Significance
The stereotyped generation and maturation of neurons during development is essential for well-coordinated brain function in maturity. This study characterizes the role of the membrane-bound receptor tyrosine kinase EphA7 in cerebral cortical dendritic elaboration and dendritic spine formation and synaptic function. Results indicate that EphA7 restricts dendritic elaboration early in corticogenesis and promotes dendritic spine formation and synaptic maturation later in a neuron’s life. These results identify EphA7 as a signaling molecule in the molecular machinery that drives neuronal maturation and synaptic function, signaling that may impact our understanding of neurodevelopmental disorders.
Keywords: dendritic spine, neurogenesis, synaptogenesis
Abstract
The process by which excitatory neurons are generated and mature during the development of the cerebral cortex occurs in a stereotyped manner; coordinated neuronal birth, migration, and differentiation during embryonic and early postnatal life are prerequisites for selective synaptic connections that mediate meaningful neurotransmission in maturity. Normal cortical function depends upon the proper elaboration of neurons, including the initial extension of cellular processes that lead to the formation of axons and dendrites and the subsequent maturation of synapses. Here, we examine the role of cell-based signaling via the receptor tyrosine kinase EphA7 in guiding the extension and maturation of cortical dendrites. EphA7, localized to dendritic shafts and spines of pyramidal cells, is uniquely expressed during cortical neuronal development. On patterned substrates, EphA7 signaling restricts dendritic extent, with Src and Tsc1 serving as downstream mediators. Perturbation of EphA7 signaling in vitro and in vivo alters dendritic elaboration: Dendrites are longer and more complex when EphA7 is absent and are shorter and simpler when EphA7 is ectopically expressed. Later in neuronal maturation, EphA7 influences protrusions from dendritic shafts and the assembling of synaptic components. Indeed, synaptic function relies on EphA7; the electrophysiological maturation of pyramidal neurons is delayed in cultures lacking EphA7, indicating that EphA7 enhances synaptic function. These results provide evidence of roles for Eph signaling, first in limiting the elaboration of cortical neuronal dendrites and then in coordinating the maturation and function of synapses.
The development of the cerebral cortex requires considerable cellular and molecular orchestration to lay the foundation for a mature neural network capable of processing sensory input, coordinating motor output, and producing thought, memory, and perception (1–3). Excitatory cortical neurons originate in the cortical ventricular zone as progenitors shift to a postmitotic state and genetic programs that promote neuronal differentiation are initiated. Newly differentiating neurons migrate radially from the germinal zone to occupy a more superficial position in the developing cortical plate (CP) in the vertical dimension and cortical area in the horizontal axis. Neurons then initiate axon and dendrite extension, eventually creating connections with pre- and postsynaptic partners (4). In parallel, inhibitory interneurons are generated in the ventral ganglionic eminences and migrate tangentially into the forming CP (5). Neuronal differentiation is coordinated by an array of molecules, some of which act at multiple points to modulate the shape and connectivity of neurons (6–8). Defects in one or more of these cellular and molecular steps are likely to contribute to neurodevelopmental disorders (9–12).
Members of the Eph receptor tyrosine kinase and ephrin ligand family mediate intercellular communication at discrete times in neuronal development (13). Eph receptors embedded in the membrane of a cell engage surface-bound ephrin ligands on neighboring neurons or glia (14, 15). Signaling is activated in the receptor- or ligand-expressing cell or in both cells (16). Eph/ephrin engagement ultimately can alter the cytoskeleton within a neuron, thus influencing both neuronal shape and contacts (13, 17). How particular Eph receptors or ephrin ligands act during the sequential steps of cerebral cortical neuronal differentiation is unknown.
Multiple Ephs and ephrins are expressed throughout the neocortex as neurons are maturing (18, 19). One receptor, EphA7, is uniquely and dynamically expressed during corticogenesis. Not expressed in the dividing cells of the dorsal telencephalon, EphA7 is present in differentiating neurons of the forming CP (18, 20). EphA7 binds ephrin-A ligands, particularly ephrin-A5, with high affinity (15, 21). Broadly expressed in development, EphA7 is present in anterior and posterior domains, whereas ephrin-A5 is restricted to a middle portion of the CP at birth (19, 20, 22). Although Ephs and ephrins modulate cell shape, influence areal parcellation, guide axonal targeting, direct dendritic elaboration, and affect synaptogenesis in discrete parts of the brain, EphA7’s role in cortical neuronal differentiation has not been studied (23).
Here, we demonstrate that EphA7 directs several discrete aspects of cortical neuronal maturation. First, EphA7 mediates dendritic avoidance of ephrin-A5 domains and inhibits dendritic growth and complexity in cortical neurons. Second, EphA7 limits protrusions from dendritic shafts early in postnatal life. Third, EphA7 promotes dendritic spine formation later in development. Finally, EphA7 promotes excitatory synaptic maturation. Thus, EphA7 is an active and complex mediator of cortical neuronal maturation and function.
Methods
Animal Husbandry and Tissue Preparation.
All animal use and care were in accordance with Georgetown University Animal Care and Use Committee protocols 09–020 (mice) and 10–044 (rats) and federal guidelines. Timed pregnant females were killed and the brains of pups were dissected and either dissociated for cell culture or fixed, frozen, and sectioned for processing. Postnatal animals were killed and the brains were dissected, fixed, frozen, and sectioned or were subjected to Golgi staining. Additional details are given in SI Methods.
In Situ Hybridization.
Embryonic day (E)17.5 mouse embryos were collected, and in situ hybridization was performed as previously described (18). Further information is given in SI Methods.
Immunohistology.
Specifics are detailed in SI Methods. Antibodies and dilutions used include goat anti-EphA7 (1:500; R&D), mouse anti-Map2 (1:1,000–2000; Sigma); rabbit anti-GFP (1:3,000; Invitrogen); mouse anti–PSD-95 clone K28/43 (1:1,000; University of California, Davis/National Institutes of Health NeuroMab Facility).
Neuronal Cultures.
Cortical neuronal cultures were generated as previously described (24); additional details re given in SI Methods. For experiments with rat neurons, hippocampi were dissected, and dissociated cells were plated at 180,000 cells per well in a 12-well dish on coverslips coated with poly-d-lysine (37.5 μg/mL) and laminin (2.5 μg/mL) in Neurobasal medium plus 2% (vol/vol) B27, 0.5 mM l-glutamine, 0.125 mM glutamate, 1% penicillin/streptomycin. Hippocampal cells were transfected at day in vitro (DIV)16 using Lipofectamine 2000 (Invitrogen). Coverslips were fixed with 4% (vol/vol) paraformaldehyde (PFA), 4% (vol/vol) sucrose at DIV18 for immunocytochemistry.
Patterned Substrate Assay.
Patterned substrates were generated as previously described (25). Details are given in SI Methods. In experiments examining Src function, 5 µM of Src reagents PP2 or PP3 (Calbiochem) was added 1 h after plating. In all cases, cells were grown for 3 DIV and then were fixed in 4% (vol/vol) PFA before being stained. For analysis, images of 10 fields per coverslip for at least three experiments were acquired at 20× magnification and imported into NeuronJ. The length of all dendrites in the field was traced and categorized as being on an unlabeled or labeled stripe. Total neurite length and the area of each stripe were calculated. A preference score [(length of labeled stripe/area of labeled stripe)/(length of unlabeled stripe/area of unlabeled stripe)] was calculated. Values from control and test groups were compared using one-way ANOVA.
Analysis of Dendritic Extent, In Vitro Gain-of-Function Paradigm.
E15.5 cortex transfected via ex utero electroporation with CMV-YFP and either control DNA (pSK+) or epitope-tagged CMV-EphA7 expression vectors generated by the M.J.D. laboratory using the gain-of-function (GOF) paradigm, and differentiated neuronal cultures were generated. At DIV7, pyramidal transfected neurons were traced (n = 30 control and n = 26 EphA7 GOF neurons from four separate experiments). Axons (thin neurites extending at least twice the length of any other neurite) were excluded from the analysis. NeuronJ was used to trace and measure length of the dendrites. The numbers of primary and secondary dendritic branches were recorded for each cell. Values were averaged, and statistical comparisons between conditions were performed using a one-way ANOVA.
Golgi Staining.
A Golgi staining kit was used according to the manufacturer’s instructions (FD Neurotechnologies). Additional details are given in SI Methods.
Analysis of Dendritic Extent, in Vivo Loss-of-Function Paradigm.
Pyramidal neurons in deep layer IV and layer V of P10 mouse cortex were traced (n = 29 WT and n = 27 EphA7−/− neurons from five animals per genotype). NeuronJ was used to count and measure dendrites. Values were averaged, and statistical comparisons between conditions were performed using a one-way ANOVA. Ten pyramidal neurons from deep IV and V cortex from each of at least three animals per genotype per time point were examined. For each cell, 50-μm segments of the primary apical or a secondary apical branch were identified, and cytoplasmic extensions from the dendrite were counted. At P10, differentiating between filopodia and immature spines was difficult; therefore the total number of protrusions was quantified. At P22 total protrusions, filopodia, (long thin extensions) and spines (extensions with mushroom head or stubby morphology) were classified according to Irwin et al. (26) and counted. Cells were averaged, and statistical comparisons between genotypes were performed using a one-way ANOVA.
Electrophysiological Recording.
Neurons with a large, pyramidal soma and three to six primary dendrites were selected for recording. Stock solutions of bicuculline methobromide (BMR), tetrodotoxin (TTX), and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt hydrate (NBQX) were prepared and diluted to final concentrations in an extracellular solution containing (in mM): 145 NaCl, 5 KCl, 1 CaCl2, 5 Hepes, 5 glucose, 25 sucrose, and 0.25 mg/L phenol red, pH adjusted to 7.35–7.45 with NaOH. Receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) were isolated by local application of 25 μM BMR and 0.5 μM TTX using Y-tube local perfusion. A subset of cells was tested with 5 μM NBQX to verify that all recorded events were AMPA receptor mediated. Details of data analysis are included in SI Methods.
Results
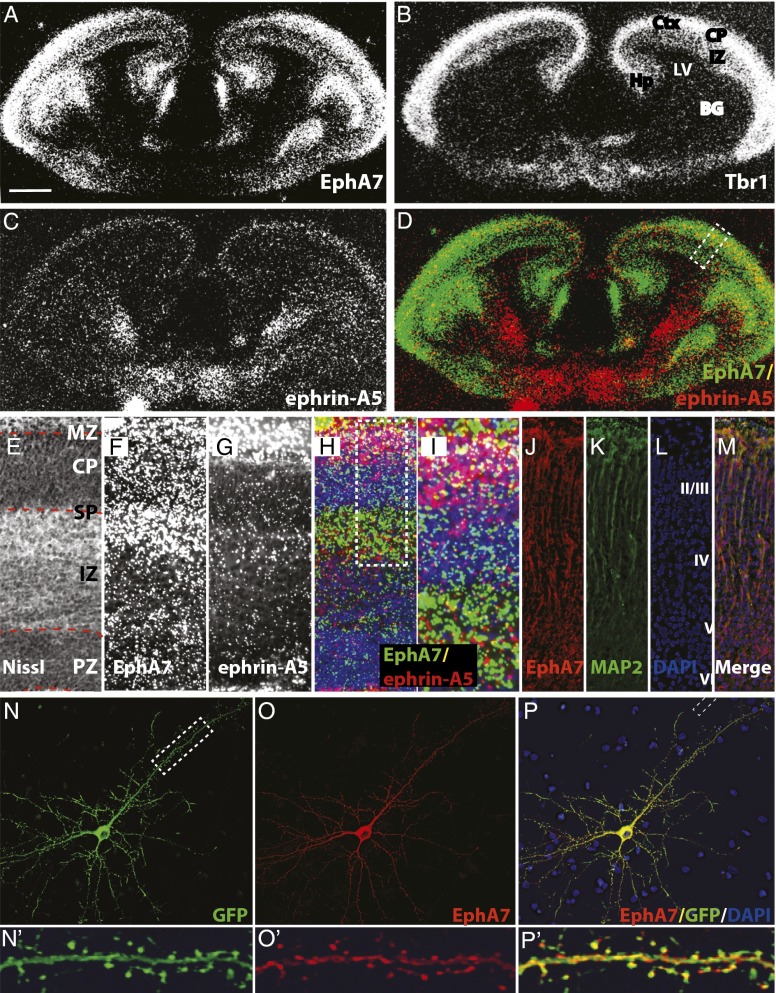
During corticogenesis, in situ hybridization reveals that receptor EphA7 is present in embryonic zones that contain differentiating cortical neurons (Fig. 1 A and F) (18, 20). Indeed, at E17.5, EphA7 is present in the intermediate zone (IZ) and CP, indicated by the CP marker TBrain-1 (Tbr-1) (Fig. 1 A, B, and F) (27, 28). The ligand ephrin-A5 also is expressed in the forming cerebral cortex at E17.5, present in the superficial CP (Fig. 1 C and G), overlapping slightly with EphA7 (yellow in Fig. 1 D, H, and I). EphA7, although expressed throughout the CP, is concentrated in deep layers, whereas ephrin-A5 is present superficially (Fig. 1I). Immunohistochemical analyses, both in vivo and in vitro, demonstrate that the EphA7 protein is present in dendrites. Indeed, in E18.5 cerebral cortex, EphA7 is present in MAP2-positive dendritic processes (Fig. 1 J–M), staining that was absent in EphA7−/− tissue (Fig. S1). In parallel, epitope-tagged EphA7 localizes to the dendritic shaft as well as to the dendritic spines of cultured hippocampal neurons (Fig. 1 N–P). Thus, EphA7 and one of its ligands are present in the CP as neurons are maturing.
Fig. 1.
Expression of EphA7 and its ligand, ephrin-A5, during corticogenesis. In situ hybridizations of EphA7 (A and F, green in D, H, and I), Tbr1 (B), or ephrin-A5 (C and G, red in H and I) in coronal sections of an E17.5 mouse brain, with the cerebral wall (E–H) or CP (I) enlarged. (A–C) In the developing cerebral cortex, both EphA7 (A) and Tbr-1 (B) are expressed in embryonic zones (IZ and CP) known to contain differentiated cortical neurons. (C and D) Ephrin-A5 is expressed by cells of the CP, overlapping with EphA7 (yellow in D). (E–I) The cerebral wall with embryonic zones marked (E). EphA7 is at highest levels in the IZ, subplate, and marginal zone (MZ) (F), and ephrin-A5 is superficially expressed (G), overlapping with EphA7 (yellow in H). Within the region of the CP (I), EphA7 is expressed in the deep CP and subplate, and ephrin-A5 is present in the upper CP and marginal zone. The overlap is shown in yellow. (J–M) Immunohistochemistry for EphA7 (J; red in M), MAP2 (K; green in M), and nuclear stain DAPI (L; blue in M) in coronal sections from an E18.5 mouse brain reveal EphA7 in dendrites of maturing cortical neurons. (N–P′) Immunocytochemistry of a hippocampal neuron transfected at DIV16 and imaged at DIV18 for the transfected actin-GFP (N and N′; green in P and P′) and EphA7 (O and O′; red in P and P′) with DAPI (blue in P and P’). EphA7 is excluded from the nucleus but is present in neurites (O and P). Magnification of the boxed area in N reveals that EphA7 is present in the dendritic shaft and localizes to dendritic spines (O′ and P′). (Scale bar: 400 μM for A–D; 100 μM for E–H; 50 μM for I–P; 10 μM for N′–P.) BG, basal ganglia; LV, lateral ventricle; PZ, proliferative zone.
Cortical Neuronal Dendrites Respond to Ephrin-A5 in Vitro via EphA7.
To study a possible role for EphA7 in cortical neuronal elaboration, a patterned substrate assay was used (25). Cortical neurons were plated on these striped substrates and grown for 3 DIV (Fig. S2D). Axons or dendrites were immunocytochemically labeled, and the lengths of either process on each substrate were measured and normalized to the stripe area. A preference score was generated and used to characterize cellular interactions with the labeled stripe (Fig. S2).
Consistent with previous studies, axons of WT cortical neurons grew evenly on control stripes but were repulsed by ephrin-A5 (Fig. S2 G, H, and K) (25, 29); this guidance was independent of EphA7, since similar repulsion was observed in WT and EphA7−/− cortical neurons (Fig. S2 I and K). In contrast, axon repulsion by ephrin-A5 relies on EphA4, because repulsion was compromised in EphA4−/− neurons (Fig. S2 J and K), as had been demonstrated previously (30). This experimental paradigm both replicates previous results and reveals previously unidentified differences between EphA4 and EphA7 function.
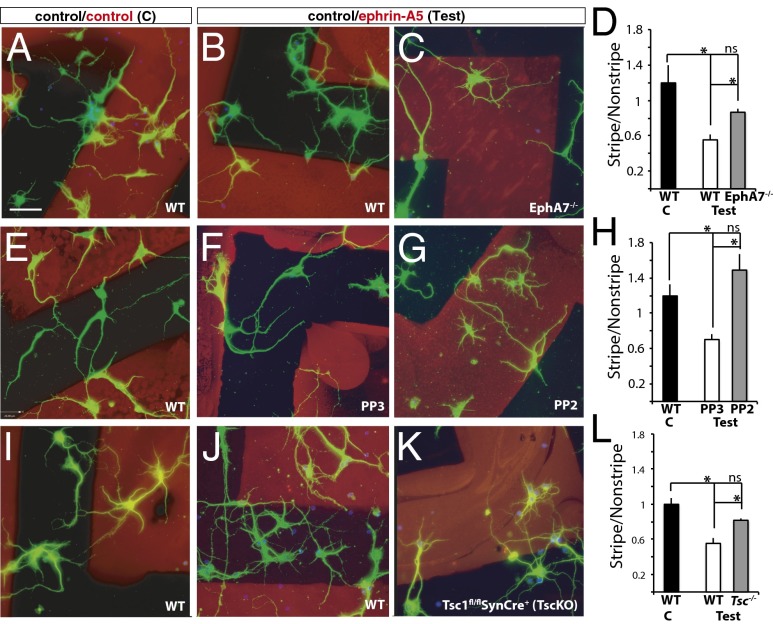
Although the roles of Eph/ephrin signaling in axon guidance have been studied extensively (25, 29, 31), much less is known about the roles of Eph/ephrin signaling in the elaboration of dendrites. Thus, patterned substrates were used to assess whether EphA signaling plays a role in dendritic elaboration. Dendrites of WT cortical neurons extended evenly on control substrates (Fig. 2 A, E, and I), producing a preference score close to 1 (black bars in Fig. 2 D, H, and L; 1.2 ± 0.12 in D, 1.18 ± 0.09 in H; 1 ± 0.07 in L). In contrast, dendrites of WT neurons avoided the ephrin-A5 substrate and preferred control protein (Fig. 2 B, F, and J), resulting in lower preference scores (white bars in Fig. 2 D, H, and L; 0.55 ± 0.06 in D, 0.7 ± 0.05 in H, and 0.58 ± 0.05 in L). The significant repulsion of dendrites of cortical neurons by ephrin-A5 ligand demonstrates dendritic sensitivity to this ligand.
Fig. 2.
Dendrites of cortical neurons avoid ephrin-A5 via EphA7 using Src family kinases and Tsc1. (A–C) Dendrites of WT (A and B), or EphA7−/− (C) cortical neurons grown on alternating stripes of control (black)/control (red) in the control condition (A) or control (black)/ephrin-A5 (red) in the test condition (B and C). (D) Preference score of WT dendrites on the control substrate (black bar) showed no selectivity (score close to 1), whereas WT dendrites on the test substrate (white bar) were repulsed from ephrin-A5 (score significantly below 1). In contrast, EphA7−/− dendrites (gray bar) were not significantly repulsed from ephrin-A5. (E–G) Dendrites of WT cortical neurons grown on control (E) or test (F and G) stripes alone (E) or in the presence of PP3, a biologically inactive compound (F), or PP2, an Src inhibitor (G). (H) Preference scores show that WT neurons grown on control stripes (black bar) had no preference. Dendrites of cortical neurons grown in the presence of PP3 (white bar) avoided ephrin-A5, but those grown in the presence of PP2 (gray bar) were unresponsive to ephrin-A5. (I–K) Compared with dendrites of control neurons, which grow evenly on control substrate (I) but are repulsed from ephrin-A5 stripes (J), dendrites of Tsc1fl/fl; SynCre+ neurons show no significant repulsion by ephrin-A5 (K). (L) The preference score revealed no preference for WT dendrites grown on control substrates (black bar), strong repulsion for WT dendrites grown on test substrates (white bar), and no significant repulsion for Tsc1fl/fl; SynCre+ dendrites grown on test substrates (gray bar). (Scale bar: 25 μM for all image panels.) *P < 0.05; ns, no significant difference.
To determine whether EphA7 plays a role in dendritic repulsion from ephrin-A5, cortical neurons from EphA7−/− mice were analyzed in the patterned substrate assay. Results indicate that dendrites of EphA7−/− neurons were less sensitive to ephrin-A5 (Fig. 2C), generating a preference score significantly different from that of WT neurons on test substrate and no different from WT neurons on control substrate (0.82 ± 0.02; gray bar in Fig. 2D). Thus, in this assay, EphA7 contributes to dendritic responsiveness to ephrin-A5.
Src and Tsc1 Participate in Dendritic Avoidance of Ephrin-A5.
How might EphA7 signal this dendritic repulsion? Several intracellular signaling pathways have been implicated in repulsive responses (17, 32–35). Here, we focus on the Src family of nonreceptor tyrosine kinases and Tsc1, a regulator of mammalian target of rapamycin (mTOR) signaling. To test whether Src family kinases play a role in dendritic repulsion of ephrin-A5, PP2, an Src inhibitor, or PP3, a structurally related inert compound, was added to neurons grown on ephrin-A5–patterned substrates. The dendrites of WT neurons grown in the presence of PP3 were significantly repulsed by the ephrin-A5 substrate on test stripes (Fig. 2F, 0.79 ± 0.06; white bar in Fig. 2H), whereas those grown with PP2 were not repulsed by ephrin-A5, generating a preference score significantly different from that of WT on test stripes and no different from WT on control stripes (1.49 ± 0.18) (Fig. 2G; gray bar in Fig. 2H). Thus, ephrin-A5–mediated repulsion of dendrites relies on the activity of Src family kinases.
Next, we examined whether Tsc1 acts in ephrin-A5–induced dendritic repulsion. Tsc1 and Tsc2 proteins normally form a complex that can regulate mTOR signaling and decrease protein synthesis and cell growth and have been implicated in axonal signaling of other Eph receptors (36). Neurons from embryos in which Tsc signaling was eliminated selectively in postmitotic neurons (Tsc1fl/fl; Synapsin-Cre+) were plated on control- or test-patterned substrates. Compared with dendrites of WT embryos (Fig. 2J), dendrites of Tsc1 mutant neurons were less repulsed by ephrin-A5 (Fig. 2K), producing a preference score significantly different from that of WT on test substrates and no different from WT on control substrates (0.55 ± 0.06) (gray bar in Fig. 2L). These data indicate that the Tsc1/mTOR pathway also is used in dendritic avoidance of ephrin-A5.
Dendritic Elaboration Is Shifted When EphA7 Signaling Is Altered.
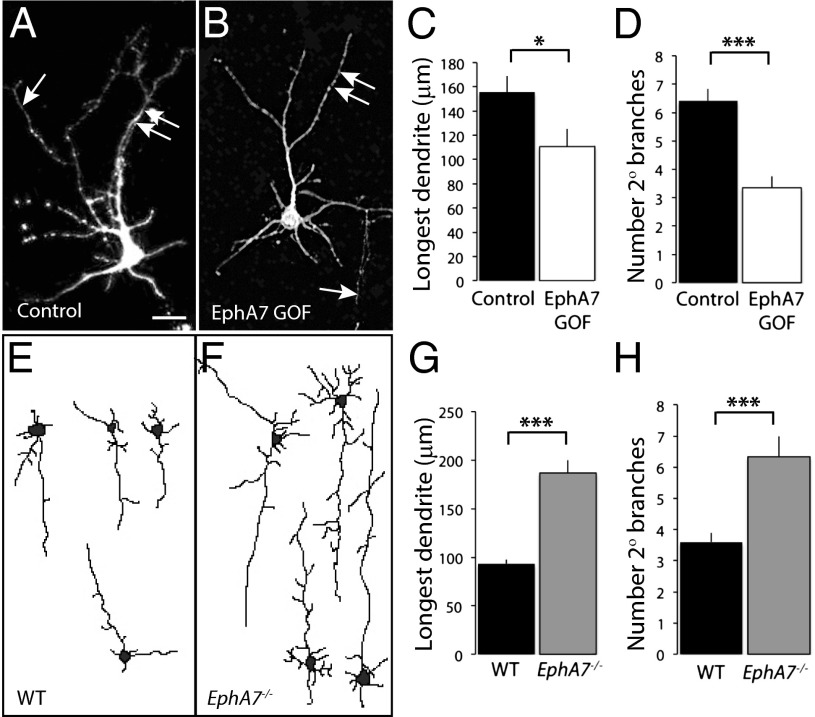
To examine the effects of EphA7 signaling in cortical neuronal elaboration, levels of EphA7 signaling were manipulated in vitro and in vivo, and cellular morphology was examined. To begin, primary cortical neurons were transfected with CMV-actin-GFP and either an inert vector (control) or an EphA7 expression construct (EphA7 GOF) that produces ligand-independent activation of forward signaling (29, 37). EphA7 GOF cells (Fig. 3B; white bars in Fig. 3 C and D) had significantly shorter dendrites than control cells (Fig. 3A; black bars in Fig. 3 C and D) (110.7 ± 14.5 μm for EphA7 GOF cells; 156.7 ± 13.4 μm for control cells). The decrease in length corresponded to less dendritic branching (6.4 ± 0.4 for control cells; 3.3 ± 0.4 for EphA7 GOF cells) (Fig. 3D). These data demonstrate that ectopic activation of EphA7 restricts dendrite length and complexity.
Fig. 3.
Dendritic length and complexity depend on EphA7 activity in vitro and in vivo. (A–D) Primary cortical neurons were transfected at the time of plating with CMV-actin-GFP and an inert vector (A) or with an EphA7 expression vector (B) and were cultured for 7 DIV before being fixed, stained for GFP staining, and analyzed. Both the average length of the longest neurite and the number of secondary branches were less for EphA7 GOF than for control neurons (quantified in C and D, respectively). A single arrow marks axons (not included in analyses), and double arrows indicate dendrites. (E–H) Golgi-stained deep-layer pyramidal neurons from P10 cerebral cortex of WT (E) or EphA7−/− (F) mice. Absence of EphA7 resulted in longer apical dendrites and an increased number of secondary branches as compared with neurons in WT cortex (quantified in G and H, respectively). (Scale bar: 25 μM in A and B; 30 μM in E and F.) *P < 0.05, ***P < 0.001.
Cellular changes also were observed when EphA7 function was eliminated in vivo. Golgi-stained neurons in the deep layers of WT cortex at postnatal day (P)10 (see Fig. 3E for representative traces) were analyzed and compared with Golgi-stained neurons of the same position in EphA7−/− cortex (see Fig. 3F for representative traces, ). Compared with WT neurons (Fig. 3E; black bars in Fig. 3 G and H), EphA7−/− neurons (Fig. 3F; gray bars in Fig. 3 G and H) had longer apical dendrites (3.1 ± 4.4 μm for control cells, 186.8 ± 13.0 μm for EphA7−/− cells) (Fig. 3G) and more branching, evidenced by more secondary branches when EphA7 was lacking (3.6 ± 0.3 for control cells, 6.3 ± 0.7 for EphA7−/− cells) (Fig. 3H). These data support an inhibitory role for EphA7 signaling in neuronal dendritic elaboration.
EphA7 Influences Dendritic Protrusions and Synaptic Components.
Once dendrites elaborate during late embryonic and early postnatal life, small dendritic protrusions become apparent during the first 2 wk of postnatal life. These protrusions tend to be long and thin early and are termed “filopodia.” Over time, and certainly by the end of first postnatal month, dendritic protrusions become more elaborate, including dendritic spines with stubby or mushroom appearances (38). One hypothesis is that dendritic filopodia expand the dendritic area available for contacts with axons, with some filopodia developing into spines (39, 40). Because EphA7 is implicated in dendritic patterning early in cortical neuronal maturation and is expressed when spines are forming, potential roles for EphA7 signaling in filopodial and spine formation were examined also.
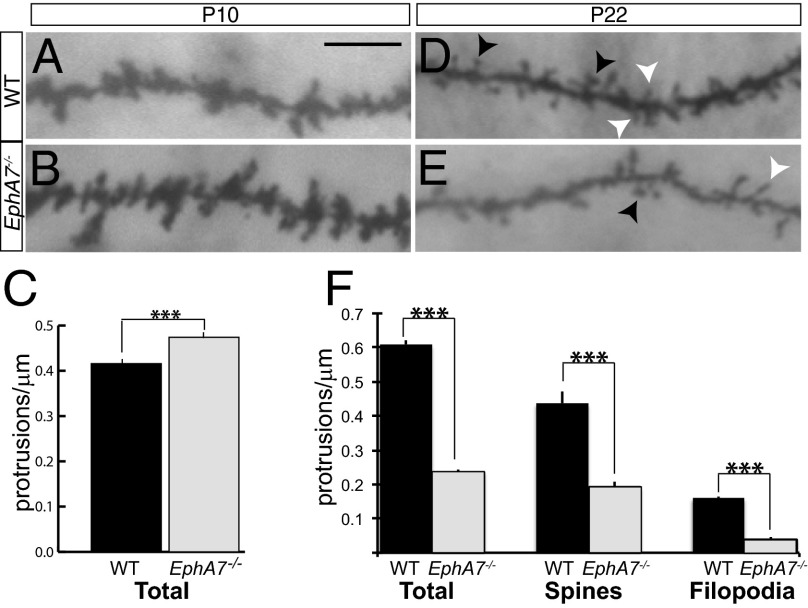
Golgi staining was used to visualize neurons in situ. Dendritic protrusions, filopodia, and spines were analyzed in WT and EphA7−/− neurons at two developmentally distinct ages: P10, when simple protrusions (filopodia) are prevalent and the development of dendritic spines is starting, and P22, when synaptic contacts have matured and protrusions have dendritic spine or filopodial morphology. At P10, WT cortical neurons extended 0.41 ± 0.011 protrusions/μm (Fig. 4A; black bar in Fig. 4C), and EphA7−/− neurons had significantly more protrusions (0.47 ± 0.016 protrusions/μm) (Fig. 4B; gray bar in Fig. 4C). Nevertheless, this result suggests that EphA7 acts to restrict dendritic protrusions early in postnatal life.
Fig. 4.
The abundance of dendritic protrusions is altered in EphA7−/− cortical neurons in vivo. (A and B) Golgi-stained P10 dendritic protrusions extend from the dendritic shaft in WT (A) and EphA7−/− (B) deep-layer cortical neurons. (C) At P10, EphA7−/− neurons (gray bar) have more dendritic protrusions than WT neurons (black bar). (D and E) Golgi-stained P22 protrusions extend from the dendritic shafts in WT (D) and EphA7−/− (E) cerebral cortical neurons. White arrowheads mark filopodia; black arrowheads mark dendritic spines. (F) EphA7−/− neurons (gray bar) have fewer total protrusions (Left), spines (Middle), and filopodia (Right) than WT neurons (black bar). (Scale bar: 10 μM for all image panels.) ***P < 0.001.
Analysis at P22 revealed that WT neurons had 0.609 ± 0.014 protrusions/μm (Fig. 4D), most of which were dendritic spines (0.437 ± 0.033 spines/μm) (black arrowheads in Fig. 4) with a small proportion of filopodia (0.172 ± 0.003 filopodia/μm) (white arrowheads in Fig. 4). In contrast, EphA7−/− neurons had significantly fewer dendritic protrusions (0.237 ± 0.005 protrusions/μm) at P22, with a large proportion classified as dendritic spines (0.191 ± 0.017 spines/μm) and relatively few filopodia (0.046 ± 0.005 filopodia/μm) (Fig. 4E). The few dendritic protrusions seen in mature EphA7−/− neurons support a role for EphA7 in promoting both filopodia and spines in late postnatal life. These differences corresponded to synaptic markers; puncta of both PSD-95, a postsynaptic marker of mature excitatory synapses, and surface GluA2, the AMPA receptor subunit, were reduced in EphA7−/− cells compared with control neurons (Fig. S3). These results indicate that neurons from EphA7−/− mutant animals have fewer mature excitatory synaptic sites at DIV18 than do control neurons.
EphA7 Influences in Vitro Synaptic Function.
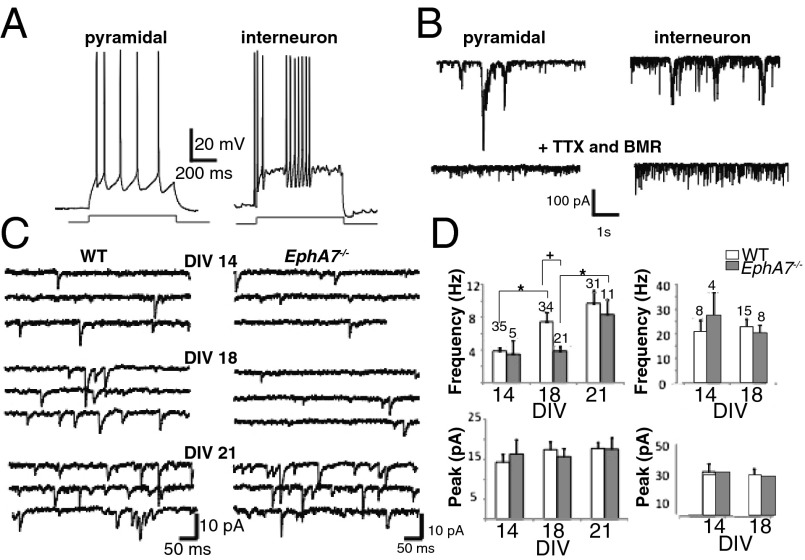
Based on changes in dendritic protrusions and synaptic markers, electrophysiological function was examined in WT and EphA7−/− primary cortical neurons. Whole-cell patch-clamp recordings were performed from large cortical neurons with pyramidal cell bodies. Because it has been reported that the morphology of excitatory pyramidal neurons and fast-spiking GABAergic interneurons can be similar in culture, we further distinguished between these cell types by studying repetitive action potentials elicited by current injection (41). Indeed, two neuronal populations were revealed: regular-spiking pyramidal neurons (Fig. 5A, Left) and fast-spiking interneurons (Fig. 5A, Right). As previously reported, pyramidal neurons were characterized by longer average spike half width (2.5 ± 0.3 ms at DIV18) than fast-spiking neurons (1.2 ± 0.1 ms at DIV18). Fewer fast-spiking interneurons were observed in culture at DIV14 than at other ages, and they were excluded from analysis. Local perfusion with TTX and BMR decreased the occurrence of spontaneous excitatory postsynaptic currents (EPSCs), revealing larger and more frequent mEPSCs in fast-spiking interneurons than in pyramidal neurons (Fig. 5B, Lower).
Fig. 5.
Delayed development of mESPCs in EphA7−/− cultured pyramidal neurons. (A) Repetitive action potentials elicited by current injection in a pyramidal neuron (Left) and an interneuron (Right) in primary culture at DIV18. (B) (Upper) Representative current traces showing spontaneous synaptic activity in a regular-spiking pyramidal neuron (Left) and a fast-spiking interneuron (Right) in primary culture at DIV19. (Lower) Perfusion with TTX and BMR revealed larger and more frequent mEPSCs in fast-spiking interneurons than in pyramidal neurons. (C). Multiple sweeps illustrating mEPSCs in neurons from WT (Left) and EphA7−/− (Right) mice at DIV14, DIV18, and DIV21 reveal age-dependent increases in WT cultures with time in vitro that was delayed in EphA7−/− cultures. (D) Quantification of data on the frequency of events (Upper) and peak mEPSC amplitude (Lower) in pyramidal neurons (Left) and fast-spiking interneurons (Right) at each day in vitro reveal age-dependent increases in the frequency but not in the amplitude of mEPSCs in WT pyramidal neurons. Time-dependent maturation is delayed in EphA7−/− neurons, with lower mEPSC frequency at both DIV14 and DIV18, although levels are similar to those in WT neurons by DIV21. The amplitude of mEPSCs in EphA7−/− neurons is similar to that in WT pyramidal neurons and is stable over time in culture. No differences were observed in the mEPSCs of fast-spiking EphA7−/− and WT interneurons. Numbers over bars indicate the number of cells analyzed. *P < 0.05.
To examine the emergence of electrophysiological characteristics, the mEPSCs of pyramidal neurons in cultures through time were examined. For WT pyramidal neurons, the frequency of mEPSCs increased significantly from DIV14–18 and remained high at DIV21 (Fig. 5C, Left; white bar in Fig. 5D). In contrast, the frequency of mEPSCs was low at DIV14 and DIV18, with WT levels eventually achieved by DIV21 in EphA7−/−cultures (Fig. 5C, Right; gray bar in Fig. 5D, Upper Left). The frequency of mEPSCs was lower in EphA7−/− neurons than in WT neurons at DIV18 (Fig. 5D, Upper Left). However, no difference was observed between genotypes in the frequency of mEPSCs in fast-spiking interneurons (Fig. 5D, Upper Right), and the amplitude of mEPSCs did not vary for based on cell type (Fig. 5C, Bottom).
Discussion
Cell surface-based communication in the brain serves to coordinate the development of neurons. Here we describe roles of EphA7 in modulating dendritic compartments and mediating synaptic connectivity in cerebral cortical neurons. The receptor EphA7 is expressed selectively in the differentiated zone of the developing cerebral cortex as neurons elaborate, overlapping with one of its ligands, ephrin-A5. EphA7 is present within dendritic shafts during cortical development and in shafts and spines in neurons in culture. Given the overlapping expression of EphA7 and ephrin-A5 and EphA7’s localization to dendrites, we hypothesized that EphA7 signaling influences dendritic elaboration. Indeed, when cortical neurons are plated on patterned substrates, both axons and dendrites avoid ephrin-A5, instead preferring the control substrate. Our data demonstrate that EphA7 mediates dendritic responses to ephrin-A5, relying on both Src and Tsc1 function, pathways that are known to regulate dendritic arborization (42–44). EphA7 activation results in less complex and shorter dendrites, whereas elimination of EphA7 signaling produces neurons with more complex, longer dendrites. Because patients and model organisms with tuberous sclerosis complex manifest neurodevelopmental symptoms (10, 45, 46), we suspect that some part of those symptoms may result from disrupted EphA7 signaling.
As neurons elaborate, axons contact dendrites, forging connections that may couple cells synaptically. A multitude of proteins, including Eph receptors, have been implicated in synaptogenesis in the mammalian forebrain (14, 47–49). With a role for EphA7 in dendritic extension defined, we next asked whether EphA7 impacts more mature neurons. To this end, we examined characteristics of dendritic protrusions in vivo. At an early age (P10), when filopodia are extending from dendritic shafts and dendritic spine formation is just beginning, pyramidal neurons from EphA7−/− cortex had more dendritic protrusions, suggesting that EphA7 normally limits dendritic extensions at this stage. Later, at P22, when filopodia and dendritic spines are morphologically distinct, dendritic protrusions (both filopodia and spines) were less dense in EphA7−/− neurons than in WT neurons. In parallel, the levels of the synaptic markers PSD-95 and GluA2 were reduced in EphA7−/− neurons, consistent with EphA7’s acting to promote the morphological and molecular maturation of synapses.
Results of electrophysiological analyses also demonstrate that cortical neuronal synaptic function relies on EphA7 signaling. Neurons cultured from WT cortex exhibited a developmental increase in mEPSC frequency from DIV14 to DIV18 to DIV21. This progressive increase was perturbed in EphA7−/− cultures; the frequency of mEPSCs was the same at DIV14 and DIV18, finally reaching control levels at DIV21. Thus, EphA7 signaling appears to coordinate the maturation of cortical synapses in excitatory pyramidal neurons. Although roles for EphB family members in synaptic function have been well characterized (47, 48, 50), a role for EphA7 in neuronal maturation and synaptic function complements the smaller group of studies that focus on EphA-mediated signaling at synapses (33, 51).
EphA7 and ephrin-A5 are expressed in cortical neurons in vivo and in vitro (18). Our data characterize population-wide responses to a perturbation in EphA7 signaling. Because it is likely that only a subset of cortical neurons use EphA7/ephrin-A5 interactions to modulate dendritic characteristics, we expect that theses results will be more pronounced once distinct populations of cortical neurons can be distinguished.
The opposing roles of EphA7 in limiting dendritic elaboration and protrusion extension early in development but promoting spine formation later in neuronal maturation are perplexing. How might one gene exert distinct effects at different stages of a neuron’s maturation? The dichotomy may be explained by the presence of two isoforms of this receptor; alternative splicing generates a repulsive full-length, signaling-competent isoform of EphA7 and a potentially attractive truncated isoform of EphA7 (52–55). During cortical development, the relative proportion of expression of these isoforms shifts (20). Because both isoforms of EphA7 are eliminated in the EphA7−/− mouse, early expression of full-length EphA7 may limit dendritic protrusions initially, whereas the later presence of truncated EphA7 may promote dendritic spine formation.
Our data demonstrate multifaceted roles for EphA7 over the course of cortical neuronal maturation. Signaling via EphA7 regulates initial neuronal dendritic elaboration as well as extension of protrusions, spine formation, and synaptic function. Use of the same signaling molecules for discrete functions over a neuron’s lifetime is an efficient way of guiding neuronal form and function.
Supplementary Material
Acknowledgments
We thank U. Drescher for providing EphA7−/− mice; H. A. North, C. Chen, L. Orefice, and S. Karam for providing helpful advice and discussion; J. Nobile and A. Roberta for conducting preliminary studies on dendritic spines; F. Vanevski, J. Baiocco, L. Jamis, and J. Espinosa for help with data analyses; and H. A. North, J. K. Kanwal, E. M. Casey, and R. Wurzman for reviewing the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323793111/-/DCSupplemental.
References
- 1.Rakic P. Neurons in rhesus monkey visual cortex: Systematic relation between time of origin and eventual disposition. Science. 1974;183(4123):425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 2.Sidman RL, Rakic P. 1982. Development of the human central nervous system. Histology and Histopathology of the Nervous System, eds Haymaker W, Adams RD (C. C. Thomas, Springfield, IL), pp 3–145.
- 3.McConnell SK. Constructing the cerebral cortex: Neurogenesis and fate determination. Neuron. 1995;15(4):761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 4.Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27(7):392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278(5337):474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 6.Shelly M, et al. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327(5965):547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 7.Arlotta P, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45(2):207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404(6778):567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 9.Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 10.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480(7375):63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X syndrome: The GABAergic system and circuit dysfunction. Dev Neurosci. 2011;33(5):349–364. doi: 10.1159/000329420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6(6):462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 14.Filosa A, et al. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 2009;12(10):1285–1292. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gale NW, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17(1):9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 16.Holland SJ, et al. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383(6602):722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 17.Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 2004;16(6):655–666. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Yun ME, Johnson RR, Antic A, Donoghue MJ. EphA family gene expression in the developing mouse neocortex: Regional patterns reveal intrinsic programs and extrinsic influence. J Comp Neurol. 2003;456(3):203–216. doi: 10.1002/cne.10498. [DOI] [PubMed] [Google Scholar]
- 19.Mackarehtschian K, Lau CK, Caras I, McConnell SK. Regional differences in the developing cerebral cortex revealed by ephrin-A5 expression. Cereb Cortex. 1999;9(6):601–610. doi: 10.1093/cercor/9.6.601. [DOI] [PubMed] [Google Scholar]
- 20.Miller K, Kolk SM, Donoghue MJ. EphA7-ephrin-A5 signaling in mouse somatosensory cortex: Developmental restriction of molecular domains and postnatal maintenance of functional compartments. J Comp Neurol. 2006;496(5):627–642. doi: 10.1002/cne.20926. [DOI] [PubMed] [Google Scholar]
- 21.Himanen JP, et al. Repelling class discrimination: Ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7(5):501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 22.Dufour A, et al. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39(3):453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- 23.North HA, Clifford MA, Donoghue MJ. ‘Til Eph do us part’: Intercellular signaling via Eph receptors and ephrin ligands guides cerebral cortical development from birth through maturation. Cereb Cortex. 2013;23(8):1765–1773. doi: 10.1093/cercor/bhs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifford MA, Kanwal JK, Dzakpasu R, Donoghue MJ. EphA4 expression promotes network activity and spine maturation in cortical neuronal cultures. Neural Dev. 2011;6:21. doi: 10.1186/1749-8104-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drescher U, et al. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82(3):359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 26.Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10(10):1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 27.Hevner RF, et al. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29(2):353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 28.Hevner RF. Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J Neuropathol Exp Neurol. 2007;66(2):101–109. doi: 10.1097/nen.0b013e3180301c06. [DOI] [PubMed] [Google Scholar]
- 29.Torii M, Levitt P. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron. 2005;48(4):563–575. doi: 10.1016/j.neuron.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Dufour A, Egea J, Kullander K, Klein R, Vanderhaeghen P. Genetic analysis of EphA-dependent signaling mechanisms controlling topographic mapping in vivo. Development. 2006;133(22):4415–4420. doi: 10.1242/dev.02623. [DOI] [PubMed] [Google Scholar]
- 31.Cheng HJ, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79(1):157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 32.Knöll B, Drescher U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J Neurosci. 2004;24(28):6248–6257. doi: 10.1523/JNEUROSCI.0985-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6(2):153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- 34.Richter M, Murai KK, Bourgin C, Pak DT, Pasquale EB. The EphA4 receptor regulates neuronal morphology through SPAR-mediated inactivation of Rap GTPases. J Neurosci. 2007;27(51):14205–14215. doi: 10.1523/JNEUROSCI.2746-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahin M, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46(2):191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Nie D, et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci. 2010;13(2):163–172. doi: 10.1038/nn.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehigh KM, Leonard CE, Baranoski J, Donoghue MJ. Parcellation of the thalamus into distinct nuclei reflects EphA expression and function. Gene Expr Patterns. 2013;13(8):454–463. doi: 10.1016/j.gep.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris KM, Kater SB. Dendritic spines: Cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 39.Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75(3):161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18(21):8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldberg EM, et al. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58(3):387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babus LW, et al. Decreased dendritic spine density and abnormal spine morphology in Fyn knockout mice. Brain Res. 2011;1415:96–102. doi: 10.1016/j.brainres.2011.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18(5):725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19(2):231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Di Nardo A, et al. Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR-dependent manner. J Neurosci. 2009;29(18):5926–5937. doi: 10.1523/JNEUROSCI.0778-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26(47):12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kayser MS, Nolt MJ, Dalva MB. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron. 2008;59(1):56–69. doi: 10.1016/j.neuron.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murai KK, Pasquale EB. Eph receptors, ephrins, and synaptic function. Neuroscientist. 2004;10(4):304–314. doi: 10.1177/1073858403262221. [DOI] [PubMed] [Google Scholar]
- 50.Torres R, et al. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21(6):1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- 51.Yu X, et al. Accelerated experience-dependent pruning of cortical synapses in ephrin-A2 knockout mice. Neuron. 2013;80(1):64–71. doi: 10.1016/j.neuron.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciossek T, Millauer B, Ullrich A. Identification of alternatively spliced mRNAs encoding variants of MDK1, a novel receptor tyrosine kinase expressed in the murine nervous system. Oncogene. 1995;10(1):97–108. [PubMed] [Google Scholar]
- 53.Ciossek T, Ullrich A, West E, Rogers JH. Segregation of the receptor EphA7 from its tyrosine kinase-negative isoform on neurons in adult mouse brain. Brain Res Mol Brain Res. 1999;74(1-2):231–236. doi: 10.1016/s0169-328x(99)00285-5. [DOI] [PubMed] [Google Scholar]
- 54.Valenzuela DM, et al. Identification of full-length and truncated forms of Ehk-3, a novel member of the Eph receptor tyrosine kinase family. Oncogene. 1995;10(8):1573–1580. [PubMed] [Google Scholar]
- 55.Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238(4834):1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.