Abstract
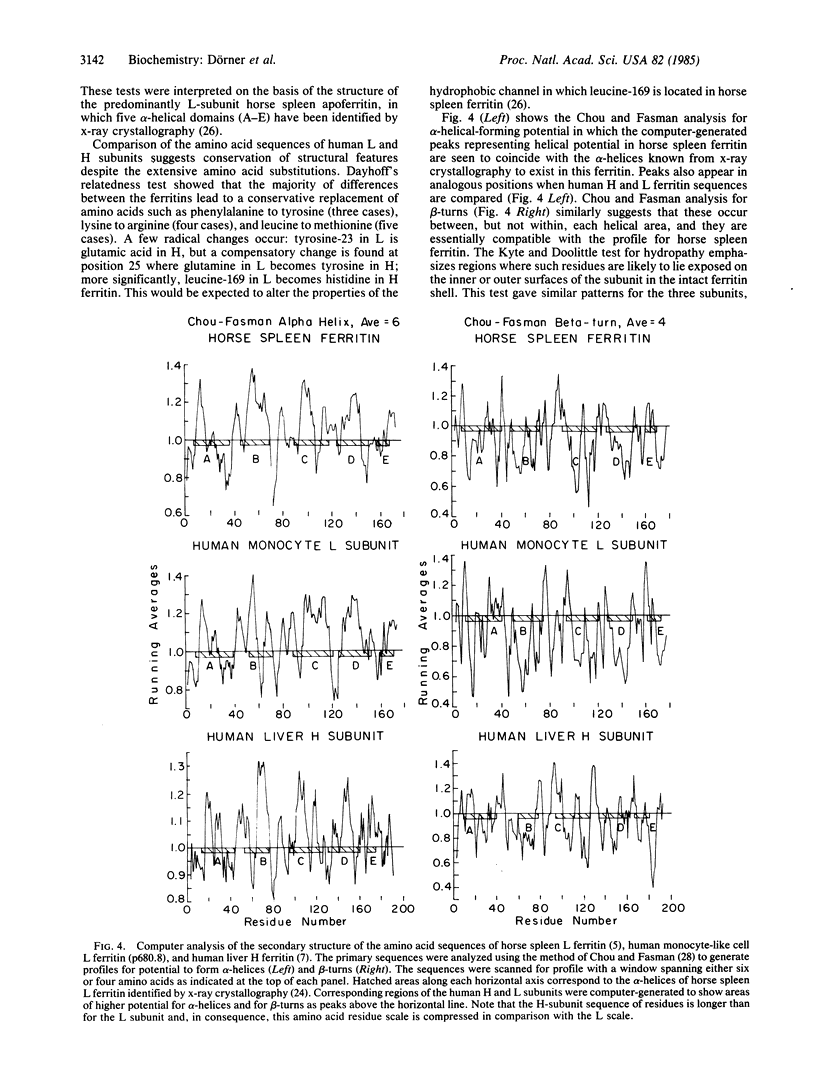
Ferritin has a protein shell of 5 X 10(6) Da consisting of 24 subunits of two types, a heavier (H) chain of 21,000 Da and a lighter (L) chain of 19,000 Da. A cDNA clone of the messenger for the L subunit has been isolated from a human monocyte-like leukemia cell line. The clone contains an open reading frame of 522 nucleotides coding for an amino acid sequence matching 97% of the published sequence of human liver ferritin L subunit determined by sequenator, but it corresponds to only 55% of the reported amino acid sequence of a human liver H-subunit clone. Nevertheless, computer analysis of the subunit conformations predicted from the open reading frames of the L and H clones shows that most of the amino acid differences are conservative and would allow both subunits to form the five alpha-helices and beta-turns established by x-ray crystallography for horse spleen ferritin subunits. This suggests that L and H subunits are structurally interchangeable in forming an apoferritin shell. The 5' untranslated region of our human ferritin L clone has considerable homology with that of the rat liver ferritin L clone in the region immediately upstream from the initiator codon, notably showing an identical sequence of 10 nucleotides at the same position in both subunit clones that may participate in regulating the known activation of ferritin mRNA after iron administration. Extensive homology, including several blocks of nucleotides, was identified between the 3' untranslated regions of the human and rat L clones. The common structural features of the H and L subunits lead us to conclude that they have diverged from a single ancestral gene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison J. M., Fitton J. E., Lewis W. G., May K., Harrison P. M. The amino acid sequence of human liver apoferritin. FEBS Lett. 1983 Nov 28;164(1):139–144. doi: 10.1016/0014-5793(83)80037-4. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford A., Berger M., Lis Y., Williams R. The iron content of human liver and spleen isoferritins correlates with their isoelectric point and subunit composition. Biochem Biophys Res Commun. 1978 Jul 14;83(1):334–341. doi: 10.1016/0006-291x(78)90436-9. [DOI] [PubMed] [Google Scholar]
- Bomford A., Conlon-Hollingshead C., Munro H. N. Adaptive responses of rat tissue isoferritins to iron administration. Changes in subunit synthesis, isoferritin abundance, and capacity for iron storage. J Biol Chem. 1981 Jan 25;256(2):948–955. [PubMed] [Google Scholar]
- Boyd D., Jain S. K., Crampton J., Barrett K. J., Drysdale J. Isolation and characterization of a cDNA clone for human ferritin heavy chain. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4751–4755. doi: 10.1073/pnas.81.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Bognacki J., Dorner M. H., de Sousa M. Identification of leukemia-associated inhibitory activity as acidic isoferritins. A regulatory role for acidic isoferritins in the production of granulocytes and macrophages. J Exp Med. 1981 Jun 1;153(6):1426–1444. doi: 10.1084/jem.153.6.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Costanzo F., Santoro C., Colantuoni V., Bensi G., Raugei G., Romano V., Cortese R. Cloning and sequencing of a full length cDNA coding for a human apoferritin H chain: evidence for a multigene family. EMBO J. 1984 Jan;3(1):23–27. doi: 10.1002/j.1460-2075.1984.tb01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner M. H., Silverstone A. E., de Sostoa A., Munn G., de Sousa M. Relative subunit composition of the ferritin synthesized by selected human lymphomyeloid cell populations. Exp Hematol. 1983 Oct;11(9):866–872. [PubMed] [Google Scholar]
- Dupont E., Vereerstraeten P., Espinosa O., Tielemans C., Dhaene M., Wybran J. Multiple transfusions and T cell subsets: a role for ferritin? Transplantation. 1983 May;35(5):508–510. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Hann H. W., Evans A. E., Cohen I. J., Leitmeyer J. E. Biologic differences between neuroblastoma stages IV-S and IV. Measurement of serum ferritin and E-rosette inhibition in 30 children. N Engl J Med. 1981 Aug 20;305(8):425–429. doi: 10.1056/NEJM198108203050803. [DOI] [PubMed] [Google Scholar]
- Heusterspreute M., Crichton R. R. Amino acid sequence of horse spleen apoferritin. FEBS Lett. 1981 Jul 6;129(2):322–327. doi: 10.1016/0014-5793(81)80193-7. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Aziz N., Brown A. J., Munro H. N. Conservation in rat liver of light and heavy subunit sequences of mammalian ferritin. Presence of unique octopeptide in the light subunit. J Biol Chem. 1984 Apr 10;259(7):4327–4334. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner Y., Hershko C., Polliack A., Konijn A. M., Izak G. Suppressive effect of ferritin on in vitro lymphocyte function. Br J Haematol. 1979 Jul;42(3):345–353. doi: 10.1111/j.1365-2141.1979.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargan D. R., Gregory S. P., Butterworth P. H. A possible novel interaction between the 3'-end of 18 S ribosomal RNA and the 5'-leader sequence of many eukaryotic messenger RNAs. FEBS Lett. 1982 Oct 18;147(2):133–136. doi: 10.1016/0014-5793(82)81026-0. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullfrog. J Biol Chem. 1982 Dec 10;257(23):14187–14191. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, function, and regulation. Adv Inorg Biochem. 1983;5:1–38. [PubMed] [Google Scholar]
- Wagstaff M., Worwood M., Jacobs A. Properties of human tissue isoferritins. Biochem J. 1978 Sep 1;173(3):969–977. doi: 10.1042/bj1730969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worwood M. Serum ferritin. CRC Crit Rev Clin Lab Sci. 1979;10(2):171–204. doi: 10.3109/10408367909147133. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]



