Significance
In Alzheimer’s disease (AD), formation of the Aβ aggregates occurs by the cleavage of the amyloid precursor protein (APP) during its trafficking inside the nerve cells. The Golgi apparatus plays a critical role in APP trafficking; fragmentation of the normally highly ordered Golgi structure occurs in nerve cells of AD patients. Here we report that Aβ accumulation triggers Golgi fragmentation by activating cyclin-dependent kinase-5 (cdk5), which phosphorylates Golgi structural proteins such as GRASP65. Rescue of Golgi structure by inhibiting cdk5 or by expressing nonphosphorylatable GRASP65 mutants reduced Aβ secretion. Our study provides a molecular mechanism for Golgi fragmentation and its effects on APP trafficking and processing, suggesting Golgi as a potential drug target for AD treatment.
Keywords: Golgi stacking, APP processing, GRASP55, amyloidogenic
Abstract
Golgi fragmentation occurs in neurons of patients with Alzheimer’s disease (AD), but the underlying molecular mechanism causing the defects and the subsequent effects on disease development remain unknown. In this study, we examined the Golgi structure in APPswe/PS1∆E9 transgenic mouse and tissue culture models. Our results show that accumulation of amyloid beta peptides (Aβ) leads to Golgi fragmentation. Further biochemistry and cell biology studies revealed that Golgi fragmentation in AD is caused by phosphorylation of Golgi structural proteins, such as GRASP65, which is induced by Aβ-triggered cyclin-dependent kinase-5 activation. Significantly, both inhibition of cyclin-dependent kinase-5 and expression of nonphosphorylatable GRASP65 mutants rescued the Golgi structure and reduced Aβ secretion by elevating α-cleavage of the amyloid precursor protein. Our study demonstrates a molecular mechanism for Golgi fragmentation and its effects on amyloid precursor protein trafficking and processing in AD, suggesting Golgi as a potential drug target for AD treatment.
Alzheimer’s disease (AD) is characterized by the accumulation of neurofibrillary tangles and beta-amyloid plaques. Beta-amyloid plaques are formed by the accumulation of β-amyloid peptide (Aβ), a proteolytic product of the amyloid precursor protein (APP), by the secretases during APP trafficking in the exocytic and endocytic pathways. Trafficking, maturation, sorting, and processing of both APP and its cleaving enzymes require proper functioning of the Golgi apparatus (1–4).
The Golgi apparatus is also linked to the dynamic vesicular network implicated in AD pathogenesis; for example, both the “Swedish” mutant of APP and mutant presenilin 1 (PS1) exert their actions in the trans-Golgi network (TGN) for Aβ generation (5). SORL1 acts as a retention factor for APP in the TGN and thus regulates APP trafficking (5). Some reports have implicated the endosomes in this process, but endosomal function could be affected by altered Golgi-mediated trafficking under disease conditions (6, 7). Furthermore, several recent reports have suggested that the amyloidogenic processing of APP, which produces Aβ, may occur in the Golgi and the late secretory pathway (2, 4). These findings support the requirement for proper Golgi functioning for APP trafficking.
Significantly, Golgi fragmentation has been observed in neurons of AD patients (8–10), but the molecular mechanism leading to Golgi fragmentation in AD remains elusive. Golgi membranes form a unique stacked structure. This structure is maintained by Golgi structural proteins (11, 12), and Golgi fragmentation may be caused by a modification of these proteins, as has been suggested by Sun et al. (13). In that study, the authors showed that cyclin-dependent kinase-5 (cdk5) is activated by the addition of synthetic Aβ peptides into the tissue culture medium, which subsequently phosphorylates GM130 and causes Golgi fragmentation. Whether other Golgi structural proteins are also phosphorylated and contribute to Golgi fragmentation in AD is unclear. More importantly, how Golgi fragmentation affects APP trafficking and Aβ production is unknown. Our previous studies found that Golgi fragmentation enhances vesicle budding from the Golgi membranes and accelerates protein trafficking (14, 15). Thus, fragmentation of the Golgi observed in AD may accelerate APP trafficking and increase Aβ production. However, GM130 is a cis Golgi protein that functions in membrane tethering (16), and a disruption of GM130 function has been shown to reduce membrane trafficking (17). Thus, the relationship between Golgi fragmentation and APP trafficking remains poorly understood.
Golgi fragmentation may impair accurate protein sorting as well. For example, several studies have detected γ-secretase activity in the TGN, cell surface, early endosome, late endosome, lysosome, and extracellular vesicles after exosome release (1–4). The dispersion of γ-secretase activity in the cell may be due in part to the disruption of protein sorting caused by Golgi fragmentation. More importantly, Golgi fragmentation may affect accurate modification, trafficking, and activation not only of APP and its processing enzymes, but also of many other proteins critical for cellular functions. Thus, determining whether, and if so, how, Golgi defects contribute to AD pathogenesis is crucial.
In the present study, we examined the cause of Golgi fragmentation and its effects on Aβ production in AD using tissue culture and transgenic mouse models. Our results demonstrate that the Golgi is fragmented in mouse brain and tissue culture cells expressing APPswe/PS1∆E9 and in hippocampal neurons treated with Aβ. Further experiments provide evidence that Golgi fragmentation results from Aβ accumulation, which causes activation of cdk5 and phosphorylation of Golgi structural proteins, such as GRASP65. Golgi fragmentation in AD in turn accelerates APP trafficking and enhances Aβ production. Significantly, the expression of nonphosphorylatable mutants of GRASP65 or its homolog GRASP55 rescues the Golgi structure and reduces Aβ40 and Aβ42 production. Thus, improving Golgi structure may alleviate the production of Aβ and decelerate the disease progression. These results not only shed light on the pathogenesis of AD, but also help refine the molecular tools necessary to correct Golgi structural defects, reduce Aβ generation, and ultimately delay disease development.
Results
Golgi Is Fragmented in APPswe/PS1∆E9 Transgenic Mice.
To confirm Golgi fragmentation in AD (8), we performed a systematic analysis of Golgi morphology in brain sections of a transgenic mouse model of AD by microscopy. We first assessed the Golgi morphology in hippocampal tissues of 12-mo-old transgenic mice expressing both the APP Swedish mutation (KM 593/594 NL, APPswe) and the exon 9 deletion mutant of human PS1 (PS1∆E9) (Jackson Laboratory; JAX stock no. 005864), a widely used AD transgenic mouse model (18). Under fluorescence microscopy, the Golgi membranes (indicated by TGN38) in the APPswe/PS1∆E9 mice were fragmented and scattered throughout the cell, in marked contrast to the highly ordered, pericentriolar, ribbon-like structure in WT mice (Fig. 1 A and B).
Fig. 1.
Golgi is fragmented in APPswe/PS1∆E9 transgenic mice. (A and B) Fluorescence images of the Golgi in neurons of APPswe/PS1∆E9 transgenic mice. Cryostat sections of hippocampal tissues from 12-mo-old transgenic (B) and WT mice (A) were immunostained for TGN38. (C–F) EM micrographs of the Golgi region in neurons of APPswe/PS1∆E9 transgenic mice. Shown are EM images from ultrathin sections of hippocampal (C and D) and cortical (E and F) tissues from 12-mo-old transgenic mice (D and F) and WT mice (C and E).
Under electron microscopy (EM), the Golgi in 12-mo-old APPswe/PS1∆E9 mice was seen to be severely fragmented in hippocampal (Fig. 1 C and D) and cortical (Fig. 1 E and F) neurons in all (100%) cells analyzed in each tissue (50 cells from cortex, 48 cells from hippocampus), with swollen cisternae and disorganized stacks, In contrast, WT mice exhibited the typical, highly organized Golgi stacks in 67% of the cortex neurons (n = 30, with n the number of neurons analyzed) and 57% of the hippocampal neurons (n = 23) evaluated (Fig. 1). The extent of Golgi fragmentation was relatively more severe in 15-mo-old APPswe/PS1∆E9 mice, suggesting that Golgi defects develop over time. These results confirm that the Golgi in neurons in the AD transgenic mouse model is fragmented and likely functionally defective.
Expression of APPswe and PS1∆E9 in Tissue Culture Cells Causes Golgi Fragmentation.
To determine whether the observed Golgi fragmentation is related to APP expression, we doubly transfected CHO cells with cDNAs encoding WT APP and PS1, APPswe and PS1∆E9 mutants, or an empty GFP vector as a control. Expression of both WT and mutant APP and PS1, but not the control vector, caused Golgi fragmentation. Expression of the mutants had a more dramatic effect, with complete fragmentation of the entire Golgi ribbon (Fig. 2 A–D). This result was confirmed in a CHO cell line stably expressing both APPswe and PS1∆E9, designated as the AD cells in the present study. Using three distinct Golgi markers—GRASP65 (cis Golgi), GRASP55 (medial), and TNG38 (trans)—we found that approximately 70% of cells had fragmented Golgi (Fig. 2 H–K), suggesting that the fragmentation extends throughout the Golgi stack. EM analysis revealed that the Golgi stack in APPswe/PS1∆E9 (AD) cells had a more dilated structure, fewer cisternae per stack, shorter cisternae, and more vesicles surrounding each stack compared with WT cells (Fig. 2 L–N), indicating unstacking and vesiculation of the Golgi membranes.
Fig. 2.
APP and PS1 expression in tissue culture cells causes Golgi fragmentation. (A–C) CHO cells transfected with control vector (A), WT APP and PS1 (B), or APPswe/PS1∆E9 mutants (C) were immunostained for GRASP65. (D) Quantitation (mean ± SEM) of A–C from three independent experiments. (E–J) WT CHO cells and cells stably expressing APPswe/PS1∆E9 were immunostained for three different Golgi markers: GRASP65 (E and H, cis-Golgi), GRASP55 (F and I, medial-Golgi), and TGN38 (G and J, trans-Golgi). (K) Quantitation (mean ± SEM) of E–J. Note the significant Golgi fragmentation in APPswe/PS1∆E9-expressing cells using all three markers. (L–N) EM images of WT and APPswe/PS1∆E9-expressing cells with quantitation. Note that the APPswe/PS1∆E9-expressing cells have fewer cisternae per stack, shorter cisternae, and more vesicles. *P < 0.05; **P < 0.01; ***P < 0.001, Student t test.
To test whether APP-induced Golgi fragmentation in AD cells is reversible, we inhibited APP expression by treating the cells briefly with cycloheximide (CHX). Because APP is a short-lived protein, the brief CHX treatment rapidly reduced APP levels, but it had no detectable effect on Golgi structural proteins, such as GRASP65, or on actin. CHX treatment effectively and reversibly suppressed Golgi disassembly (Fig. S1), supporting our hypothesis that APP expression causes Golgi defects and identifying the cause as reversible modifications (e.g., phosphorylation), rather than degradation of Golgi structural proteins.
Golgi Fragmentation in APPswe/PS1∆E9 Cells Is Caused by Aβ Accumulation.
To determine whether the Golgi fragmentation observed in this study is caused by either holo-APP or its processed fragments, we inhibited APP cleavage by secretase inhibitors. Treatment of cells with the β-site APP-cleaving enzyme inhibitor (BACEi) or the γ-secretase inhibitor N-[N- (3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT) significantly reduced Golgi fragmentation in AD cells (Fig. 3 A–E), whereas the α-secretase inhibitor TNF-α processing inhibitor (TAPI) had no effect on the Golgi structure (Fig. 3B), indicating that Golgi fragmentation is caused by the downstream products of amyloidogenic γ-cleavage [P3, APP intracellular domain (AICD), and Aβ], but not the products of the nonamyloidogenic pathway (sAPPα, C83, AICD, and P3). Treatment with secretase inhibitors had no effect on Golgi morphology in WT cells (Fig. S2 A–D).
Fig. 3.
Aβ accumulation causes Golgi fragmentation in CHO cells and primary hippocampal neurons. (A–D) Inhibition of Aβ production reduces Golgi fragmentation. APPswe/PS1∆E9-expressing CHO cells were cultured in growth medium containing DMSO (A), 20 µM α-secretase inhibitor TAPI (B), 1 µM β-secretase inhibitor BACEi (C), or 5 µM γ-secretase inhibitor DAPT (D) for 8 h and then analyzed by fluorescence microscopy for GRASP65. Note that inhibition of β- and γ-secretases, but not of α-secretase, reduced Golgi fragmentation. (E) Quantitation (mean ± SEM) of A–D. Statistical significance was assessed by comparison of secretase inhibitors to DMSO treatment. (F–H) Treatment with synthetic Aβ peptides leads to Golgi fragmentation in WT cells. WT CHO cells were treated without (control, F) or with 1 µM Aβ40 (G) or Aβ42 (H) for 6 h and immunostained for GRASP65. (I) Quantitation of F–H. Statistical significance was assessed by comparing Aβ-treated and PBS-treated cells. (J–L) EM images (J and K) and quantitation (L) of WT CHO cells treated with PBS or 1 µM Aβ40 as in F–G. (M–P) Cultured primary hippocampal neurons were treated with PBS (control, M), 1 µM Aβ40 (N), or Aβ42 (O) for 6 h and immunostained for GRASP65 in green and the neuronal marker NeuN in red. Fluorescence images are shown in A–C, and quantitation is shown in P. Statistical significance was assessed by comparing Aβ-treated and PBS-treated cells. (Q–S) EM images and quantitation of hippocampal neurons treated with PBS or with 1 µM Aβ42, as in M and O. **P < 0.01; ***P < 0.001, Student t test.
The foregoing results suggest that an APP fragment generated by γ-cleavage, likely Aβ, triggers Golgi disassembly. This was confirmed by direct application of synthetic Aβ peptides to the cell culture medium of WT CHO cells. Aβ treatment induced Golgi fragmentation in WT CHO cells in a dose-dependent manner (Fig. 3 F–I), with approximately 60% of cells containing fragmented Golgi when exposed to as low as 50 nM Aβ peptides, a significantly greater percentage than seen in PBS-treated cells (18.3 ± 0.5%) (Fig. 3F). Because 1 µM Aβ produced the strongest effect on Golgi morphology, we used this concentration in subsequent experiments.
Golgi fragmentation was also indicated by the increased number of Golgi elements per cell after Aβ treatment compared with controls, an alternative method of quantifying Golgi fragmentation (Fig. S3A). EM results confirmed that Aβ treatment caused fragmentation of the Golgi ribbon, reduced the number and length of the cisternae in the stacks, and resulted in significant vesiculation of Golgi membranes in CHO cells treated with Aβ (Fig. 3 J–L). Similar effects were observed in normal rat kidney cells after Aβ treatment (Fig. S4 A–D). Because Aβ secreted by AD cells accumulates in the medium, we tested whether removal of Aβ from the medium could reduce Golgi fragmentation, and found that replacing the overnight culture with fresh medium for 4 h significantly reduced Golgi fragmentation in AD cells (Fig. S4 E–H). Consistently, treatment of WT CHO cells with AD cell-conditioned medium (from a 24-h incubation) caused significant Golgi fragmentation in a time-dependent fashion (Fig. S4 I–O). Taken together, these findings confirm that Aβ accumulation in the tissue culture medium causes Golgi fragmentation.
Aβ Treatment Causes Golgi Fragmentation in Hippocampal Neurons in Culture.
Because Aβ accumulates in the brain of AD mice, we evaluated whether Aβ is sufficient to cause Golgi fragmentation in neurons. We isolated hippocampal neurons from newborn mice (1 d old), cultured them on poly-l-lysine–coated coverslips for 4 d, and then added synthetic Aβ peptides to the neuronal culture medium. As shown in Fig. 3 M–P and Fig. S3B, Aβ treatment caused severe fragmentation of the Golgi in neurons in a dose-dependent manner, whereas untreated control neurons showed intact Golgi. This observation was further confirmed by EM showing that Aβ treatment caused fragmentation of the Golgi ribbon, reduced the number and length of the cisternae in the stacks, and resulted in significant vesiculation of Golgi membranes (Fig. 3 Q–S). These results suggest that Aβ accumulation in the brain induces Golgi fragmentation in the neurons.
Aβ-Induced Golgi Fragmentation Is Caused by cdk5 Activation.
The next important question that we addressed is how Aβ peptide accumulation causes Golgi fragmentation in the cell. The stacked structure of the Golgi is maintained by Golgi structural proteins, and either mitotic phosphorylation or apoptotic cleavage of these proteins can cause Golgi fragmentation (11, 19). Because Aβ-induced Golgi fragmentation is rapidly reversible (Fig. 3 and Fig. S1), phosphorylation of one or more Golgi structural proteins is the likely underlying mechanism. Previous reports have demonstrated cdk5 activation in AD (13, 20) associated with tau hyperphosphorylation (21) and neurodegeneration (22).
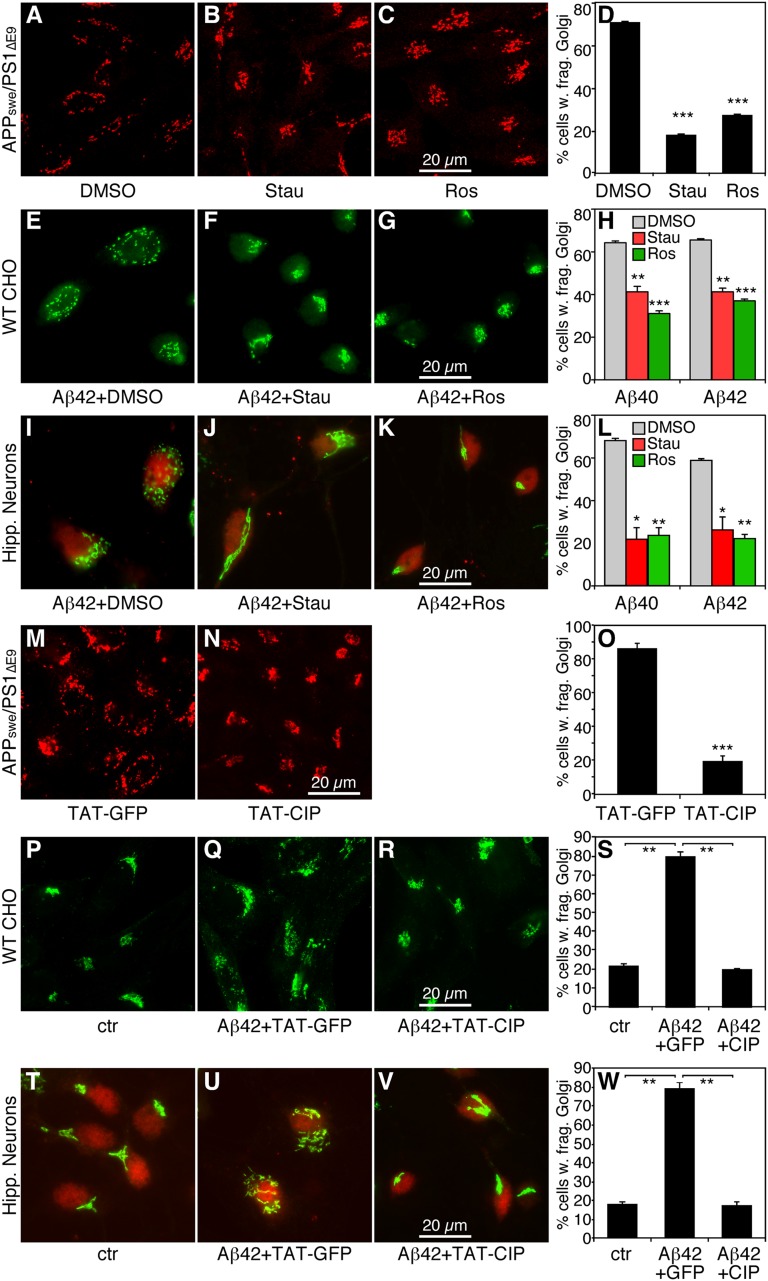
Significantly, activated cdk5 in AD also phosphorylates GM130 (13), a Golgi tethering protein that interacts with GRASP65. Thus, we investigated whether activation of cdk5 by Aβ accumulation could be the cause of Golgi fragmentation in different model systems, including AD cells and neuronal cultures from mice. As shown in Fig. 4 A–D, treatment of cells with staurosporine, a general kinase inhibitor, or roscovitine, a cdk-specific inhibitor, reduced Golgi fragmentation in AD cells. We observed similar effects of kinase inhibitors in WT CHO cells treated with either Aβ peptides (Fig. 4 E–H) or AD cell-conditioned medium (Fig. S4 I–Q). Aβ−treated hippocampal neurons followed a similar rescue pattern by both staurosporine and roscovitine (Fig. 4 I–L). In these experiments, we treated the cells with kinase inhibitors only briefly (up to 2 h), to avoid apoptosis. Of note, treatment of WT cells with same kinase inhibitors had no effect on Golgi morphology (Fig. S2 E–G).
Fig. 4.
Kinase inhibitors reduce Aβ-induced Golgi fragmentation. (A–C) Kinase inhibitors reverse Golgi fragmentation in APPswe/PS1∆E9-expressing (AD) cells. Cells cultured overnight were treated with DMSO (A), 1 µM staurosporine (B), or 15 µM roscovitine (C) for 2 h. Chemicals were added directly to the tissue culture dishes without changing the media. Cells were immunostained for GRASP65. (D) Quantitation of A–C. (E–G) Kinase inhibitors reverse Golgi fragmentation in Aβ-treated WT CHO cells. WT CHO cells were treated with Aβ in the presence of DMSO (E), 1 µM staurosporine (F), or 8 µM roscovitine (G) for 2 h, and then stained for GRASP65. (H) Quantitation of E–G. (I–K) Kinase inhibitors reverse Aβ-induced Golgi fragmentation in hippocampal neurons. Primary hippocampal neurons were treated with Aβ peptides in the presence of DMSO (I), 1 µM staurosporine (J), or 8 µM roscovitine (K) for 2 h, and then stained for GRASP65. (L) Quantitation of I–K. (M and N) TAT-CIP treatment reverses Golgi fragmentation in AD cells. Cells cultured overnight were treated with 600 nM TAT-GFP (M) or TAT-CIP (N) (added directly into the medium) for 12 h, then immunostained for GRASP65. (O) Quantitation of M and N. (P–R) TAT-CIP inhibits Golgi fragmentation in Aβ-treated WT CHO cells. WT CHO cells were treated with 1 µm control peptide (P) or Aβ42 for 12 h in the presence of 600 nM TAT-GFP (Q) or TAT-CIP (R) added 30 min before Aβ42, then immunostained for GRASP65. (S) Quantitation of P–R. (T–W) TAT-CIP reverses Aβ-induced Golgi fragmentation in hippocampal neurons. Primary hippocampal neurons were treated with either control peptide (T) or 1 μM Aβ42 for 12 h in the presence of 600 nM TAT-GFP (U) or TAT-CIP (V) added 30 min before Aβ42, stained for GRASP65, and quantified (W). *P < 0.05; **P < 0.01; ***P < 0.001, Student t test.
Because roscovitine is not specific for cdk5, we applied the cdk5-specific inhibitor peptide (CIP) to AD cells, as well as to Aβ-treated WT CHO cells and hippocampal neurons. CIP is a 126-aa peptide containing p35154–279, which blocks cdk5 interaction with its activator p25, thereby specifically inhibiting cdk5. The CIP is fused to an 11-aa HIV TAT sequence (TAT-CIP) for better temporal and dosage control when applied to cells (14). TAT-GFP served as a control. Compared with TAT-GFP treatment, TAT-CIP treatment of AD cells significantly reduced the percentage of cells with Golgi fragmentation (Fig. 4 M–O), as well as the number of Golgi elements per cell (Fig. S3C). TAP-CIP treatment also rescued the Golgi structure in WT CHO cells (Fig. 4 P–S and Fig. S3D) and hippocampal neurons (Fig. 4 T–W and Fig. S3E) treated with Aβ peptides. These results strongly suggest that activated cdk5 in AD causes Golgi fragmentation.
Cdk5 activation is mediated by the cleavage of p35 to generate the cdk5 activator p25. It has been proposed that Aβ can trigger Ca2+ influx (23), which activates calpain, a Ca2+-dependent protease that cleaves p35 to produce p25 for cdk5 activation (24). Western blot analysis revealed reduced p35 levels and increased p25 levels in hippocampal neurons treated with Aβ peptide and in AD mouse brain, with the relative p25/p35 ratio increased by more than twofold compared with controls (Fig. 5A). Taken together, these results support the view that Aβ accumulation activates cdk5, which causes Golgi fragmentation in AD, as reported previously (13). Thus, identifying the substrates of cdk5 on the Golgi membranes is important.
Fig. 5.
Activation of cdk5 by Aβ accumulation phosphorylates GRASP65. (A) Cdk5 is activated by Aβ treatment. Hippocampal neurons in culture were treated with DMSO (lane 1) or 2 μM Aβ42 (lane 2) for 20 h. Lysates of these cells, of 12-mo-old control (lane 3), or of AD mouse brain (lane 4) were evaluated for p35/p25 and actin by Western blot analysis. Note the increased p25 levels in the Aβ-treated cells and AD mouse brain. (B) GRASP65 is phosphorylated in APP-expressing cells on Aβ accumulation. APPswe/PS1∆E9 cells stably expressing GFP-tagged GRASP65 WT protein (p65FL) were cultured overnight to accumulate Aβ in the media. The media for cells in lanes 1 and 4 were changed every hour for 4 h to remove Aβ from the medium, whereas cells in lanes 2, 3, and 5 were maintained in the old media. In lane 3, cells were treated with 15 μm roscovitine (Ros) in the old medium for another 2 h. After treatment, cells were lysed, immunoprecipitated using an anti-GRASP65 polyclonal antibody (lanes 1–3) or with control rabbit IgG (lanes 4 and 5), and evaluated by Western blot analysis for total GRASP65 (Lower) or phosphorylated GRASP65 (Upper). Note that GRASP65 is phosphorylated on Aβ accumulation (lane 2), and that this phosphorylation is inhibited by roscovitine (lane 3). (C) Aβ treatment-triggered GRASP65 phosphorylation depends on cdk5. Cells stably expressing GFP-tagged GRASP65 WT protein (p65FL) were treated with 4 µM control peptide (lane 1) or Aβ42 (lanes 2–4) for 12 h. During the last 2 h of incubation, 15 μM roscovitine (Ros) (lane 3) or 600 nM TAT-CIP (lane 4) was added directly into the medium. Cells were lysed and evaluated by Western blot analysis for phospho-GRASP65 and total GRASP65 as in B. (D) Activated cdk5 by p25 phosphorylates GRASP65. SH-SY5Y cells were transfected with cdk5 alone (lanes 2 and 3) or with cdk5+p25 (lanes 4–8). Cdk5 was immunoprecipitated and used to treat His-tagged recombinant GRASP65 in the presence or absence of roscovitine or TAT-CIP. The p25-activated cdk5 (lanes 4 and 5) phosphorylated the recombinant GRASP65 to a greater extent than the nonactivated cdk5 (lanes 2 and 3) or the nontransfected cdk5 (lane 1). Roscovitine (lane 6) and CIP (lanes 7 and 8) significantly reduced GRASP65 phosphorylation. (E–I) Activation of cdk5 by p25 causes Golgi fragmentation and enhances Aβ production. SH-SY5Y cells were transfected with cdk5 (F) and cdk5+p25 (G and H) for 16 h. Cells in H were subsequently treated with 600 nM TAT-CIP for 12 h. Nontransfected cells served as a negative control (E). Cells were stained for GRASP65, and images were quantified (I). (J and K) ELISA measurement of Aβ40 (J) and Aβ42 (K) secretion by the cells described in E–I. Shown are the average results from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, Student t test.
Aβ-Induced Golgi Fragmentation Is Caused by Phosphorylation of GRASP65 by Activated cdk5.
Formation of the highly organized stacked Golgi structure is mediated by Golgi structural proteins (12), including GRASP65 (25), GRASP55 (26), GM130 (16), golgin-160 (27), and p115 (28). Among these proteins, GRASP65 and GRASP55 play essential roles in Golgi structure formation, whereas the others are important for trafficking across the Golgi stack. GRASP65 forms oligomers that function as a protein “glue” to tether the cisternae into stacks (25, 29) and ribbons (30). GRASP65 is the major target of mitotic kinases on the Golgi membranes. Phosphorylation of GRASP65 by the mitotic kinases such as cdk1 disassembles GRASP65 oligomers, causing the Golgi to disassemble in mitosis. Conversely, dephosphorylation of GRASP65 restacks cisternae (11, 31).
To examine whether Aβ accumulation triggers GRASP65 phosphorylation, we expressed full-length GRASP65-GFP in AD cells, immunoprecipitated the protein, and then performed Western blot analysis using an antibody (LX108) that recognizes GRASP65 phosphorylated on T220/T224 (32). The results show that GRASP65 was highly phosphorylated in AD cells (Fig. 5B). Removing Aβ by changing the medium or adding roscovitine to the medium led to significantly reduced GRASP65 phosphorylation. GRASP65 phosphorylation in AD cells was confirmed by immunofluorescence microscopy using R3F2, an antibody that recognizes GRASP65 phosphorylated on S376 (32). R3F2 immunoreactivity was detected in cells only after Aβ accumulation, and was abolished in the presence of roscovitine (Fig. S5).
To identify the specific role of cdk5 in GRASP65 phosphorylation on Aβ accumulation, we treated cells with Aβ42 (lane 2) and with a control peptide (lane 1) of similar length as Aβ42 in the presence or absence of kinase inhibitors and evaluated GRASP65 phosphorylation as described in Fig. 5B. As shown in Fig. 5C, GRASP65 phosphorylation occurred when the cells were treated with Aβ42 (lane 2), but not with control peptide or PBS (lane 5), and was abolished by the addition of roscovitine (lane 3) or TAT-CIP (lane 4).
To provide evidence that activated cdk5 directly phosphorylates GRASP65, we transfected SH-SY5Y cells with cdk5 alone or with its activator p25, immunoprecipitated these cells with cdk5, and then incubated them with purified recombinant GRASP65 (25). After incubation, we evaluated GRASP65 phosphorylation by Western blot analysis using the phosphospecific antibody LX108 (32). As shown in Fig. 5D, strong phosphorylation of GRASP65 required both cdk5 and p25 (lanes 4 and 5) and was significantly reduced by roscovitine (lane 6) and TAT-CIP (lanes 7 and 8). Consistent with these results, expression of both cdk5 and p25 caused severe Golgi fragmentation in SH-SY5Y cells. This effect was dependent on p25; expression of cdk5 alone had little, if any, effect on Golgi morphology. TAT-CIP treatment also rescued Golgi morphology in cdk5/p25-expressing SH-SY5Y cells (Fig. 5 E–I).
We then measured the Aβ level in the media of these cells by ELISA. Expression of cdk5/p25 increased both Aβ40 and Aβ42 levels, whereas rescue of Golgi structure by TAT-CIP treatment significantly reduced Aβ40 and Aβ42 production (Fig. 5 J and K). Taken together, these results strongly suggest that Aβ accumulation activates cdk5, which phosphorylates GRASP65 and causes Golgi fragmentation.
In addition to phosphorylation, another mechanism known to cause Golgi fragmentation is caspase-mediated cleavage of Golgi structural proteins, including GRASP65 (33), GM130 (34), Golgin 160 (35), and p115 (28), during apoptosis. Previous studies have shown that p115 levels are reduced by Aβ treatment (13). However, we detected no cleavage of GRASP65, GM130, Golgin 160, or p115 in AD mouse brain or cells, and found that the levels of other Golgi structural proteins, such as GARSP55 and syntaxin 5, remain unchanged compared with those in the control mice or cells (Fig. S6). In addition, treatment of AD cells with a pan-caspase inhibitor, Z-VAD, did not rescue the Golgi structure in AD cells. Taken together, these results demonstrate that Aβ-induced Golgi fragmentation occurs through phosphorylation, but not through cleavage or degradation, of Golgi structural proteins.
Three Methods for Rescuing the Golgi Structure in APP-Expressing Cells.
Our results so far suggest that Aβ accumulation increases cdk5 activity, which phosphorylates Golgi structural proteins like GRASP65 and causes Golgi fragmentation. If this interpretation is correct, then we should be able to rescue Golgi structure in several ways: by reducing APP expression (Fig. S1) and cleavage (Fig. 3), by inhibiting cdk5 (Fig. 4 and Fig. S4 I–L and Q), and by expressing nonphosphorylatable mutants of GRASP65. To test the latter prediction, we stably transfected AD cells with GFP-tagged full-length GRASP65 (p65FL) or GRASP55 (p55FL) or with the respective GRASP domains p65GD and p55GD (Fig. 6). GRASP65 localizes and functions on the cis side of the Golgi stack, whereas its homolog GRASP55 stacks medial- to trans-Golgi in a similar way (26, 36). Both GRASP domains can form oligomers but lack essential phosphorylation sites, and thus inhibit mitotic Golgi disassembly during mitosis (26, 32). Expression of GFP had no effect on the Golgi, whereas p65GD and p55GD expression significantly reduced Golgi fragmentation. Expression of p65FL or p55FL caused a less significant reduction of Golgi fragmentation in cells (Fig. 6 A–F and Fig. S3F). In this experiment, not all cells expressed the indicated GRASP constructs. The GFP-negative cells in the GRASP dishes also showed less Golgi fragmentation than those in the GFP dish (Fig. S3 G and H), although to a lesser degree than the transfected cells (Fig. 6 A–F and Fig. S3F), indicating a possibility that rescue of the Golgi structure by GRASP expression may reduce Aβ production as well.
Fig. 6.
Golgi structure and function are rescued by expression of nonphosphorylatable GRASP mutants. (A–E) Expression of GRASP55, GRASP65, and their nonphosphorylatable mutants rescues the Golgi structure in AD cells. AD cells stably expressing APPswe/PS1∆E9 were transfected with GFP (A), GFP-tagged GRASP-domains p55GD (B) or p65GD (D), or full-length proteins p55FL (C) or p65FL (E) of GRASP55 or GRASP65. Shown are fluorescent images of GFP and GM130. Note that the Golgi is more compact in cells expressing GRASP55/65 and their mutants (positive cells indicated by asterisks). (F) Quantitation of A–E. (G) Western blot of the cells in A–E for indicated GFP and GFP-tagged proteins using a GFP antibody. Actin served as a loading control. *P < 0.05; **P < 0.01; ***P < 0.001, Student t test.
Rescue of the Golgi Structure by GRASP Expression Reduces Aβ Production.
We next examined how rescue of the Golgi structure in AD cells by GRASP expression affects APP trafficking and Aβ production. On Western blot analysis, expression of GRASP constructs did not decrease APP or PS1 levels in AD cells (Fig. S7 A and B). Expression of p65GD or p55GD significantly reduced Aβ40 and Aβ42 production as determined by ELISA (Fig. 7 A–D and Fig. S7 C–F). Compared with GFP-expressing cells, expression of p65GD or p55GD reduced Aβ40 and Aβ42 production by >90%. Expression of full-length GRASP65 or GRASP55 led to a significant, albeit less dramatic, reduction in Aβ40 and Aβ42 production.
Fig. 7.
Rescue of the Golgi structure reduces Aβ production. (A–D) Expression of GRASPs and their nonphosphorylatable mutants reduces Aβ production. AD cells transfected with GFP, GFP-tagged GRASP domain (p55GD/p65GD), or the full-length protein (p55FL/p65FL) of GRASP55 or GRASP65 were incubated overnight, after which Aβ40 (A and C) and Aβ42 (B and D) were measured in the media by ELISA. Shown are the average results from three independent experiments. (E and F) Expression of nonphosphorylatable GRASP mutants reduces Aβ production. (E) AD cells transfected with indicated constructs were labeled by 35S-met/cys for 4 h at 37 °C, after which APP in the cell lysate and Aβ in the media were immunoprecipitated and analyzed by SDS/PAGE and autoradiography. (F) Quantitation of E from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, Student t test.
To confirm the effect of GRASP expression on Aβ production, we labeled the cells with [35S]methionine/cysteine (met/cys), immunoprecipitated full-length APP from the cell lysate and Aβ from the media, and performed autoradiography and phosphoimaging. As shown in Fig. 7 E and F, expression of p65GD or p55GD significantly reduced Aβ production, but not APP synthesis. Expression of full-length GRASP proteins also reduced Aβ production, but to a less significant degree.
GRASP Expression Affects APP Trafficking and Promotes α-Cleavage of APP.
We next asked how rescue of the Golgi structure by GRASP expression reduces Aβ production. To determine the effect of GRASP expression on APP trafficking and subcellular localization, we used a sucrose density gradient to fractionate subcellular components. Expression of p65GD significantly increased the amount of APP in the Golgi fractions relative to the denser fractions, presumably the endosomes and plasma membrane (Fig. 8 A and B). This accumulation of APP in the Golgi suggests the possibility of slow intra-Golgi and Golgi-to-plasma membrane trafficking.
Fig. 8.
Rescue of the Golgi structure enhances APP α-cleavage. (A and B) Expression of nonphosphorylatable GRASP mutants results in APP accumulation in the Golgi. (A) APPswe/PS1∆E9-expressing AD cells expressing GFP, GFP-tagged GRASP domain (p65GD), or full-length GRASP65 (p65FL) were homogenized, fractionated by a sucrose equilibrium gradient, and evaluated by Western blot analysis for APP and syntaxin 5 (syn5) as a Golgi marker. (B) Quantitation of A for % APP in the Golgi fractions (fractions 3 and 4 of the sucrose gradient). Note that the relative amount of APP in Golgi-enriched fractions is increased in p65GD-expressing cells. (C) Expression of GRASP constructs increases sAPPα production. AD cells expressing indicated GRASP constructs were treated with CHX for the indicated times, after which sAPPα in the medium and APP in the cell lysate were evaluated by Western blot analysis. Note the increased sAPPα signal in the medium in GRASP-expressing cells (lanes 5, 6, 11, and 12) compared with GFP-expressing cells (lanes 4 and 10). (D) Expression of the GRASP domain of GRASP65 (p65GD) increases sAPPα production. APPswe/PS1∆E9-cells transfected with GFP or p65GD were pulse-labeled by 35S-met/cys for 15 min and then chased for the indicated times. APP from the cell lysate and sAPPα from the media were immunoprecipitated using the 6E10 antibody, followed by analysis with SDS/PAGE and autoradiography. Note the increased sAPPα signal in the p65GD cell medium (lane 6 vs. lane 5). (E) Working hypothesis. APP expression and processing cause Aβ accumulation (1), which induces Golgi fragmentation through modification of Golgi structural proteins (2), which in turn increases Aβ production by enhancing amyloidogenic cleavage (3). This deleterious feedback loop would impair the integrity of the secretory pathway and compromise neuronal function; thus, rescue of the Golgi structure and function may reduce Aβ production and thereby delay AD development (4).
To further investigate how rescue of the Golgi structure may affect protein trafficking, we tracked the trafficking of the temperature-sensitive mutant of the vesicular stomatitis virus glycoprotein (VSV-G ts045) (37). To do so, we infected GFP- or p65GD-expressing AD cells with adenoviruses encoding VSV-G ts045-GFP at 40.5 °C for 16 h and then at 32 °C (with CHX to block new protein synthesis), to release VSV-G from the endoplasmic reticulum (ER) to the Golgi. We then treated the samples with endoglycosidase H (EndoH), which removes sugars from the glycoprotein when it is in ER high-mannose form, but fails to do so when VSV-G is further modified by the Golgi sugar transferases. This difference is shown by a band-shift on Western blot analysis and thus can be used to monitor VSV-G trafficking (15). As shown in Fig. S8, rescue of the Golgi structure by GRASP65 expression reduced VSV-G trafficking at different time points of release. This result is consistent with our previous finding that disruption of Golgi enhances protein trafficking (14, 15), in contract to the disruption of GM130 function, which slows trafficking (17).
To determine the effect of GRASP expression on APP α-cleavage, we treated cells with CHX and tracked APP in the cell and sAPPα secretion into the medium by Western blot analysis. Expression of GRASPs and especially of the GRASP domains significantly increased sAPPα production (Fig. 8C, lane 5 vs. lane 4 and lane 11 vs. lane 10). To confirm this result, we labeled the cells with 35S- met/cys for 15 min, followed by a chase of different time periods; immunoprecipitated APP from cell lysate and sAPPα from media; and analyzed the precipitates by SDS/PAGE and autoradiography. As shown in Fig. 8D, expression of p65GD significantly increased sAPPα levels in the media. After a 90-min chase, the sAPPα level was 2.5-fold greater in the p65GD-expressing AD cells compared with the GFP cells based on three independent experiments. These results demonstrate that p65GD expression promotes nonamyloidogenic α-cleavage. The finding that APP level in the cells was not decreased by p65GD expression (Fig. 7E and Fig. S7 A and B) suggests that p65GD may reduce β and γ cleavage. Taken together, these results demonstrate that rescue of the Golgi structure by inhibition of cdk5 or by expression of nonphosphorylatable forms of GRASPs reduces Aβ production by shifting APP cleavage toward the nonamyloidogenic pathway.
Discussion
The Golgi is an essential membrane organelle in the trafficking and processing of both APP and its processing enzymes. Golgi defects in AD were reported more than a decade ago, but the underlying mechanism and the biological consequences have remained largely unexplored. In this study, we examined the structural and functional defects of the Golgi in AD mouse and tissue culture models and found that Aβ accumulation by APP expression and processing causes Golgi structural defects that directly affect APP trafficking and processing.
At the molecular level, Aβ accumulation increases cdk5 activity by enhancing the cleavage of p35 and production of p25. Activated cdk5 then phosphorylates Golgi structural proteins and causes Golgi fragmentation, which in turn accelerates APP trafficking and increases Aβ production. This deleterious feedback loop impairs the integrity of the secretory pathway and compromises neuronal functions.
In this respect, our findings reveal Golgi fragmentation as an important mechanism through which Aβ may exert its toxic effects. A major potential unrecognized source of Aβ toxicity may be compromised Golgi integrity and interruption of the proper trafficking and processing of numerous proteins essential for neuronal function. Importantly, we have developed molecular tools to rescue the Golgi structure, which significantly reduces Aβ production (Figs. 5–8).
We found that Aβ-triggered Golgi fragmentation most likely occurs through phosphorylation rather than degradation of Golgi structural proteins, at least in the early stages. Golgi fragmentation is rapidly reversible, rescued largely by treatment of AD cells with cdk5 inhibitors. It has been proposed that Aβ can trigger Ca2+ influx (23). Subsequently, Ca2+ activates calpain, a Ca2+-dependent protease that cleaves p35 to produce the cdk5 activator p25 (24). We found increased p25 levels in hippocampal neurons treated with Aβ and in AD mouse brains. Previous studies have implicated activated cdk5 in tau hyperphosphorylation (22, 38). Cdk5 also phosphorylates GM130 in AD (13). Similar to tau, the GRASP proteins have multiple phosphorylation sites that are phosphorylated by cdk1 during mitosis (32, 39, 40). Consistently, GRASP65 is phosphorylated in APPswe/PS1∆E9-expressing cells, which is inhibited by the cdk inhibitors roscovitine and CIP, and activated cdk5 directly phosphorylates GRASP65 in vitro.
Importantly, both inhibition of cdk5 and expression of the nonphosphorylatable GRASP domain of the GRASP proteins were found to rescue the Golgi structure. The GRASP domain has been shown to form oligomers, but it cannot be phosphorylated (29, 41), and thus its oligomerization cannot be interrupted by activated kinases. These characteristics make the GRASP domain a perfect tool for rescuing the Golgi structure.
In addition to GM130 phosphorylation, previous work also revealed p115 degradation in Golgi fragmentation in AD (13). This does not seem to be the cause in our model systems, given that we detected no reduction in protein levels of p115 or other Golgi structural proteins that we evaluated. Similar to GM130, p115 is involved in membrane tethering; disruption of p115 function also reduces membrane trafficking (42, 43). However, our results using VSV-G as a marker demonstrated accelerated protein trafficking in cells with fragmented Golgi, whereas rescue of the Golgi structure by GRASP expression reduced protein trafficking (Fig. S8). These results support our hypothesis that Golgi fragmentation in AD is caused mainly by phosphorylation of the GRASP proteins.
Consistent with our observation that disruption of the Golgi accelerates protein trafficking (14, 15), rescue of the Golgi led to enrichment of APP in the Golgi, promotes nonamyloidogenic cleavage, and thus reduces Aβ production (Figs. 7 and 8 and Fig. S7 C–F). One plausible explanation for this finding is that in the Golgi stacks, vesicles can be generated only from the rims of the cisternae; unstacking or fragmentation of the Golgi increases the surface area for vesicle formation and thus accelerates protein trafficking. It is possible that cells with a compact Golgi structure have reduced trafficking, whereas partial fragmentation of the Golgi enhances protein transport. It has been shown that knockdown of either GOLPH3 or MYO18A results in a compact Golgi structure and reduced trafficking of VSV-G (44).
Golgi fragmentation has been observed under physiological conditions, such as during migration, on treatment with growth factor, and in neurons with increased neuronal activity (45, 46), suggesting that Golgi fragmentation may be not an immediate pathological response, but rather a compensatory reaction to allow rapid transport of proteins to their final destinations when the cells are under stress. Golgi fragmentation affects protein glycosylation and sorting (14, 15), however. In AD neurons, Golgi fragmentation and dysfunction not only may affect trafficking, modification, and processing of APP and its processing enzymes, but also may cause global changes on the cell surface. For example, defects in protein and lipid glycosylation on the cell surface may result in activation of immune cells that recognize these neurons as nonself, and the subsequent inflammatory responses may induce neuronal death. Indeed, infiltration of immune cells and inflammatory responses are often observed in regions where neurons undergo apoptosis in AD. In this respect, our study has expanded the traditional amyloid hypothesis to the defects of the secretory pathway that affect many more proteins essential for neuronal functions.
In addition, Golgi fragmentation and the resulted sorting defects may be the reason why γ-secretase activity has been detected in virtually every membrane organelle. Rescue of the Golgi structure by expression of nonphosphorylatable GRASP proteins shifts APP from amyloidogenic cleavage to nonamyloidogenic cleavage, possibly because of the reduced APP trafficking or the proper sorting of APP and its processing enzymes. This is an interesting topic for further investigation.
The present study identifies the Golgi or Golgi structural proteins as potential drug targets for AD treatment. Given that Golgi fragmentation has been observed in many other diseases besides AD, our findings may contribute to a better understanding of the pathogenesis of related diseases as well.
Materials and Methods
Reagents.
APPswe/PS1∆E9 mice and age-matched C57BL/6J control mice (Jackson Laboratory) were kindly provided by Raymond Scott Turner and Henry Paulson (Michigan Alzheimer’s Disease Research Center, Ann Arbor, MI). C57BL/6J mice for hippocampal neuron cultures were bred, maintained, and treated in the University of Michigan’s animal facility following a protocol approved by the University’s Committee on Use and Care of Animals (UCUCA PRO 00001300). The cdk5-specific inhibitor TAT-CIP and the control TAT-GFP were kindly provided by Kavita Shah (Purdue University, West Lafayette, IN).
Cell Transfection and Biochemical Analysis.
To establish stable cell lines, AD cells were transfected with GFP, GFP-tagged p65GD, p65FL, p55GD, or p55FL plasmid. GFP-positive cells were enriched by FACS at 72 h after transfection, cultured for 2 wk, and then enriched again by FACS for stable transfection. Hippocampal neurons were prepared from 1-d-old WT C57BL/6J mice as described previously (47).
Cell Treatment and Microscopy.
Hippocampal neurons from 1-d-old pups were cultured for 3 d in vitro and then, on the fourth day, treated with Aβ as described in the figure legends. Cells were processed for immunofluorescence microscopy. For cdk5 activation, hippocampal neurons were treated with DMSO or 2 µM Aβ42 for 20 h. Cells were lysed in SDS and evaluated by Western blot analysis.
For kinase inhibitor treatment, CHO AD cells were cultured for 12 h. Staurosporine (1 μM) and roscovitine (15 μM) were directly added into the medium, followed by incubation for another 2 h and then processing for immunofluorescence microscopy. For TAT-CIP treatment, TAP-CIP or the equivalent concentration of TAT-GFP, as indicated in the figure legends, was added directly to the tissue culture medium of AD cells. When cells were treated with both TAP-CIP and Aβ, TAP-CIP was added 30 min before Aβ treatment.
For quantitation, fragmented Golgi was defined as scattered dots (not connected) in the perinuclear region or multiple mini-Golgi (isolated dots) dissociated from the major Golgi apparatus. A gallery of AD cells and hippocampal neurons with normal and fragmented Golgi is displayed in Fig. S9. For the critical experiments, we also quantified the number of Golgi elements per cell using ImageJ software (30); results are shown in Fig. S3. Quantification was performed using more than 300 cells per experiment. Results are presented as mean ± SEM from three independent experiments (n = 3).
GRASP65 Phosphorylation.
For examining whether Aβ accumulation in the cell culture medium causes GRASP65 phosphorylation, AD cells stably expressing GFP-tagged GRASP65 WT protein (p65FL) were cultured overnight, placed in fresh growth medium with 4 µM Aβ42, and then incubated for another 10 h. PBS or the control peptide served as a control. For kinase inhibition, cells were treated with roscovitine for an additional 2 h in the presence of Aβ42. For TAT-CIP treatment, cells were pretreated with 600 nM TAT-CIP or TAT-GFP for 30 min, Aβ42 was added, and the cells were incubated for another 12 h. Cells were lysed and evaluated by Western blot analysis using antibodies for phospho-GRASP65 (LX108) or total GRASP65 (Mary) (32, 48).
Online Supplemental Information.
Online supplemental materials include SI Materials and Methods, along with nine figures. Fig. S1 shows that inhibition of APP expression by CHX reduces Golgi fragmentation. Fig. S2 shows that treatment of WT cells with secretase and kinase inhibitors does not affect Golgi morphology. Fig. S3 shows the number of Golgi items (or fragments) per cell after different treatments, another method of quantifying Golgi fragmentation. It also presents quantitation results for the GFP-negative cells shown in Fig. 6. Fig. S4 shows that AD cell-conditioned medium induces Golgi fragmentation in WT cells, and that the effect is time-dependent and inhibited by kinase inhibitors. Fig. S5 shows that GRASP65 is phosphorylated in AD cells, and Fig. S6 shows that Golgi proteins are not degraded in AD cells and mouse tissues. Fig. S7 shows that GRASP expression reduces Aβ production, but not APP and PS1 levels, in the cell. Fig. S8 shows that rescue of the Golgi structure reduces VSV-G trafficking in AD cells. Fig. S9 presents a gallery of AD cells and hippocampal neurons with normal or fragmented Golgi.
Supplementary Material
Acknowledgments
We thank Drs. Blaise Boles, Xiao-Wei Chen, Cunming Duan, Henry Paulson, Saiprasad Ramnarayanan, Indu Saluja, Edward Stuenkel, and Scott Turner for providing cDNA constructs, cell lines, transgenic mice, antibodies, and other reagents. We thank Dr. Kavita Shah for providing us with the TAT-CIP and TAT-GFP constructs and for useful suggestions, and Patricia V. Burgos for suggestions on detecting Aβ by radiolabeling. We thank Graham Atkin, Jack Hunt, Danming Tang, Yi Xiang, Xiaoyan Zhang, and members of the Y.W. laboratory for stimulating discussions, suggestions, and constructive technical support. We also thank Dr. Robert Fuller, Geoffrey Murphy, and Henry Paulson for their critical reading of the manuscript. We acknowledge the continuing support and encouragement from Dr. Henry Paulson. This work was supported by the National Institutes of Health (Grant GM087364), the American Cancer Society (Grant RGS-09-278-01-CSM), a University of Michigan Rackham faculty research grant, the National Institutes of Health-funded Michigan Alzheimer’s Disease Research Center (Grant P50 AG08761), MCubed and the Protein Folding Disease Initiative of the University of Michigan, and an anonymous donation to Y.W.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.B.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320192111/-/DCSupplemental.
References
- 1.Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS. Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells: Evidence that cleavage at the “beta-secretase” site occurs in the Golgi apparatus. J Biol Chem. 1996;271(16):9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- 2.Choy RW, Cheng Z, Schekman R. Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid β (Aβ) production in the trans-Golgi network. Proc Natl Acad Sci USA. 2012;109(30):E2077–E2082. doi: 10.1073/pnas.1208635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dries DR, Yu G. Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer’s disease. Curr Alzheimer Res. 2008;5(2):132–146. doi: 10.2174/156720508783954695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhu Y, et al. Adaptor protein 2-mediated endocytosis of the β-secretase BACE1 is dispensable for amyloid precursor protein processing. Mol Biol Cell. 2012;23(12):2339–2351. doi: 10.1091/mbc.E11-11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt V, et al. SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J Biol Chem. 2007;282(45):32956–32964. doi: 10.1074/jbc.M705073200. [DOI] [PubMed] [Google Scholar]
- 6.Das U, et al. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron. 2013;79(3):447–460. doi: 10.1016/j.neuron.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stieber A, Mourelatos Z, Gonatas NK. In Alzheimer’s disease the Golgi apparatus of a population of neurons without neurofibrillary tangles is fragmented and atrophic. Am J Pathol. 1996;148(2):415–426. [PMC free article] [PubMed] [Google Scholar]
- 9.Huse JT, et al. Beta-secretase processing in the trans-Golgi network preferentially generates truncated amyloid species that accumulate in Alzheimer’s disease brain. J Biol Chem. 2002;277(18):16278–16284. doi: 10.1074/jbc.M111141200. [DOI] [PubMed] [Google Scholar]
- 10.Dal Canto MC. The Golgi apparatus and the pathogenesis of Alzheimer’s disease. Am J Pathol. 1996;148(2):355–360. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Seemann J. Golgi biogenesis. Cold Spring Harb Perspect Biol. 2011;3(10):a005330. doi: 10.1101/cshperspect.a005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang Y, Wang Y. New components of the Golgi matrix. Cell Tissue Res. 2011;344(3):365–379. doi: 10.1007/s00441-011-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun KH, et al. Novel genetic tools reveal Cdk5’s major role in Golgi fragmentation in Alzheimer’s disease. Mol Biol Cell. 2008;19(7):3052–3069. doi: 10.1091/mbc.E07-11-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wei JH, Bisel B, Tang D, Seemann J. Golgi cisternal unstacking stimulates COPI vesicle budding and protein transport. PLoS ONE. 2008;3(2):e1647. doi: 10.1371/journal.pone.0001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Y, et al. Regulation of protein glycosylation and sorting by the Golgi matrix proteins GRASP55/65. Nat Commun. 2013;4:1659. doi: 10.1038/ncomms2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowe M, et al. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998;94(6):783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- 17.Seemann J, Jokitalo EJ, Warren G. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol Biol Cell. 2000;11(2):635–645. doi: 10.1091/mbc.11.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrissette DA, Parachikova A, Green KN, LaFerla FM. Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem. 2009;284(10):6033–6037. doi: 10.1074/jbc.R800030200. [DOI] [PubMed] [Google Scholar]
- 19.Tang D, Wang Y. Cell cycle regulation of Golgi membrane dynamics. Trends Cell Biol. 2013;23(6):296–304. doi: 10.1016/j.tcb.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang KH, Vincent F, Shah K. Deregulated Cdk5 triggers aberrant activation of cell cycle kinases and phosphatases inducing neuronal death. J Cell Sci. 2012;125(Pt 21):5124–5137. doi: 10.1242/jcs.108183. [DOI] [PubMed] [Google Scholar]
- 21.Lee MS, et al. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163(1):83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundaram JR, et al. Specific inhibition of p25/Cdk5 activity by the Cdk5 inhibitory peptide reduces neurodegeneration in vivo. J Neurosci. 2013;33(1):334–343. doi: 10.1523/JNEUROSCI.3593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30(36):11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MS, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405(6784):360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Seemann J, Pypaert M, Shorter J, Warren G. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 2003;22(13):3279–3290. doi: 10.1093/emboj/cdg317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188(2):237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hicks SW, Machamer CE. The NH2-terminal domain of Golgin-160 contains both Golgi and nuclear targeting information. J Biol Chem. 2002;277(39):35833–35839. doi: 10.1074/jbc.M206280200. [DOI] [PubMed] [Google Scholar]
- 28.Chiu R, Novikov L, Mukherjee S, Shields D. A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J Cell Biol. 2002;159(4):637–648. doi: 10.1083/jcb.200208013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang D, Yuan H, Wang Y. The role of GRASP65 in Golgi cisternal stacking and cell cycle progression. Traffic. 2010;11(6):827–842. doi: 10.1111/j.1600-0854.2010.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8(3):238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- 31.Tang D, Mar K, Warren G, Wang Y. Molecular mechanism of mitotic Golgi disassembly and reassembly revealed by a defined reconstitution assay. J Biol Chem. 2008;283(10):6085–6094. doi: 10.1074/jbc.M707715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang D, Yuan H, Vielemeyer O, Perez F, Wang Y. Sequential phosphorylation of GRASP65 during mitotic Golgi disassembly. Biol Open. 2012;1(12):1204–1214. doi: 10.1242/bio.20122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane JD, et al. Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol. 2002;156(3):495–509. doi: 10.1083/jcb.200110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker A, et al. Golgi fragmentation during Fas-mediated apoptosis is associated with the rapid loss of GM130. Biochem Biophys Res Commun. 2004;316(1):6–11. doi: 10.1016/j.bbrc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Mancini M, et al. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol. 2000;149(3):603–612. doi: 10.1083/jcb.149.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shorter J, et al. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999;18(18):4949–4960. doi: 10.1093/emboj/18.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Presley JF, et al. ER-to-Golgi transport visualized in living cells. Nature. 1997;389(6646):81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 38.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40(3):471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 39.Lin CY, et al. Peripheral Golgi protein GRASP65 is a target of mitotic polo-like kinase (Plk) and Cdc2. Proc Natl Acad Sci USA. 2000;97(23):12589–12594. doi: 10.1073/pnas.220423497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preisinger C, et al. Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling. EMBO J. 2005;24(4):753–765. doi: 10.1038/sj.emboj.7600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Satoh A, Warren G. Mapping the functional domains of the Golgi stacking factor GRASP65. J Biol Chem. 2005;280(6):4921–4928. doi: 10.1074/jbc.M412407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puthenveedu MA, Linstedt AD. Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc Natl Acad Sci USA. 2004;101(5):1253–1256. doi: 10.1073/pnas.0306373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grabski R, et al. Identification of a functional domain within the p115 tethering factor that is required for Golgi ribbon assembly and membrane trafficking. J Cell Sci. 2012;125(Pt 8):1896–1909. doi: 10.1242/jcs.090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dippold HC, et al. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139(2):337–351. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bisel B, et al. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol. 2008;182(5):837–843. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thayer DA, Jan YN, Jan LY. Increased neuronal activity fragments the Golgi complex. Proc Natl Acad Sci USA. 2013;110(4):1482–1487. doi: 10.1073/pnas.1220978110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleene R, et al. NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments. J Neurosci. 2010;30(32):10784–10798. doi: 10.1523/JNEUROSCI.0297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vielemeyer O, et al. Direct selection of monoclonal phosphospecific antibodies without prior phosphoamino acid mapping. J Biol Chem. 2009;284(31):20791–20795. doi: 10.1074/jbc.M109.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.