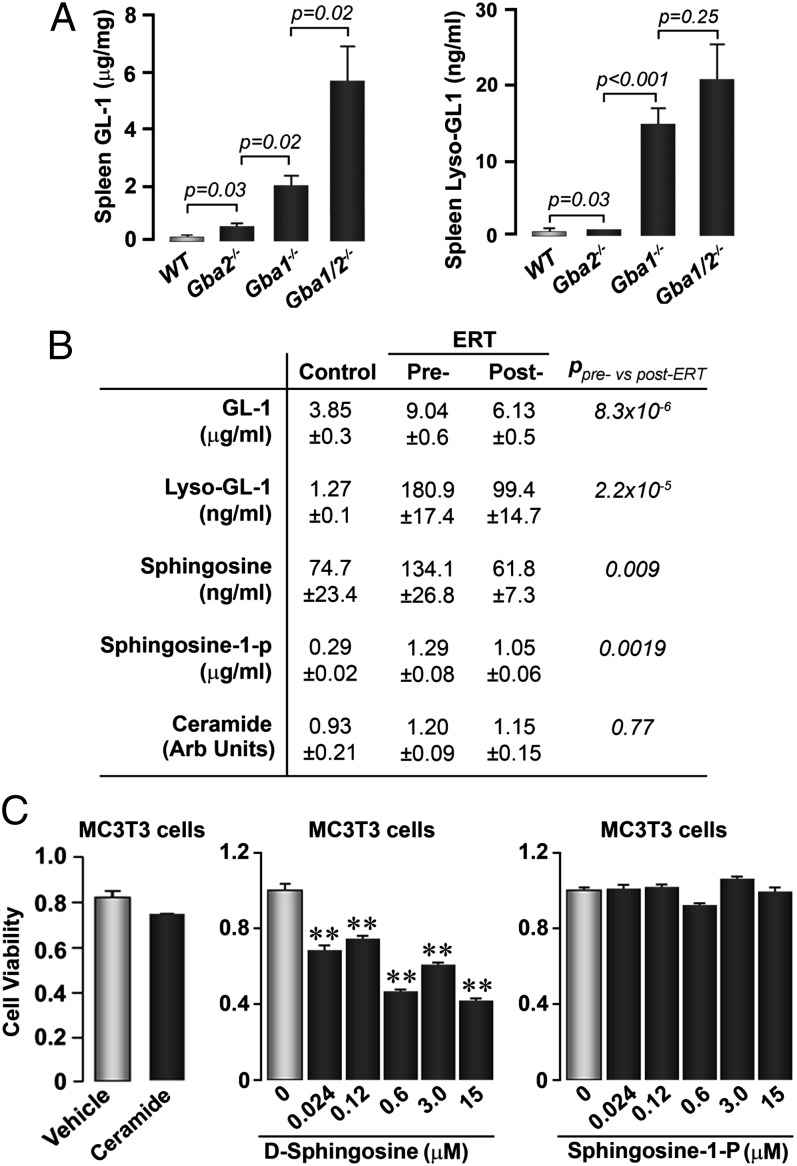
Sphingosine is a likely candidate for osteoblast inhibition in GD1. (A) Splenic GL-1 and gLysoGL-1 levels in wild-type (WT; n = 12), Mx1–Cre+:GD1 (GD1; n = 12), Gba2−/− (n = 12), and GD1:Gba2−/− (n = 12) mice. (B) Serum levels of GL-1, LysoGL-1, sphingosine, sphingosine-1-phosphate (S1P), and ceramide in GD patients before (Pre-) and after (Post-) ERT with Imiglucerase (n = 41), as well as an unaffected control group (unpaired Student t test; P values are shown). (C) MTT assay showing that sphingosine, but not ceramide (15 µM), or S1P (doses shown), reduce MC3T3.E1 cell viability. **P ≤ 0.01 vs. zero dose.
These rescue studies clearly demonstrate that selective targeting of Gba2 can reverse certain GD1 phenotypes in mice. As predicted in our hypothesis (Fig. 1C), we show that GL-1 and LysoGL-1 are elevated in the double-mutant Mx1–Cre+:GD1:Gba2−/− mice, even beyond the significant increases noted in Gba or Gba2 deficiency (Fig. 4A). This finding implies that the modulation of GL-1 and/or LysoGL-1 cannot explain the rescue of organomegaly and cytopenia noted in Mx1–Cre+:GD1:Gba2−/− mice.
We therefore measured GL-1 and LysoGL-1 levels, as well as lipids downstream of Gba2—namely, ceramide, sphingosine, and sphingosine-1-phosphate (S1P)—in sera of GD1 patients before and after ERT with imiglucerase (Fig. 4B). Imiglucerase therapy resulted in a highly significant reduction, not only of serum GL-1 and LysoGL-1, but also of sphingosine and S1P. Ceramide levels remained within the normal range (Fig. 4B). Imiglucerase has also been shown to improve bone disease in GD1 patients (18).
From our findings with the Mx1–Cre+:GD1:Gba2−/− mouse, it seems unlikely that GL-1 and LysoGL-1 are the sole direct mediators of the GD1 phenotype. We therefore tested whether Gba2-regulated sphingolipids—namely, ceramide, sphingosine, or S1P—played a role in the cellular dysfunction noted in GD1. Toward this end, we used MC3T3.E1 preosteoblasts based on previous data showing attenuated osteoblast function in Mx1–Cre+:GD1 mice (4) as well as studies in human GD1 revealing predominant bone formation defects (19). We found that, whereas ceramide and S1P did not affect cell viability in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Fig. 4C), sphingosine potently inhibited cell survival in a concentration-dependent manner (Fig. 4C). This finding suggested that, at least for one of the cell types affected in GD1—namely, the osteoblast—sphingosine, but not ceramide or S1P, was the active inhibitor. Our finding that S1P does not impair cell viability is not in contradiction with reports showing positive effects of this lipid on the differentiation of osteoblasts (20). It is possible, however, that in GD1, among other yet-uncharacterized mediators, sphingosine reduces osteoblast viability (Fig. 4C), and this effect might overcome any stimulation of cell differentiation by its downstream product S1P (20). This process, we believe, ultimately leads to the bone formation defect and low-turnover osteoporosis in GD1.
Discussion
Gba2 is present at normal or elevated levels in GD1 (21). The deficiency of GBA2 in a murine GD model has been reported to enhance GL-1 accumulation in the spleen (22). However, its role in GD pathophysiology is not known. Our results show that the concomitant deletion of the Gba2 gene in GD1 mice rescues, fully or in part, the hepatosplenomegaly, cytopenia, osteopenia, and hypercytokinemia, albeit with persistent Gaucher cells and further elevations in spleen GL-1 and LysoGL-1 levels. There was unexpected dichotomy in the liver in that it was protected from storage. This effect may be due to its ability to excrete GL-1 into bile, which was enhanced upon Gba2 deletion in GD1 mice. The function of Gba2 in the liver is poorly understood, although it has potent bile acid 3-o-β-glucosidase activity, and its deficiency has been linked with impaired liver regeneration (23, 24).
Despite full reversal of hepatosplenomegaly, cytopenia, and skeletal disease in Mx1–Cre+:GD1:Gba2−/− mutants, we observed only partial rescue of hypercytokinemia. The hypercytokinemia was either sensitive to Gba or Gba2 deletion or insensitive to either enzyme. A group of B-cell–derived cytokines—namely, IL-1α, -10, and -12, which were elevated in GD1 mice—were restored to normal by Gba2 deletion. This finding suggests that elevation of these cytokines is driven by lipids downstream of GL-1 and LysoGL-1, notably sphingosine and/or S1P. In contrast, certain T-cell and macrophage-derived cytokines—namely, IL-3, -13, MCP-1, and TNF-α—were not sensitive to Gba2, suggesting that the elevation of these cytokines was driven by GL-1 and/or LysoGL-1. Furthermore, compared with wild-type mice, Gba2 deletion caused an impressive reduction of IL-2, -6, G-CSF, and KC. Although the significance of this latter finding remains unknown, we speculate that GBA2 may determine the susceptibility to inflammatory stimuli via its ability to generate bioactive lipids in the extralysosomal compartment. Overall, however, the different patterns of rescue of hypercytokinemia by Gba2 deletion suggest that extralysosomal neutral glucocerebrosidase is critical for engaging both innate and adaptive immunity in GD1 pathophysiology and that targeting Gba2 may provide additional therapeutic benefit in reducing the systemic inflammation.
Gba2 converts GL-1 and LysoGL-1 to ceramide and sphingosine, respectively. The latter are catalyzed by specific kinases to their 1-phosphate analogs. S1P binds to S1P receptors to stimulate the differentiation, migration, and survival of a variety of cell types (25). This process makes it highly unlikely that S1P contributes to the inhibition of osteoblast function that we observe in Mx1–Cre:GD1 mice and GL-1– and LysoGL-1–exposed cultures (4). GL-1 and LysoGL-1 themselves are equally unlikely candidates, because Gba2 deletion, which further increases GL-1 and LysoGL-1 levels in Mx1–Cre:GD1 mice, rescues the hepatosplenomegaly, cytopenia, and skeletal disease. Focusing on the skeletal disease, we find that sphingosine, even at nanomolar concentrations, decreases the viability of MC3T3.E1 preosteoblasts, whereas S1P, ceramide, or sphingomyelin do not, even at higher micromolar levels. Sphingosine similarly impairs the differentiation of other cell types and can cause cell-cycle arrest and apoptosis (26). In concordance, we find that serum sphingosine in GD1 patients is in the ∼0.4-µM (0.45 ± 0.09 µM) range. It thus seems likely that sphingosine is responsible, at least in part, for the osteoblast defect seen in GD1. Should sphingosine be proven as a global culprit for GD1, the GBA2-encoded extralysosomal glucocerebrosidase could be targeted to ameliorate certain disabling manifestations. Specific and potent inhibitors of neutral glucocerebrosidase, such as N-(5-adamantane-yl-methoxy)pentyl)-deoxynojirimycin (IC50 ∼ 2 nM), have been developed (27). Such inhibitors merit further consideration as potential therapies for GD1. In the brain, however, inhibition of GBA2 may not be therapeutically beneficial, because severe mutations in GBA2 have recently been associated with autosomal recessive ataxia (28, 29).
Like other Mendelian disorders highly prevalent in Ashkenazi Jews, including congenital adrenal hyperplasia (CAH), GD1 is recognized for the extraordinary variability of clinical phenotype (30). Furthermore, similar to CAH, severity cannot be predicted accurately from the knowledge of mutations alone (30, 31). That the primary storage material can, via extralysosomal Gba2, generate an array of bioactive lipids suggests multiple steps in the pathway that may underlie phenotype variability in GD1. Our delineation of a pivotal role for GBA2 in GD1 pathophysiology calls for further adequately powered studies to determine whether differences in the activity of neutral glucocerebosidase in patients harboring identical GBA mutations may, in fact, account for the variable expressivity of GD1. Significant interindividual variability of neutral glucocerebrosidase in cells and tissues of GD1 patients has been reported (21). Finally, delineation of steps from lysosomal accumulation of substrates to the generation of bioactive lipids and ensuing cellular effects might reveal candidate biomarkers that may be more informative than currently available surrogates. Our data compel us, in particular, to evaluate the candidacy of serum sphingosine and S1P as pathophysiologically relevant biomarkers of disease activity, which should permit improved monitoring of GD1 patients while on therapy or under observation.
Materials and Methods
All procedures involving animals were reviewed and approved by Institutional Animal Care and Use Committees of the Yale School of Medicine and the Mount Sinai School of Medicine. Studies on serum sphingolipids in GD patients were approved by the Human Investigations Committee of the Yale School of Medicine. The generation of GD1 mice has been described (4). The GBA2−/− mice did not show a clinical visceral or hematologic phenotype. For the current protocol, Gba2+/− mice, generated from embryos as described (17), were crossed with Gbafl/fl mice, and resulting Gbafl/fl:Gba2−/− mice were bred with Mx1–Cre mice to yield Mx1–Cre:GD1:Gba2−/− mutants. Deletion of LoxP-flanked Gba allele was achieved in week 1 by administering polyinosinic:polyctytidylic acid [poly(I:C)] (Sigma) daily intraperitoneally. This process resulted in the deletion of Gba exons 8–11 in hematopoietic and mesenchymal tissues of the compound mutants (4). For skeletal phenotyping, mice received two sequential injections of calcein (15 mg/kg) and xylelol orange (90 mg/kg) 5 d apart. The WT mice were LoxP/WT/Cre− or LoxP/LoxP/Cre− littermate controls that were not given poly(I:C). These mice were of the same genetic background as the test mice; they were bred from 129/C57 B6 chimera to a mixed background with estimated >75% C57 B6.
The mice were killed with CO2, and visceral organs, including vertebrae, were removed and fixed overnight in 10% (vol/vol) formaldehyde (pH 7.4), followed by embedding in paraffin and sectioning for routine histopathology as described (32). In addition, static and dynamic histomorphometry was performed on frozen sections to calculate BV/TV, MS, MAR, and BFR as described in Zhu et al. (33). Th1 and Th2 cytokines were measured by using a Bioplex-23 Cytokine Array. Sphingolipids were quantitated by using an API 4000 triple-quadrupole mass spectrometer interfaced with Agilent 1200 HPLC. Eight dominant isoforms of GL-1 and LysoGL-1 were identified by spectral analysis under the multiple reaction monitoring mode and quantitated as the ratio of the sum of the eight isoforms to the internal standard (Fig. S1). The MTT assay was performed by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide kit (Sigma), as described in Sun et al. (32).
The data were analyzed by one-way ANOVA with Bonferroni’s correction. Results are expressed as mean ± SEM with significance at P ≤ 0.05. In certain instances, P values are stated. For comparison of pre- and post-ERT values of serum sphingolipid levels, the unpaired Student t test was used.
Supplementary Material
Acknowledgments
We thank David W. Russell (University of Texas Southwestern Medical Center) for providing Gba2−/− mice. This study was supported in large part by National Institutes of Arthritis, Musculoskeletal and Skin Diseases Grant AR 65932 (to M.Z. and P.K.M.). This work was also supported by National Institutes of Health Grants AG23176, DK80490, and AG40132 (to M.Z. and L.S.); National Institutes of Health Grant DK66306 (to P.K.M.); Silvio O. Conte Digestive Diseases Research Core Center Grant DK34989 (to J.L.B. and A.M.); and the Children’s Hormone Foundation (M.I.N.). P.K.M. is also supported by a Genzyme, a Sanofi Company, Center of Excellence Grant in Clinical Translational Research.
Footnotes
Conflict of interest statement: P.K.M. and M.Z. have received honoraria and/or consulting fees from Genzyme.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400768111/-/DCSupplemental.
References
- 1.Grabowski GA. Gaucher disease and other storage disorders. Hematology (Am Soc Hematol Educ Program) 2012;2012:13–18. doi: 10.1182/asheducation-2012.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Brady RO. Benefits from unearthing “a biochemical Rosetta Stone”. J Biol Chem. 2010;285(53):41216–41221. doi: 10.1074/jbc.X110.197954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sidransky E. Gaucher disease: Insights from a rare Mendelian disorder. Discov Med. 2012;14(77):273–281. [PMC free article] [PubMed] [Google Scholar]
- 4.Mistry PK, et al. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc Natl Acad Sci USA. 2010;107(45):19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker N, et al. Elevated plasma glucosylsphingosine in Gaucher disease: Relation to phenotype, storage cell markers, and therapeutic response. Blood. 2011;118(16):e118–e127. doi: 10.1182/blood-2011-05-352971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lachmann RH, Grant IR, Halsall D, Cox TM. Twin pairs showing discordance of phenotype in adult Gaucher’s disease. QJM. 2004;97(4):199–204. doi: 10.1093/qjmed/hch036. [DOI] [PubMed] [Google Scholar]
- 7.Enquist IB, et al. Effective cell and gene therapy in a murine model of Gaucher disease. Proc Natl Acad Sci USA. 2006;103(37):13819–13824. doi: 10.1073/pnas.0606016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizukami H, et al. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002;109(9):1215–1221. doi: 10.1172/JCI14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid beta-glucosidase deficiency: The defect in Gaucher disease. Am J Pathol. 2003;163(5):2093–2101. doi: 10.1016/s0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, et al. Gaucher disease gene GBA functions in immune regulation. Proc Natl Acad Sci USA. 2012;109(25):10018–10023. doi: 10.1073/pnas.1200941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen T, et al. Disease-drug pairs revealed by computational genomic connectivity mapping on GBA1 deficient, Gaucher disease mice. Biochem Biophys Res Commun. 2012;422(4):573–577. doi: 10.1016/j.bbrc.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze H, Sandhoff K. Lysosomal lipid storage diseases. Cold Spring Harb Perspect Biol. 2011;3(6) doi: 10.1101/cshperspect.a004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasagasako N, Kobayashi T, Yamaguchi Y, Shinnoh N, Goto I. Glucosylceramide and glucosylsphingosine metabolism in cultured fibroblasts deficient in acid beta-glucosidase activity. J Biochem. 1994;115(1):113–119. doi: 10.1093/oxfordjournals.jbchem.a124284. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Sasagasako N, Goto I, Kobayashi T. The synthetic pathway for glucosylsphingosine in cultured fibroblasts. J Biochem. 1994;116(3):704–710. doi: 10.1093/oxfordjournals.jbchem.a124584. [DOI] [PubMed] [Google Scholar]
- 15.Elleder M. Glucosylceramide transfer from lysosomes—the missing link in molecular pathology of glucosylceramidase deficiency: A hypothesis based on existing data. J Inherit Metab Dis. 2006;29(6):707–715. doi: 10.1007/s10545-006-0411-z. [DOI] [PubMed] [Google Scholar]
- 16.Boot RG, et al. Identification of the non-lysosomal glucosylceramidase as beta-glucosidase 2. J Biol Chem. 2007;282(2):1305–1312. doi: 10.1074/jbc.M610544200. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz Y, et al. Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J Clin Invest. 2006;116(11):2985–2994. doi: 10.1172/JCI29224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims KB, et al. Improvement of bone disease by imiglucerase (Cerezyme) therapy in patients with skeletal manifestations of type 1 Gaucher disease: Results of a 48-month longitudinal cohort study. Clin Genet. 2008;73(5):430–440. doi: 10.1111/j.1399-0004.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Dussen L, et al. Markers of bone turnover in Gaucher disease: Modeling the evolution of bone disease. J Clin Endocrinol Metab. 2011;96(7):2194–2205. doi: 10.1210/jc.2011-0162. [DOI] [PubMed] [Google Scholar]
- 20.Lotinun S, et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest. 2013;123(2):666–681. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aureli M, et al. Cell surface associated glycohydrolases in normal and Gaucher disease fibroblasts. J Inherit Metab Dis. 2012;35(6):1081–1091. doi: 10.1007/s10545-012-9478-x. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz Y, et al. Functional and genetic characterization of the non-lysosomal glucosylceramidase 2 as a modifier for Gaucher disease. Orphanet J Rare Dis. 2013;8:151. doi: 10.1186/1750-1172-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Carmona MA, et al. Beta-glucosidase 2 knockout mice with increased glucosylceramide show impaired liver regeneration. Liver Int. 2012;32(9):1354–1362. doi: 10.1111/j.1478-3231.2012.02841.x. [DOI] [PubMed] [Google Scholar]
- 24.Harzer K, et al. Beta-glucosidase 1 (GBA1) is a second bile acid β-glucosidase in addition to β-glucosidase 2 (GBA2). Study in β-glucosidase deficient mice and humans. Biochem Biophys Res Commun. 2012;423(2):308–312. doi: 10.1016/j.bbrc.2012.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zu Heringdorf DM, Ihlefeld K, Pfeilschifter J. Pharmacology of the sphingosine-1-phosphate signalling system. Handbook Exp Pharmacol. 2013;(215):239–253. doi: 10.1007/978-3-7091-1368-4_13. [DOI] [PubMed] [Google Scholar]
- 26.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 27.Overkleeft HS, et al. Generation of specific deoxynojirimycin-type inhibitors of the non-lysosomal glucosylceramidase. J Biol Chem. 1998;273(41):26522–26527. doi: 10.1074/jbc.273.41.26522. [DOI] [PubMed] [Google Scholar]
- 28.Hammer MB, et al. Mutations in GBA2 cause autosomal-recessive cerebellar ataxia with spasticity. Am J Hum Genet. 2013;92(2):245–251. doi: 10.1016/j.ajhg.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin E, et al. Loss of function of glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia. Am J Hum Genet. 2013;92(2):238–244. doi: 10.1016/j.ajhg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.New MI, et al. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 2013;110(7):2611–2616. doi: 10.1073/pnas.1300057110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haider S, et al. Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia. Proc Natl Acad Sci USA. 2013;110(7):2605–2610. doi: 10.1073/pnas.1221133110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 33.Zhu LL, et al. Vitamin C prevents hypogonadal bone loss. PLoS ONE. 2012;7(10):e47058. doi: 10.1371/journal.pone.0047058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.