Significance
Ras small GTPases including ARFs act as molecular switches to modulate signaling pathways involved in hematopoiesis. Decreased guanine nucleotide exchange factor activity or increased GTPase-activating protein activation are associated with many leukemias, myelodysplastic syndromes, and myeloproliferative disorders. Drosophila is a good model to address questions related to aberrant hematopoiesis. Using Drosophila genetics and gene expression analysis we show tissue-specific function of the ubiquitously expressed endocytic protein, Drosophila ARF1 by interaction of ARF1–GTP with a blood-cell–expressed endocytic protein Asrij. The ARF1–Asrij axis brings about endosomal regulation of multiple signaling pathways in hematopoiesis.
Abstract
Drosophila melanogaster larval hematopoiesis is a well-established model to study mechanisms that regulate hematopoietic niche maintenance and control of blood cell precursor (prohemocyte) differentiation. Molecules that perturb niche function affect the balance between prohemocytes and differentiated hemocytes. The conserved hemocyte-specific endosomal protein Asrij is essential for niche function and prohemocyte maintenance. Elucidating how subcellular trafficking molecules can regulate signaling presents an important challenge. Here we show that Asrij function is mediated by the Ras family GTPase Arf79F, the Drosophila homolog of ADP ribosylation factor 1 (ARF1), essential for clathrin coat assembly, Golgi architecture, and vesicular trafficking. ARF1 is expressed in the larval lymph gland and in circulating hemocytes and interacts with Asrij. ARF1-depleted lymph glands show loss of niche cells and prohemocyte maintenance with increased differentiation. Inhibiting ARF1 activation by knocking down its guanine nucleotide exchange factor (Gartenzwerg) or overexpressing its GTPAse-activating protein showed that ARF1–GTP is essential for regulating niche size and maintaining stemness. Activated ARF1 regulates Asrij levels in blood cells thereby mediating Asrij function. Asrij controls crystal cell differentiation by affecting Notch trafficking. ARF1 perturbation also leads to aberrant Notch trafficking and the Notch intracellular domain is stalled in sorting endosomes. Thus, ARF1 can regulate Drosophila blood cell homeostasis by regulating Asrij endocytic function. ARF1 also regulates signals arising from the niche and differentiated cells by integrating the insulin-mediated and PDGF-VEGF receptor signaling pathways. We propose that the conserved ARF1–Asrij endocytic axis modulates signals that govern hematopoietic development. Thus, Asrij affords tissue-specific control of global mechanisms involved in molecular traffic.
The Drosophila larval lymph gland is a hematopoietic organ, which is segmented into a primary lobe and few posterior secondary and tertiary lobes. Primary lobe hematopoiesis is well studied and depends on maintenance of the hematopoietic stem cells (prohemocytes) in the medullary zone (MZ) by cells of the niche (posterior signaling center, PSC). The cortical zone (CZ) consists of terminally differentiated hemocytes namely plasmatocytes that phagocytose, crystal cells that mediate melanization and lamellocytes that encapsulate foreign material (1). Active signals including those of the Wg, Hh, and JAK/STAT pathways emanate from the PSC to maintain a fine balance between the prohemocytes and differentiated populations (2). Without a functional niche, prohemocytes differentiate as seen during infection or metamorphosis (1). Several signaling molecules and transcription factors required to maintain blood cell homeostasis have been studied. However, mechanisms that orchestrate this interplay of signals are poorly understood.
Endocytic intracellular trafficking of signaling receptors, which are then subjected to endosomal sorting, leading to either recycling or degradation, is an extremely effective way of signal transduction (3). Lately, endosomes have also been shown to facilitate generation of new signals on the endosome or to bring about overactivation or termination of existing signals, thereby conferring the ability for signal regulation (4, 5). The unique environment of the endosome and the clustering of receptors and effectors facilitate interactions that may not occur otherwise. The endocytic protein Asrij is expressed specifically in the Drosophila blood system where it functions to maintain the hematopoietic niche and regulate hematopoiesis (6). Upon Asrij depletion, Collier+ cells in the niche decrease, resulting in a reduced stem cell population with a concomitant increase in differentiation. In the case of crystal cell specification, the increased crystal cell number in asrij null mutants is due to entrapment of Notch intracellular domain (NICD) in the hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) positive sorting endosomes, leading to overactivation of Notch (6). Asrij acts as a scaffold for endosomal regulation of STAT3 activation, thereby regulating JAK-STAT signaling in mouse stem cells and Drosophila hematopoiesis (7). Here we explore how Asrij integrates with the generic endocytic machinery to regulate larval lymph gland hematopoiesis.
A genome-wide yeast two-hybrid screen reported ADP ribosylation factor 1 (ARF1) as an interacting partner of Asrij (8). Our preliminary analysis indicated that ARF1 depletion leads to hematopoietic defects. ARF1 is a ubiquitous, highly conserved rat sarcoma (Ras) family GTPase (9, 10). Drosophila ARF1 (Arf79F) was shown to be the homolog of mammalian ARF1. ARF1 was first identified as a cofactor required for ADP ribosylation of the alpha subunit of heterotrimeric G proteins (11). The endocytic function of ARF1 was shown recently where reduced ARF1 levels or inactive ARF1 inhibits glycosylphosphatidylinositol-anchored proteins (GPI-APs) and fluid phase endocytosis but does not affect the clathrin-dependent and -independent mechanisms of endocytosis (12).
ARF1 cycles between the GTP-bound active and the GDP-bound inactive forms. In Drosophila the GDP-to-GTP exchange of ARF1 is catalyzed by the guanine nucleotide exchange factor (GEF) called Gartenzwerg (Garz). In the salivary gland, Garz depletion disrupts the secretory pathway, inhibiting surface delivery of adhesion molecules such as Drosophila E-cadherin (DE-cadherin) and Flamingo. Deregulated trafficking affects the positioning and elongation of epithelial cells leading to disorganized salivary glands (13). Interestingly, DE-cadherin is expressed in prohemocytes of the primary lymph gland lobe (1) and asrij depletion reduces the expression of DE-cadherin leading to premature differentiation (6). Garz modulates membrane trafficking of coat protein I vesicles between the Golgi and ER, controlling epithelial secretion and tracheal tube morphogenesis (14). The Drosophila GTPase activating protein (GAP), Gap69c induces hydrolysis of GTP bound to ARF1. However, its functional details remain largely uncharacterized (15).
In this study we show that ARF1 regulates Drosophila hemocyte homeostasis and this requires Asrij function. Further, perturbation of Garz and Gap69c in the lymph gland shows that Asrij function depends on the ARF1–GTP activity. Using crystal cell differentiation as an example, we show that ARF1 activity is essential in NICD trafficking. We also show that ARF1 balances insulin signaling in the niche and augments Pvr (PDGF/VEGF receptor) signaling in the CZ to regulate hematopoietic progenitor maintenance. We propose a model for hematopoietic niche and stem cell maintenance by the ARF1–Asrij axis.
Materials and Methods
Fly Strains.
Fly stocks were maintained according to standard rearing conditions. Transgenic parental expression lines were used as controls where appropriate. Details are in SI Materials and Methods.
Immunostaining, Microscopy, and Analysis.
Lymph glands and hemocytes were dissected, immunostained, and analyzed as previously described (6). DAPI was used as nuclear stain. See SI Materials and Methods for antibody details. Images were captured with a Zeiss LSM510-Meta confocal microscope and analyzed using LSM510 processing software (Carl Zeiss). Antennapedia (Antp) positive cells in the PSC and phosphohistone H3 (H3P) positive cells in the lymph gland lobes of respective genotypes were manually counted using LSM510 processing software (Carl Zeiss); graphs were plotted accordingly. Hemocyte counts, statistical analyses, and additional methods are described in SI Materials and Methods.
Results
Drosophila ARF1 Is Expressed in the Larval Hematopoietic Organ and in Hemocytes.
Drosophila ARF1 (Arf79F) is ubiquitously expressed (10) yet its expression in the Drosophila hematopoietic system has not been reported. Immunolocalization with specific antibodies (Fig. S1) showed that ARF1 is expressed in several tissues including all larval and adult blood cell types (Fig. S2) and colocalizes with endosomal, Golgi and lysosomal markers (Fig. S3). Hence ARF1 could serve as an important molecular link to probe the role of endocytosis in the ontogeny of blood cells.
ARF1 Interacts with the Drosophila Blood Cell Factor Asrij.
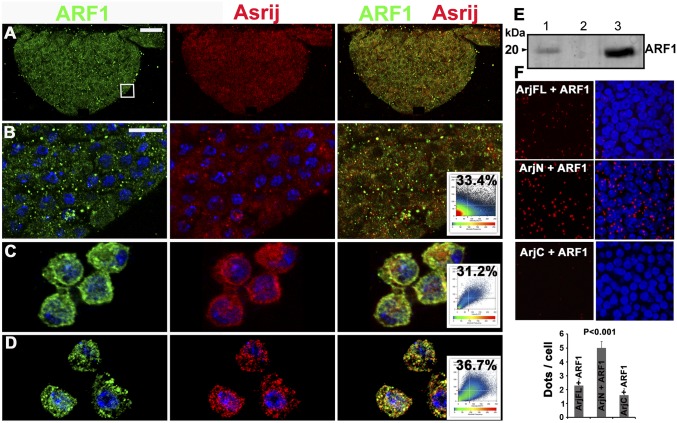
A proteome-wide yeast two-hybrid screen-based protein interaction map of Drosophila showed four interactors of Asrij including ARF1, with a significant confidence score (8), although their expression in hematopoiesis was not known. Our analysis shows that ARF1 is expressed in the Drosophila hematopoietic system. Hence we checked for possible interactions between Asrij and ARF1. By immunolocalization analysis we found that Asrij and ARF1 colocalize in the lymph gland, circulating hemocytes and S2R+ cells (Fig. 1 A–D). Immunoprecipitation experiments showed that Asrij and ARF1 also interact biochemically (Fig. 1E). This indicates a direct interaction between Asrij and ARF1, suggesting that the two proteins may function together. We showed earlier that the Asrij N-terminal half containing the conserved ovarian carcinoma immunoreactive antigen (OCIA) domain is essential for STAT3 interaction and Asrij function (7). We similarly checked for interaction with ARF1 using the proximity ligation assay (PLA) (SI Materials and Methods) and found that the ARF1–Asrij interaction is through the OCIA domain (Fig. 1F). This suggests that Asrij can mediate interaction between ARF1 and STAT92e.
Fig. 1.
ARF1 interacts with the pan hemocyte marker Asrij. (A–D) ARF1 (green) and Asrij (red) colocalization in the lymph gland (A, and magnified boxed region in B), hemocytes in circulation (C), and S2R+ cells (D). Colocalization plots are as indicated. (E) Coimmunoprecipitation (co-IP) of Asrij and ARF1 from S2R+ protein extracts with anti-Asrij antibodies. Immunoblot was probed with anti-ARF1 antibody. (Lane 1) Input control (10% of the total protein). (Lane 2) IP with preimmune serum. (Lane 3) IP with anti-Asrij antibodies. (F) In situ proximity ligation assay on wild-type lymph glands using antibodies against ARF1 and Asrij full length (ArjFL) or ArjN or ArjC. Graph shows PLA signal (red dots/cell). n = 10. Nuclei were stained with DAPI (blue). [Scale bar, 20 μm (A) and 5 μm (B–D and F).]
ARF1 Depletion in the Lymph Gland Affects Cell Proliferation and Leads to Abnormal Hematopoiesis.
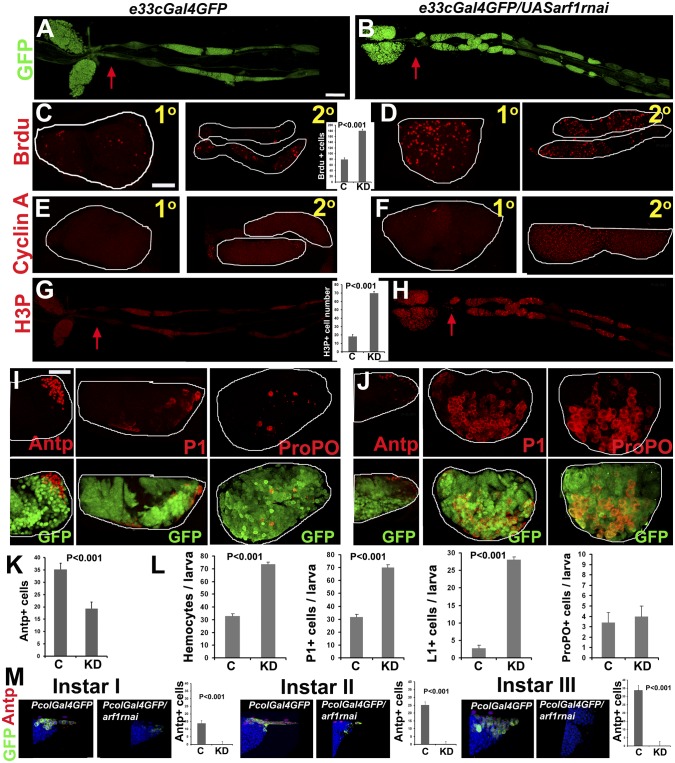
To test ARF1 function in the lymph gland we used the UAS–GAL4 system to achieve knockdown of ARF1 in the entire lymph gland (e33cGal4), PSC (PCol85Gal4), MZ (domeGal4), or CZ (PxnGal4 and HmldeltaGal4), with coexpression of UAS-GFP in all cases, to mark the GAL4 expression domain (SI Materials and Methods). Note that the CZ-expressing GAL4s also express in the circulating hemocytes, as discussed later. Knockdown was validated by immunostaining (Fig. S1G). A striking phenotype was an increased number of secondary lobes and tissue overgrowth. All of the knockdown larvae examined (n = 10) showed hyperproliferation of secondary lymph gland lobes upon e33cGal4-mediated ARF1 knockdown (Fig. 2 A and B) reminiscent of the asrij null phenotype. This suggests that ARF1 regulates the cell cycle to control proliferation and tissue overgrowth. To test this we assessed entry of cells into the S phase by assaying BrdU incorporation in the lymph gland. Only differentiating CZ cells of the third instar are known to incorporate BrdU, whereas quiescent precursors of the MZ do not show BrdU label (1). All ARF1-depleted lobes had a significantly larger population of cells in S phase, suggesting an effect on entry into the cell cycle (Fig. 2 C and D; n = 10). Cyclin A accumulates during the transition from late S/G2 phase to mitosis (16). Abundant cyclin A was seen in ARF1-depleted lobes, indicating the cells were ready to enter mitosis (Fig. 2 E and F). Staining for H3P showed increased mitotically active (H3P+) cells (≥70 cells) in ARF1 knockdown lobes compared with controls (≤20 cells) (Fig. 2 G and H; n = 10). These observations raised the question of whether the lymph glands with increased proliferation show increased differentiation and a reduced quiescent stem cell population.
Fig. 2.
ARF1 perturbation affects blood cell homeostasis. (A–K) Lymph gland whole mounts showing primary (1°) or secondary (2°) lobes of control (e33cGal4GFP) or ARF1 knockdown (e33cGal4GFPUASarf1rnai) third instar larvae. Knockdown shows excess of 2° lobes (B), BrdU+ cells (D), cyclin A staining (F), and H3P+ cells (H), also reduced Antp+ niche with increased differentiation (P1, ProPO staining) (J) compared with controls (A, C, E, G, and I, respectively). Graph shows number of BrdU+ cells (C and D), H3P+ cells (G and H), and Antp+ cells (K). GFP expression (green) in I and J is shown (Lower). (L) Total and differential hemocyte counts in circulation as indicated. (M) Antp+ cells coexpressing GFP (green) seen in control (PCol85Gal4GFP) are absent in PCol85Gal4GFPUASarf1rnai in all larval instars. Graphs represent Antp+ cells/larva. n = 10. P values are as indicated. Red arrows in A, B, G, and H indicate start of the 2° lobes. Genotypes are as indicated. [Scale bar, 50 μm (A, B, G, and H) and 20 μm (C–F, I, J, and M).] Also see Figs. S4 and S5.
To examine whether ARF1 depletion with e33cGal4 affects the population of niche, precursor, and differentiated cells, we stained lymph gland and hemocytes in circulation with the plasmatocyte marker P1 and crystal cell marker ProPO to assess the extent of differentiation. ARF1-depleted primary lobes showed an expanded CZ, indicating premature differentiation of prohemocytes into plasmatocytes and crystal cells compared with parental controls (Fig. 2 I–K). The PSC, marked by Antp, is responsible for prohemocyte maintenance. ARF1-depleted primary lobes showed reduced Antp+ cells (≤26 cells) compared with parental control (35–40 cells) (Fig. 2K; n = 10), suggesting that lack of or reduction in the niche cells results in premature hemocyte differentiation. Total hemocyte number in circulation increased from the second larval instar, reflecting the increase in plasmatocyte and lamellocyte numbers (Fig. 2L). Also see Fig. S4.
A Cell-Autonomous Role for ARF1 in Niche Maintenance.
We next performed zone-specific depletion of ARF1 using specific Gal4 drivers listed above. PSC-specific depletion of ARF1 with PCol85-Gal4 caused a complete loss of the Antp+ niche from the first instar (Fig. 2M) and increased differentiation to P1- and ProPO-expressing cells (Fig. S5). Depletion in the MZ prohemocytes showed no change in the niche, whereas depletion in the CZ caused a moderate reduction in the niche, both with increased differentiation (SI Results and Figs. S6–S8). This suggests an essential cell-autonomous role for ARF1 in maintaining PSC cells and a nonautonomous role mediated via the CZ and possibly circulating hemocytes.
PSC- or MZ-specific depletion showed no change in circulating hemocyte number (Figs. S5 and S6). However, ARF1 depletion with PxnGal4 perturbs the hemocyte balance resulting in fewer P1+ cells and increased lamellocytes in circulation (Fig. S7). As all of the genotypes included GFP as a tracer for Gal4 expressing cells we could track the location of ARF1-depleted cells in lymph gland, sessile pockets of the body wall, and in circulation (Figs. S4–S8). Further the lymph glands also showed no sign of disintegration (Figs. S4–S8). This suggests contribution from embryonic hemocytes to the increased count in larval circulation. Because PxnGAL4 and HmldeltaGAL4 also express in circulating hemocytes, ARF1 knockdown phenotype may result from a nonautonomous effect of circulating hemocytes on lymph gland hematopoiesis. To test this we used the GcmGAL4 driver as it drives UASGFP in circulating hemocytes but not in lymph gland hemocytes (Fig. S4 D–F) (17). ARF1 depletion with GcmGAL4 increased differentiation in the lymph gland. However, there was no change in the Antp+ cell number in the niche (Fig. S4G), indicating a nonautonomous control on the lymph gland by cross-talk of signals from circulating hemocytes. The total and differential count of circulating hemocytes was also increased, indicating a role for ARF1 here too (Fig. S4H).
ARF1–GTP Activity Is Required for Niche Maintenance and Regulation of Hematopoiesis.
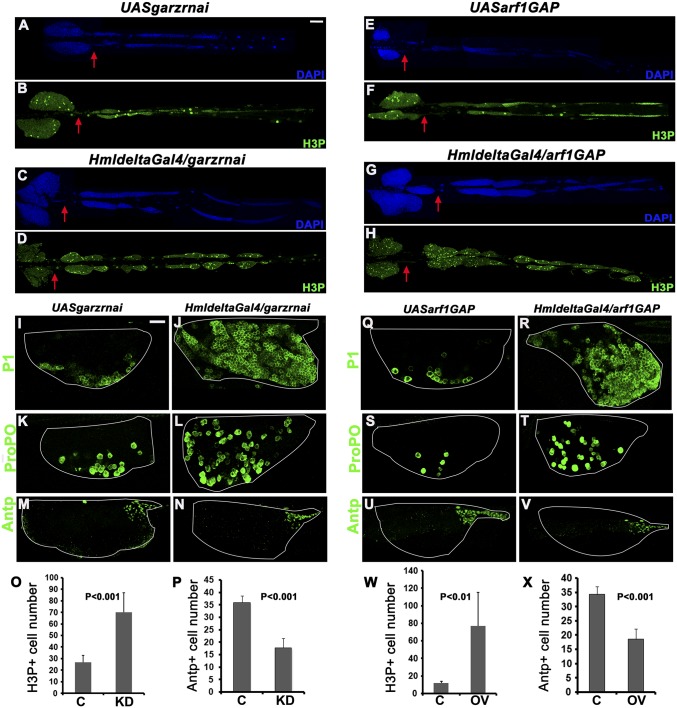
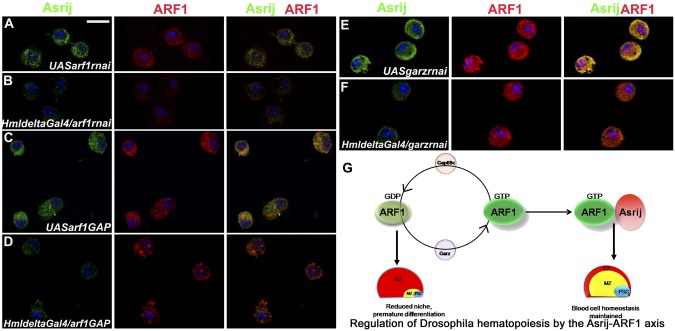
ARF1 bound to GTP is required to effect its GTPase activity and mediate cellular function in the context of salivary gland morphogenesis (13). The inactive form of ARF1 is GDP bound. To test whether ARF1–GTP activity is essential for hematopoiesis, we depleted Garz (ARF1GEF) or overexpressed Gap69c (ARF1GAP) in the lymph gland and examined third instar larvae for the effect of inactivating ARF1. Either GEF depletion or GAP overexpression caused phenotypes similar to and more severe than ARF1 knockdown. In either case over 70% of the lymph glands (n = 10 each) showed increase in secondary lymph gland lobe number and tissue overgrowth, respectively (Fig. 3 A–D and E–H). Hyperproliferation was accompanied by increase in mitotically active cell numbers. Seventy–eighty H3P+ cells were seen in Garz knockdown or GAP overexpression compared with 10–25 in the respective parental controls (Fig. 3 O and W). In addition, there was premature differentiation of prohemocytes into plasmatocytes and crystal cells in the primary lymph gland lobes (Fig. 3 I–L and Q–T). This corresponded with a highly reduced Antp expressing niche—15–20 Antp+ cells in Garz knockdown or arf1GAP overexpression compared with 35–36 in respective parental controls (Fig. 3 M, N, U, V, P, and X), showing that ARF1–GTP activity is essential for maintenance of the niche cells and hence the prohemocytes.
Fig. 3.
Perturbation of ARF1–GTP activity leads to aberrant hematopoietic phenotypes. (A–H) Whole mounts showing secondary lobe tissue overgrowth marked with DAPI (blue) and increase in mitotically active cells (H3P+, green) in HmldeltaGal4-mediated gartenzwerg (garz) knockdown and Gap69c (arf1GAP) overexpression lymph glands (C, D, G, and H) compared with controls (A, B, E, and F). Graphs show H3P+ cells/lymph gland (O and W). Antp+ (green) niche cells are reduced in garz knockdown and arf1GAP overexpression (N and V) compared with control (M and U) also represented graphically (P and X). Premature differentiation into plasmatocytes (P1, green) (J and R) and crystal cells (ProPO, green) (L and T) in garz knockdown and arf1GAP overexpression compared with controls (I, K, Q, and S), respectively. Red arrows indicate start of the secondary lymph gland lobes. Genotypes are as labeled. C, control; KD, knockdown. [Scale bar, 50 μm (A–H) and 20 μm (I–N and Q–V).]
Perturbation of ARF1 and Its Activated Form Leads to Aberrant Notch Trafficking.
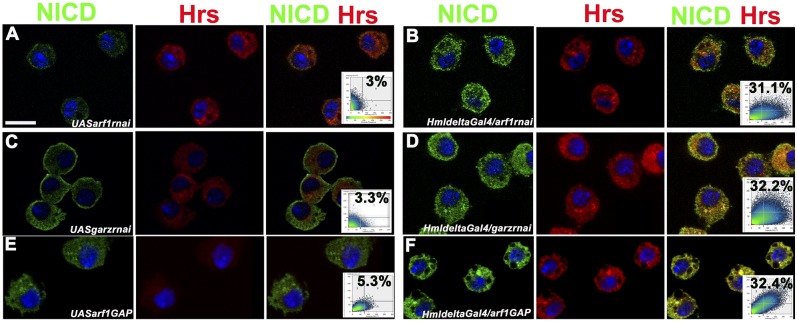
ARF1 is a mediator of many endocytic processes. The effect of depleting active ARF1 in the lymph gland suggested that it may regulate trafficking of signals that control hematopoiesis in the lymph gland. Serrate-mediated Notch signaling is essential for crystal cell specification (18). The endosomal sorting complex required for transport (ESCRT) machinery is essential for receptor/cargo sorting thus affecting a range of developmental processes (19). Transport of the NICD for recycling or degradation is mediated by the ESCRT complex of proteins, mutations in which often activate or down-regulate Notch signaling (20). Mutants of the ESCRT complex show entrapment of NICD in endosomes leading to an N gain-of-function phenotype (21). We showed earlier that Asrij depletion causes NICD entrapment in Hrs+ endosomes and results in increased crystal cell differentiation indicating N gain of function (6). As ARF1 is intimately linked with endocytosis, we checked the effect of depleting ARF1 or blocking ARF1-GTP on NICD trafficking in the circulating hemocytes. In all cases we found entrapment of NICD in Hrs+ endosomes (Fig. 4 A–F) reminiscent of the Asrij phenotype. Further, this was manifested as increased crystal cell lineage specification. Thus, ARF1 activity mediates endocytic trafficking in Drosophila lymph gland hematopoiesis.
Fig. 4.
Perturbation of ARF1 and its activity affects Notch trafficking. NICD expression (green) and Hrs expression (red) in larval circulating hemocytes from control (A, C, and E) and arf1 knockdown (B), garz knockdown (D), and arf1GAP overexpressing (F) larvae. Right panels are merged images and respective colocalization plots. Nuclei were stained with DAPI (blue). Genotypes are as indicated. [Scale bar, 5 μm (A–F).]
ARF1 Is Essential for Asrij Expression and Function.
The phenotypes of ARF1 depletion or activity mutants mirror those of the endocytic protein Asrij. To test the interdependence of ARF1 and Asrij we perturbed/depleted one and studied the effect on the other. Knockdown of ARF1 specifically in the hemocytes or perturbation of the activated form of ARF1 by knockdown of its GEF or overexpression of GAP caused reduction in Asrij levels in the circulating hemocytes (Fig. 5 A–F). However, asrij depletion had no effect on ARF1 levels (Fig. S3 F–I). This indicates that Asrij requires active ARF1 to stabilize it and mediate its activity as depicted in Fig. 5G.
Fig. 5.
ARF1–GTP regulates Asrij expression in hemocytes. (A–F) Third instar larval hemocytes showing expression of Asrij (green) and ARF1 (red) in controls (A, C, and E) and (B) arf1 knockdown and (D and F) ARF1-GTP perturbed conditions in (D) garz knockdown and (F) arf1GAP overexpression. Genotypes are as labeled. Right panels are merged images. [Scale bar, 5 μm (A–F).] (G) Proposed model for the role of ARF1 in lymph gland hematopoiesis. ARF1 cycles between GDP- and GTP-bound states depending on the activity of its GAP (Gap69c) and GEF (Garz), respectively. ARF–GDP does not support niche maintenance and promotes premature differentiation possibly due to absence of Asrij interaction. ARF–GTP interacts with and stabilizes Asrij thereby supporting maintenance of blood cell homeostasis by Asrij.
ARF1 Balances Insulin/IGF Signaling in the Niche.
Recently insulin signaling was shown to control prohemocyte maintenance (22) and an ARF–GEF Steppke is speculated to regulate the insulin signaling pathway by activating ARF (23), thereby completing the cycle of ARF activation and inactivation. Steppke is thought to act upstream of PI3K (24). Hence it is likely that an ARF regulates hematopoiesis via its control of insulin signaling. As we found ARF1 and its GEF (Garz) regulate niche maintenance and hematopoiesis we tested whether the effect of perturbing insulin signaling in the niche can be overcome by ARF1 (Fig. S9). PSC-specific overexpression of Pten, an inhibitor of insulin signaling causes a reduced niche and reduced differentiation (22). We observed that overexpression of ARF1 alone in the niche (PCol85Gal4GFP/UASarf1) does not affect niche cell number (Fig. S9C); however, hemocyte differentiation is reduced (Fig. S9A). Overexpression of ARF1 along with Pten (UASPten/PCol85Gal4GFP; UASarf1), shows a Pten overexpression phenotype in the niche, suggesting that ARF1 cannot rescue the inhibition imposed by Pten (Fig. S9A). Arf1 depletion (PCol85Gal4GFP/UASarf1rnai) causes complete loss of the niche and increased differentiation, which cannot be rescued by Pten (UASPten/PCol85Gal4GFP; UASarf1rnai) (Fig. S9B) but can be partially rescued by a constitutively active PI3Kcaax overexpression (UASPI3Kcaax/PCol85Gal4GFP; UASarf1rnai) (Fig. S9D). Simultaneous overexpression of ARF1 and PI3K results in an enlarged niche; however, differentiation is still excess (UASPI3Kcaax/PCol85Gal4GFP; UASarf1) (Fig. S9 C and D). This suggests ARF1 and Pten independently regulate and balance the outcome of insulin signaling thereby affecting PI3K activation.
ARF1 Can Overcome Perturbed Pvr Signaling in the CZ.
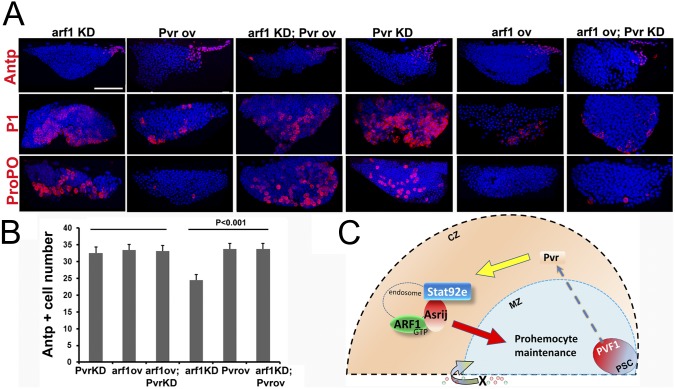
Prohemocyte maintenance is achieved by a balanced response to signals from the niche and from differentiated cells of the CZ (25). Because ARF1 depletion in the CZ affects niche maintenance non-cell autonomously and also results in induction of differentiation, we studied the effect of modulating ARF1 levels on the Pvf/Pvr/Stat/AdgfA axis that signals from the niche to the CZ and back to MZ prohemocytes (25). Because Asrij has a conserved role in endosomally regulating JAK/STAT signaling to maintain stem cell potency in Drosophila hematopoiesis, we investigated whether ARF1 is involved in this axis. Pvr is the receptor for Pvf1 and a key mediator of signaling from the CZ to the MZ for prohemocyte maintenance. Perturbation of Pvr signaling in the CZ results in loss of prohemocyte maintenance and premature differentiation, a phenotype similar to ARF1 depletion in the CZ. To test whether endosomal regulation is active at the level of the Pvr receptor, we overexpressed UAS–ARF1 (SI Materials and Methods and Fig. S1C) in the CZ while simultaneously depleting Pvr (UASPvrrnai; UASarf1/HmldeltaGal4). Although the niche was unaffected (Fig. 6 A and B) plasmatocyte and crystal cell differentiation was suppressed, indicating that homeostasis was restored upon ARF1 overexpression (Fig. 6A). However, the converse (Pvr overexpression in ARF1 knockdown) (UASPvr; UASarf1rnai/HmldeltaGal4) could not rescue the increased differentiation phenotype (Fig. 6A), indicating a role for ARF1 downstream of Pvr.
Fig. 6.
ARF1 modulates multiple signaling pathways to control hematopoiesis. (A) Effect of knockdown (KD) or overexpression (OV) of ARF1 in the CZ singly or in combination with Pvr: Whole mount lymph gland primary lobe showing expression of Antp, P1, or ProPo in the different genotypes as indicated. Note increase in P1+ and ProPo+ cells seen in Arf1KD,PvrOV is rescued in Arf1OV,PvrKD. (B) Effect on Antp+ cell numbers. See text for detailed genotypes. (Scale bar, 20 μm.) (C) Proposed model for nonautonomous ARF1 function in progenitor maintenance. Pvf1 emanating from the niche activates Pvr signaling in the CZ. This causes Stat92e activation, which is aided by the ARF1–Asrij complex on endosomes. Thus, signals are generated and/or relayed to the MZ for prohemocyte maintenance. Hence loss of ARF1 from the CZ causes differentiation of MZ progenitors. In addition a signal “X” mediated by non–lymph-gland hemocytes also regulates prohemocyte maintenance by acting on the MZ and/or CZ.
Discussion
The importance of housekeeping functions such as endocytic trafficking in actively regulating signaling is becoming increasingly apparent (4, 5). This study highlights the role of a ubiquitous endocytic molecule in a tissue-specific developmental scenario. We show a previously undocumented role of ARF family GTPases and their associated GEFs and GAPs, in cellular differentiation. By tissue-specific interactions these molecules afford fine control of blood cell homeostasis regulating the hematopoietic stem cell niche and stem cell quiescence. Our study provides an example of how endogenous mechanisms can be recruited tissue specifically to actively regulate signaling in specific cell fate determination. Asrij or ARF1 depletion or inactivation, both lead to a leukemia-like situation with increased blood cell proliferation and premature differentiation. The ARF1–Asrij axis now allows one to screen for genetic and chemical modulators of this interaction and identify potential avenues for therapy. Similar interactions are likely to regulate key events of other signaling pathways as well.
Depletion of ARF1 or its inactivation causes premature differentiation in the primary lymph gland lobe. Mutant lobes comprise almost entirely of differentiated plasmatocytes and crystal cells. Thus, ARF1 negatively regulates differentiation and we found that this is due to its effect on the hematopoietic stem cell niche (PSC). Recent studies showed that lymph gland hematopoiesis is regulated by autonomous as well as systemic factors (26, 27). Our analysis shows an essential resident role for ARF1 in the niche as well as for communication from the non–lymph-gland hemocytes. Given the important role of ARF1 in mediating secretion and trafficking, it could regulate the delivery of a systemic signal(s) “X” to the lymph gland that could activate positive regulators of homeostasis or suppress mediators of differentiation (Fig. 6C). Because GcmGAL4 also drives expression in other tissues such as glial cells, it is possible that systemic signals could emanate from nonhematopoietic tissues. Communication between the nervous system and the hematopoietic compartment is already reported (28). However, because gcm is expressed in the lymph gland, we cannot exclude the possibility that GcmGAL4 has low undetectable activity in the lymph gland, which may be sufficient to deplete ARF1 to levels below the threshold for progenitor maintenance. The paucity of suitable GAL4 drivers makes this difficult to resolve at present.
The niche is specified by the homeobox transcription factor Antp (29) and maintained by the early B-cell factor ortholog Collier and the endocytic protein Asrij (6, 30). Collier activates JAK/STAT signaling to maintain prohemocytes. We recently showed that Asrij can maintain the niche independently of Col and promotes STAT activation (7). Whereas collier mutants lack an Antp+ niche, asrij mutants do not affect Antp+ cell specification but reduce Col+ cell maintenance. Depletion of ARF1 or its inactivation, results in a reduced Antp+ niche, indicating an important role in maintaining optimum niche cell number. ARF1 could deregulate the signaling circuit that governs niche maintenance and is likely to be epistatic to Asrij. This corroborates earlier results showing that Asrij plays a vital role in niche maintenance. Further we show here that this function of Asrij is mediated by its interaction with ARF1, a well-known trafficking protein whose active form is GTP bound. Thus, the ARF1–Asrij axis is indispensable for niche maintenance and prohemocyte quiescence. Several ARF family proteins are expressed in vertebrates. Our study now opens up avenues to investigate similar interactions in vertebrate hematopoiesis that should help identify novel control points for this important process.
Mechanisms that govern the patterning and development of the posterior lymph gland lobes are not characterized. The posterior lobes house undifferentiated prohemocytes (1). Supernumerary and hyperproliferated posterior lobes are seen in many cases where primary lobe hematopoiesis is perturbed. For example, sumoylation defective mutants like Ubc9 show posterior lymph gland tissue overgrowth (31). Pten mutants also display secondary lymph gland lobe overgrowth with numerous proliferating cells (22). Even in asrij-depleted posterior lobes stemness is lost and cells differentiate (6). Perturbation of ARF1 expression or its active GTP-bound form specifically in the blood lineage leads to drastic secondary and tertiary lymph gland tissue overgrowth, correlating with increased mitoses. Increase in the number of cells entering the S phase and G2/M transition suggests perturbation of signals that control cell proliferation like JAK/STAT, Notch, and MAPK pathways. Here we show that ARF1 suppresses posterior lobe prohemocyte proliferation by regulating JAK/STAT and Notch signals, mediated by Asrij. The ARF1– Asrij axis can serve as an important tool to identify and investigate signaling pathways that remain elusive in the context of the posterior lobes.
ARF1 regulates dynamin-independent and clathrin-independent modes of endocytosis (12, 32) and could be involved in the endocytic regulation of signaling pathways that modulate hemocyte homeostasis. Notch is required for crystal cell lineage specification (18) and NICD entrapment in sorting endosomes results in increased crystal cells, an N gain-of-function phenotype, as seen in asrij mutants (6). Perturbation of ARF1 or ARF1-GTP leads to a similar phenotype of entrapment of NICD in Hrs+ endosomes and concurrent increase in crystal cells. This suggests that perturbed trafficking due to loss of ARF1 activity affects NICD localization and this could be Asrij dependent or independent. However, the similarity in phenotypes of ARF1 and Asrij and the fact that they interact, strongly suggests that ARF1 function in sorting NICD is mediated by Asrij. Further, Asrij expression is dependent on ARF1 but not vice versa, suggesting that Asrij acts downstream of ARF1 in endocytic control of hematopoiesis. The interaction with activated ARF1 may be required to stabilize Asrij (Fig. 6C). Additional genetic and biochemical analysis should reveal whether there are other mediators of this interaction that help make the choice between recycling and degradation. For example, ESCRT mutants show NICD entrapment in Hrs+ endosomes leading to overactivation of Notch signals (21) and can now be tested for their interaction with Asrij and ARF1.
Dissecting mechanisms of coordinated spatial and temporal regulation of the multiple signaling pathways that operate in the lymph gland to achieve hemocyte homeostasis is a major challenge. Molecular communication across pathways is essential to maintain optimal levels of signaling, and endosomal proteins are well positioned to aid these interactions. Our analysis shows that ARF1 acts both in a cell-autonomous and a nonautonomous manner to maintain the niche and to control hemocyte homeostasis. The severity of the niche-specific ARF1 depletion phenotype suggests that it could regulate additional molecules required for niche maintenance. Our analysis shows that ARF1 and Pten have to strike a balance between activation and inactivation of PI3K. Partial rescue of the ARF1-depleted phenotype and the inability of ARF1 to overcome the PI3K overexpression phenotype, suggests that ARF1 may act upstream of PI3K (as has been reported for stepkke) along with other players or it may have an indirect effect on insulin signaling. Being endocytic in nature, ARF1 could bring about this fine regulation at multiple levels with a tissue-specific interactor like Asrij. Because ARF1 depletion has a pronounced phenotype on hemocyte homeostasis it may have an indirect role in insulin signaling, which needs to be tested.
An example of nonautonomous regulation is Pvr signaling, which activates Stat92e to control prohemocyte potency. Earlier we have shown that Asrij controls Stat activity. Here, we show that ARF1 acts downstream of Pvr and the ARF1–Asrij axis could probably be controlling Stat92e activation, which could be a result of activation from the niche, as Collier is known to activate JAK/STAT, or from the CZ via Pvr. Because Asrij and ARF1 express in the entire lymph gland as well as circulating hemocytes, the ARF1–Asrij axis can regulate at multiple levels. However, Pvr overexpression did restore the ARF1-depleted niche loss, indicating that an alternate mechanism for STAT activation may operate from the CZ or circulating hemocytes. The control of AdgfA activity by Stat has been demonstrated (25). We have shown that Stat92e activation is mediated by Asrij (7). In this report we show that Asrij interacts with ARF1 to achieve homeostasis. Hence we propose that ARF1 functions in concert with Asrij and Stat as shown in Fig. 6C.
Deregulation of Notch signaling in Asrij mutants is manifested as increase in Lozenge+ (Lz) crystal cells in the lymph gland (6). The ARF1–Asrij axis is essential for regulating NICD traffic and thereby crystal cells, which are specified by Lz expression. Lz is the Drosophila homolog of the vertebrate Runx1/AML1 transcription factor and is associated with blood cell disorders in a Drosophila model of leukemia. Mammalian Asrij is also expressed in blood stem cells (33) and perturbed asrij expression in humans is seen in several aggressive carcinomas (34, 35) and leukemias like T-cell acute lymphoblastic leukemia (36), multiple myeloma (37), and neutrophilia (38). The power of Drosophila genetics can now be harnessed to draw parallels with vertebrate hematopoiesis and unravel similar mechanisms that were hitherto difficult to dissect.
Supplementary Material
Acknowledgments
We thank the Drosophila community for fly stocks and antibodies, National Centre for Biological Sciences common imaging facility for confocal access, our laboratory members for valuable discussions, and the anonymous reviewers for valuable suggestions. This work was funded by the Jawaharlal Nehru Centre for Advanced Scientific Research and the Department of Science and Technology, Government of India.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. U.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303559111/-/DCSupplemental.
References
- 1.Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132(11):2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 2.Crozatier M, Vincent A. Drosophila: A model for studying genetic and molecular aspects of haematopoiesis and associated leukaemias. Dis Model Mech. 2011;4(4):439–445. doi: 10.1242/dmm.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 4.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463(7280):464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 5.Sorkin A, von Zastrow M. Endocytosis and signalling: Intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10(9):609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni V, Khadilkar RJ, Magadi SS, Inamdar MS. Asrij maintains the stem cell niche and controls differentiation during Drosophila lymph gland hematopoiesis. PLoS ONE. 2011;6(11):e27667. doi: 10.1371/journal.pone.0027667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha A, Khadilkar RJ, S VK, Roychowdhury Sinha A, Inamdar MS. Conserved regulation of the Jak/STAT pathway by the endosomal protein asrij maintains stem cell potency. Cell Rep. 2013;4(4):649–658. doi: 10.1016/j.celrep.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giot L, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302(5651):1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson JG, Honda A. Localization and function of Arf family GTPases. Biochem Soc Trans. 2005;33(Pt 4):639–642. doi: 10.1042/BST0330639. [DOI] [PubMed] [Google Scholar]
- 10.Murtagh JJ, Jr, et al. Molecular characterization of a conserved, guanine nucleotide-dependent ADP-ribosylation factor in Drosophila melanogaster. Biochemistry. 1993;32(23):6011–6018. doi: 10.1021/bi00074a012. [DOI] [PubMed] [Google Scholar]
- 11.Kahn RA, Gilman AG. ADP-ribosylation of Gs promotes the dissociation of its alpha and beta subunits. J Biol Chem. 1984;259(10):6235–6240. [PubMed] [Google Scholar]
- 12.Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol. 2008;10(1):30–41. doi: 10.1038/ncb1666. [DOI] [PubMed] [Google Scholar]
- 13.Szul T, et al. The Garz Sec7 domain guanine nucleotide exchange factor for Arf regulates salivary gland development in Drosophila. Cell Logist. 2011;1(2):69–76. doi: 10.4161/cl.1.2.15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armbruster K, Luschnig S. The Drosophila Sec7 domain guanine nucleotide exchange factor protein Gartenzwerg localizes at the cis-Golgi and is essential for epithelial tube expansion. J Cell Sci. 2012;125(Pt 5):1318–1328. doi: 10.1242/jcs.096263. [DOI] [PubMed] [Google Scholar]
- 15.Frolov MV, Alatortsev VE. Molecular analysis of novel Drosophila gene, Gap69C, encoding a homolog of ADP-ribosylation factor GTPase-activating protein. DNA Cell Biol. 2001;20(2):107–113. doi: 10.1089/104454901750070319. [DOI] [PubMed] [Google Scholar]
- 16.Minakhina S, Druzhinina M, Steward R. Zfrp8, the Drosophila ortholog of PDCD2, functions in lymph gland development and controls cell proliferation. Development. 2007;134(13):2387–2396. doi: 10.1242/dev.003616. [DOI] [PubMed] [Google Scholar]
- 17.Avet-Rochex A, et al. An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis. BMC Dev Biol. 2010;10:65. doi: 10.1186/1471-213X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duvic B, Hoffmann JA, Meister M, Royet J. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr Biol. 2002;12(22):1923–1927. doi: 10.1016/s0960-9822(02)01297-6. [DOI] [PubMed] [Google Scholar]
- 19.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9(5):687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Giebel B, Wodarz A. Tumor suppressors: Control of signaling by endocytosis. Curr Biol. 2006;16(3):R91–R92. doi: 10.1016/j.cub.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180(4):755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benmimoun B, Polesello C, Waltzer L, Haenlin M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139(10):1713–1717. doi: 10.1242/dev.080259. [DOI] [PubMed] [Google Scholar]
- 23.Fuss B, Becker T, Zinke I, Hoch M. The cytohesin Steppke is essential for insulin signalling in Drosophila. Nature. 2006;444(7121):945–948. doi: 10.1038/nature05412. [DOI] [PubMed] [Google Scholar]
- 24.Hahn I, et al. The Drosophila Arf GEF Steppke controls MAPK activation in EGFR signaling. J Cell Sci. 2013;126(Pt 11):2470–2479. doi: 10.1242/jcs.120964. [DOI] [PubMed] [Google Scholar]
- 25.Mondal BC, et al. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147(7):1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat Cell Biol. 2012;14(4):394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shim J, et al. Olfactory control of blood progenitor maintenance. Cell. 2013;155(5):1141–1153. doi: 10.1016/j.cell.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makhijani K, Alexander B, Tanaka T, Rulifson E, Brückner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138(24):5379–5391. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446(7133):320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crozatier M, Ubeda JM, Vincent A, Meister M. Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2004;2(8):E196. doi: 10.1371/journal.pbio.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalamarz ME, Paddibhatla I, Nadar C, Govind S. Sumoylation is tumor-suppressive and confers proliferative quiescence to hematopoietic progenitors in Drosophila melanogaster larvae. Biol Open. 2012;1(3):161–172. doi: 10.1242/bio.2012043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta GD, et al. Analysis of endocytic pathways in Drosophila cells reveals a conserved role for GBF1 in internalization via GEECs. PLoS ONE. 2009;4(8):e6768. doi: 10.1371/journal.pone.0006768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips RL, et al. The genetic program of hematopoietic stem cells. Science. 2000;288(5471):1635–1640. doi: 10.1126/science.288.5471.1635. [DOI] [PubMed] [Google Scholar]
- 34.Shen C, et al. Molecular cloning, identification and analysis of lung squamous cell carcinoma-related genes. Lung Cancer. 2002;38(3):235–241. doi: 10.1016/s0169-5002(02)00300-8. [DOI] [PubMed] [Google Scholar]
- 35.Usary J, et al. Mutation of GATA3 in human breast tumors. Oncogene. 2004;23(46):7669–7678. doi: 10.1038/sj.onc.1207966. [DOI] [PubMed] [Google Scholar]
- 36.Jundt F, et al. Aberrant expression of Notch1 interferes with the B-lymphoid phenotype of neoplastic B cells in classical Hodgkin lymphoma. Leukemia. 2008;22(8):1587–1594. doi: 10.1038/leu.2008.101. [DOI] [PubMed] [Google Scholar]
- 37.Arai H, Emson PC, Mountjoy CQ, Carassco LH, Heizmann CW. Loss of parvalbumin-immunoreactive neurones from cortex in Alzheimer-type dementia. Brain Res. 1987;418(1):164–169. doi: 10.1016/0006-8993(87)90974-7. [DOI] [PubMed] [Google Scholar]
- 38.Nigrovic PA, et al. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173(6):1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.