Significance
Diapause has evolved as a specific subtype of dormancy in most insect species and as a seasonal polyphenism that ensures survival under unfavorable environmental conditions and synchronizes populations. In Bombyx mori, embryonic diapause is induced transgenerationally as a maternal effect. However, the molecular mechanisms involved in the perception of environmental temperature and in linking thermal information to neuroendocrine functions are still unknown. Here, we show that the Bombyx transient receptor potential A1 (TRPA1) could be thermally activated during embryogenesis, and an unknown signaling pathway linked to the release of diapause hormone may then be activated to affect the induction of diapause in progeny. The Bombyx TRPA1 acts as a molecular switch for the development of an alternative phenotype in an animal with seasonal polyphenism.
Abstract
In the bivoltine strain of the silkworm, Bombyx mori, embryonic diapause is induced transgenerationally as a maternal effect. Progeny diapause is determined by the environmental temperature during embryonic development of the mother; however, its molecular mechanisms are largely unknown. Here, we show that the Bombyx TRPA1 ortholog (BmTrpA1) acts as a thermosensitive transient receptor potential (TRP) channel that is activated at temperatures above ∼21 °C and affects the induction of diapause in progeny. In addition, we show that embryonic RNAi of BmTrpA1 affects diapause hormone release during pupal-adult development. This study identifying a thermosensitive TRP channel that acts as a molecular switch for a relatively long-term predictive adaptive response by inducing an alternative phenotype to seasonal polyphenism is unique.
Seasonal polyphenism is the differential expression of alternative phenotypes from a single genotype, depending upon environmental conditions, such as photoperiod, temperature, and nutrition (1, 2). This differential expression is a form of developmental phenotypic plasticity that evolved for seasonal adaptation. In some cases, this type of environmental response improves fitness at a later stage of development, or of progeny more distant than the next generation; this is known as a predictive adaptive response and is matched to the environment predicted to be experienced at a later phase in the organism’s life history (3, 4). However, the molecular basis for seasonal polyphenism is largely unknown.
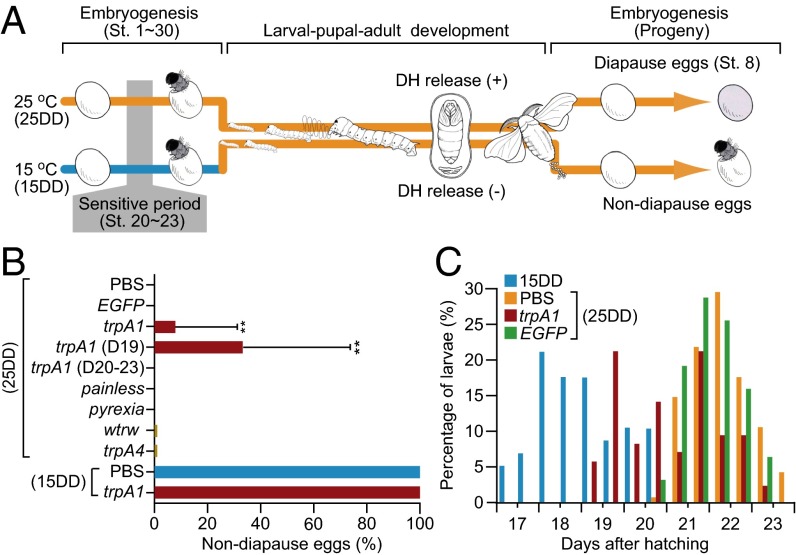
Diapause is a type of seasonal polyphenism and represents one of the most dramatic developmental switches that has been described in the field of biology. Diapause has evolved as a specific subtype of dormancy in most insect species that ensures survival under unfavorable environmental conditions and that synchronizes populations (5). In many insects, environmental cues that signal future environmental changes induce the development of alternative seasonal phenotypes. These alternative phenotypes are accompanied by changes in energy metabolism or storage to improve cold/stress tolerance in later life-history stages or in progeny (6, 7). In populations of Bombyx mori that have two generations per year, embryonic diapause is induced transgenerationally as a maternal effect (Fig. 1A). Progeny diapause is determined by the environmental temperature during embryonic development of the mother. When eggs are incubated at 25 °C under continuous darkness, the resultant female moths (25DD) lay diapause eggs. In contrast, incubation of eggs at 15 °C in dark condition results in moths (15DD) that lay nondiapause eggs (8, 9). Although nondiapause eggs complete their embryogenesis 10 d after oviposition at 25 °C, diapause eggs remain dormant until exposed to appropriate diapause-breaking conditions. For example, exposure of diapause eggs to a temperature as low as 5 °C for over 60 d completely terminates diapause, and when transferred to 25 °C, the eggs resume embryogenesis (10). The diapause/nondiapause phenotype is determined only during a sensitive period. This period lasts for 3 d in 25DD or for 9 d in 15DD and corresponds to stages 20–23 of embryogenesis (Fig. 2A). Transient incubation—or incubation for only part of the sensitive period—of eggs at 25 °C or 15 °C never results in eggs that are uniformly diapause or nondiapause (8, 11). Thus, the diapause of B. mori progeny can be controlled by changing the environmental temperature during the sensitive period of embryonic development, but the temperature must continuously remain in the appropriate range for a relatively long and restrictive period.
Fig. 1.
Embryonic RNAi knockdown of BmTrpA1 induces nondiapause eggs in progeny. (A) Schematic representation of embryonic diapause in the progeny of the silkworm, B. mori. Progeny diapause is determined by environmental temperature during the sensitive period [stages (st.) 20–23] of the mother’s embryonic development. When eggs are incubated at 25 °C, the resultant female moths (25DD) lay pigmented diapause eggs. In contrast, incubation at 15 °C causes the resultant moths (15DD) to lay nondiapause eggs. Nondiapause eggs complete their embryogenesis ∼9 d after oviposition at 25 °C. In contrast, diapause eggs remain in the diapause stage (stage 8). In 25DD pupae, DH released into the hemolymph acts on a G protein-coupled receptor, DH receptor. (B) Effect of dsRNA injection on diapause-egg–inducing activity. Double-stranded RNA of TrpA subfamily genes (trpA1, painless, pyrexia, wtrw, and trpA4) and EGFP were injected into newly laid, nondiapause eggs. Nondiapause egg-inducing activity is represented as the percentage of oviposited eggs in each batch that were nondiapause eggs; trpA1 (D19) and trpA1 (D20–23) indicate BmTrpA1 RNAi-injected larvae that initiated spinning at 19 d and at 20–23 d, respectively. Data represent means ± SD; **P < 0.01 vs. PBS (25DD). (C) Effect of BmTrpA1 dsRNA injection on initiation of spinning. Larval growth rates are represented as days between hatching and initiation of spinning in final- (fifth-) instar larvae. The larvae were checked twice daily at 8:00 AM and 5:00 PM. In B and C, n (egg batch) = 84 (PBS, 25DD); n = 31 (EGFP, 25DD); n = 84 (trpA1, 25DD); n = 81 (others, 25DD); n = 10 (PBS and trpA1, 15DD); and n = 81 (noninjected control, 15DD) in C.
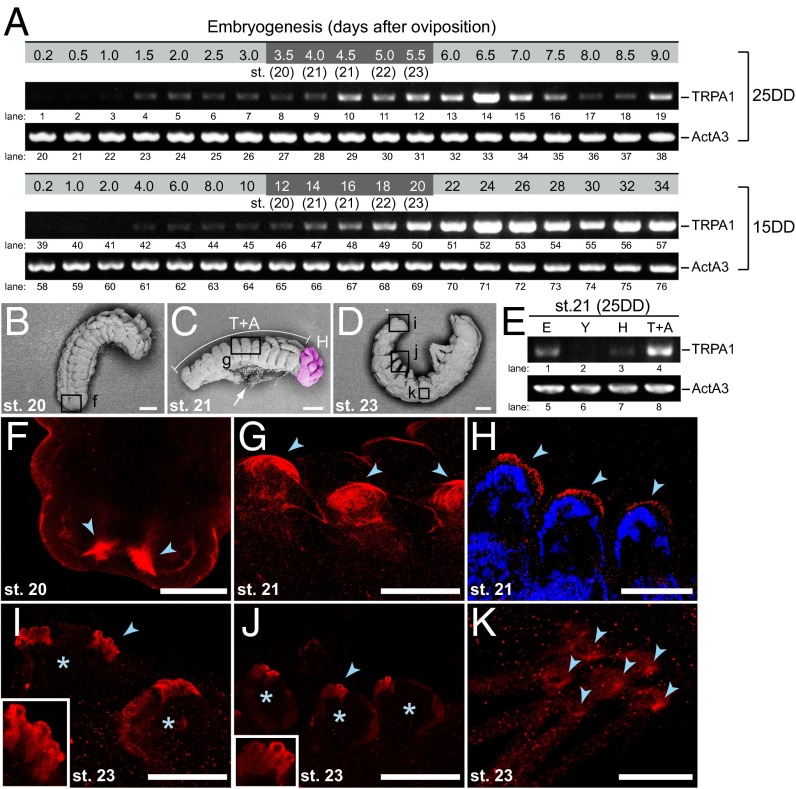
Fig. 2.
Developmental expression profiles of BmTrpA1. (A) RT-PCR analysis was performed on 25DD and 15DD during embryonic development. The mRNA levels of BmTrpA1 in 25DD and 15DD (TRPA1, lanes 1–19 and 39–57, respectively) and ActinA3 in 25DD and 15DD (ActA3, lanes 20–38 and lanes 58–76, respectively) were examined. The shaded area indicates the sensitive periods. (B–D) Scanning electron microscope observation of the lateroventral view at stage 20 (B), and lateral view at stages 21 (C) and 23 (D) of embryonic development. The head in C is indicated by red pseudocolor. Each box indicates the body part examined using immunohistochemistry in F, G, and I–K. The arrow indicates yolk granules taken from around the dorsal closure. (Scale bars, 100 µm.) (E) Distribution of BmTrpA1 mRNA in embryos. Whole embryos (E) and yolk (Y) were separated from stage-21 eggs of 25DD and the embryos were dissected to separate the head (H, indicated in C) and thorax and abdomen complex (T+A, indicated in C). Dissected tissues were subjected to RT-PCR analysis of BmTrpA1 (TRPA1, lanes 1–4) and ActA3 (ActA3, lanes 5–8) mRNAs. (F–K) Whole-mount immunohistochemistry was performed using anti-BmTRPA1 antibody at embryo stages 20–23. Immunoreactive signals (red) were detected in the caudal region of stage-20 embryos (F), anal prolegs of stage-23 embryos (I), prolegs of stage 21 embryos (G and H), prolegs of stage-23 embryos (J), and the hump with bristle (socket cell) in stage-23 embryos (K). Arrowheads indicate the immunoreactive signals in each specimen. Asterisks indicate each proleg. The prolegs of stage-21 embryos were stained with DAPI (blue) (H). Magnified images of the tips of prolegs are shown (Insets) (I and J). (Scale bars, 25 µm in F–J and 10 µm in K.)
At the molecular level, diapause in B. mori are regulated hormonally by diapause hormone (DH), a member of the FXPRLa neuropeptide family that is responsible for diapause induction. DH acts on a G protein-coupled receptor in the developing ovaries of females during pupal-adult development (12, 13), and is produced in DH-pheromone-biosynthesis-activating neuropeptide-producing neurosecretory cells (DHPCs) located within the subesophageal ganglion (SG) (14–16). DH levels in the SG and SG-brain complex are markedly lower in 25DD pupae than in 15DD pupae, although level of mRNA expression is higher in 25DD than in the 15DD pupae at the early to middle stages of pupal-adult development (11, 17). The labial neuromere cell of the SG (SLb) somata are DHPCs, and transport of DH from the SLb somata is actively modified in response to temperature and light conditions during the embryonic development of 25DD pupae. These changes can result in active release of DH into the hemolymph during the middle pupal stages, during which the developing ovaries are sensitive to DH (12, 16). Thus, environmental thermal information is stored for a long time period (approximately 1 mo) until the middle pupal stage, and is transduced to neuroendocrine signals (DH release) to induce progeny diapause. However, the molecular mechanisms involved in perception of environmental temperature and in linking thermal information to neuroendocrine functions are still unknown.
Thermosensitive transient receptor potential (TRP) channels are key players in thermal sensation in both vertebrate and invertebrate organisms (18–21). In insects, TRPA1 and other TRPA subtypes mediate the sensing of increases in temperature. One such TRPA, Drosophila TRPA1, is activated at a threshold of ∼27 °C and regulates thermotaxis (22–24). Furthermore, it has been reported that other Drosophila TRP channels are involved in cold sensation and cold-avoidance behavior (25, 26). Anopheles TRPA1 is activated by temperature increases from 25 °C to ∼37 °C, and it is hypothesized that this activation allows insects to detect increasing temperature gradients derived from host body heat during crucial, close-range host-seeking behaviors (27). Another TRPA subfamily member, AmHsTRPA, is activated in honeybees at a threshold of 34 °C. It has been proposed that AmHsTRPA mediates detection of brood-nest temperatures >36 °C and induces nest-cooling behavior (28). Therefore, in insects the thermosensitive TRPA channels are involved in the detection of, and the short-term and immediate response to, extreme variability in temperature.
Here, we report that a Bombyx TRPA1 (BmTRPA1) ortholog acts as a thermosensitive TRP channel that is activated at a threshold of ∼21 °C and determines progeny diapause during maternal embryonic development. To our knowledge, this is the first report of a thermosensitive TRP channel that acts as a molecular switch for a relatively long-term predictive-adaptive response by inducing an alternative phenotype to seasonal polyphenism, but that does not result in an immediate organismal response.
Results
Embryonic BmTrpA1 Is Involved in Progeny Diapause Induction.
Consistent with previous reports (29), we found genes for 13 TRP channel superfamily members in the Bombyx genome. We successfully cloned the full-length cDNA of five TRPA subfamily genes, BmTrpA1, BmPainless, BmPyrexia, BmWtrw, and BmTrpA4, using a PCR-based strategy. We then evaluated the effects of RNAi on progeny embryos to determine whether any of these Bombyx TRPA subfamily members were involved in diapause induction. To accomplish this, each double-stranded RNA (dsRNA) corresponding to the Bombyx TrpA subfamily genes was injected into newly laid nondiapause eggs, and the eggs were incubated at 25 °C during embryogenesis. The eclosed moths were allowed to oviposit, and the percentages of nondiapause eggs were determined (Fig. 1B, 25DD). We predicted that the TRP channel would be important in progeny diapause induction; this would be demonstrated if nondiapause eggs were laid by 25DD dsRNA-injected moths. Although 15DD and 25DD moths laid all nondiapause or all diapause eggs, respectively, injection of one dsRNA sequence that corresponded to BmTrpA1 and incubation at 25 °C resulted in nondiapause eggs in the next generation (25DD, trpA1, 9.7 ± 26.1%) (Fig. 1B and Fig. S1A). Similarly, injection of another dsRNA sequence corresponding to BmTrpA1 also resulted in the development of nondiapause eggs (4.5 ± 15.6%) (Figs. S1B and S2A). Few or no nondiapause eggs developed after injection of PBS, the dsRNA corresponding to EGFP, or the dsRNA corresponding to other TrpA family genes (Fig. 1B and Figs. S1A and S2A). Furthermore, the nondiapause-type was not disrupted by injection of dsRNA corresponding to BmTrpA1 and incubation at 15 °C (Fig. 1B and Fig. S1A, 15DD). We confirmed that both of the dsRNAs corresponding to BmTrpA1 strongly suppressed the level of BmTrpA1 mRNA (Fig. S2B), and observed that BmTrpA1 RNAi affected the duration of larval development (Fig. 1C). Generally, 15DD larvae initiate spinning earlier than 25DD larvae, as indicated in Fig. 1C. The peak of spinning initiation for 15DD larvae occurred 18 d (AM) after hatching. However, the 25DD larvae initiated spinning 20 d (PM) after hatching and the peak occurred at 22 d (AM). BmTrpA1 larvae exhibited two peaks in spinning initiation (Fig. 1C, trpA1). Interestingly, larvae that initiated spinning at 19 d oviposited nondiapause eggs (35.3 ± 40.2%), whereas larvae that initiated spinning at 20–23 d did not lay any nondiapause eggs (Fig. 1 B and C). EGFP injection did not affect initiation of spinning. These results suggest that embryonic BmTrpA1 is involved in both progeny diapause induction and the duration of larval development.
Developmental Expression Profile of BmTRPA1 During Embryogenesis.
We examined the expression of BmTrpA1 during the embryonic development of eggs incubated at 25DD or 15DD using RT-PCR analysis. BmTrpA1 mRNA was slightly expressed during early embryonic stages and increased during the middle embryonic stages, including the sensitive periods, under both temperature conditions (Fig. 2A, lanes 8–12 and 46–50). We then examined the distribution of BmTrpA1 mRNA in eggs during developmental stage 21, which is the sensitive period (Fig. 2E). Scanning electron microscope observations indicated that differentiation and histogenesis of larval tissues occurs during stages 20–23 and the head and thorax of the embryo become distinguishable after stage 20 (Fig. 2B). Blastokinesis then proceeds actively, and dorsal closure is completed during stage 21 (Fig. 2C). During stage 22, complete embryonic reversal occurs and trichogenous cell masses appear in stage 23 (Fig. 2D). Expression of BmTrpA1 was predominantly detected in mRNA derived from a mixture of thoracic and abdominal tissues (Fig. 2E, lane 4).
We subsequently examined the developmental profiles and localization of BmTRPA1 by using the anti-BmTRPA1 antibody. First, we performed immunostaining on HEK293 cells transfected with BmTrpA1 to confirm the immunoreactivity of anti-BmTRPA1 (Fig. S3 A–C). Then, using Western blot analysis, the immunoreactive bands were detected at ∼130 kDa in 4.0, 5.5, and 9.0 d, including the sensitive periods after oviposition (Fig. S3D, lanes 1–4; arrow). These bands were similar to the predicted molecular weight of BmTRPA1 (131.363 Da), suggesting that they were derived from BmTRPA1. Furthermore, the intensity of these bands was lower in dsRNA-injected embryos (Fig. S3D, lanes 5 and 6), suggesting that RNAi of BmTrpA1 was effective for decreasing BmTRPA1 accumulation and that the anti-BmTRPA1 antibody recognized BmTRPA1 in embryos. We examined the localization of BmTRPA1 in embryogenesis by whole-mount immunohistochemistry (Fig. 2 F–K). With advancing embryogenesis, immunoreactive signals were first detected in the ventral surface of the caudal portion of the embryo in stage 20 (Fig. 2F), and intensive signals were detected in both sides of the tips of anal prolegs in stage 23 (Fig. 2I). In addition, intensive signals were detected in the tips of prolegs during stages 21 and 23 (Fig. 2 G, H, and J, respectively). In a single section with DAPI staining, immunoreactive signals were clearly detected in the epidermal surfaces of proleg tips (Fig. 2H, arrowheads). Furthermore, immunoreactive signals were observed in stage 23 trichogenous tormogen cells that formed the bristle sockets (Fig. 2K). To confirm the specificity of immunoreactive signals in embryos, we compared the intensity of the signals to dsRNA-injected embryos (Fig. S3 E–L). Immunoreactive signals disappeared or decreased in RNAi embryos. Thus, BmTRPA1 is predominantly and ubiquitously localized in the epidermal nonneural cells of various tissues.
BmTrpA1 RNAi Affects Immunoreactivity of DH in Pupal Neurosecretory Cells.
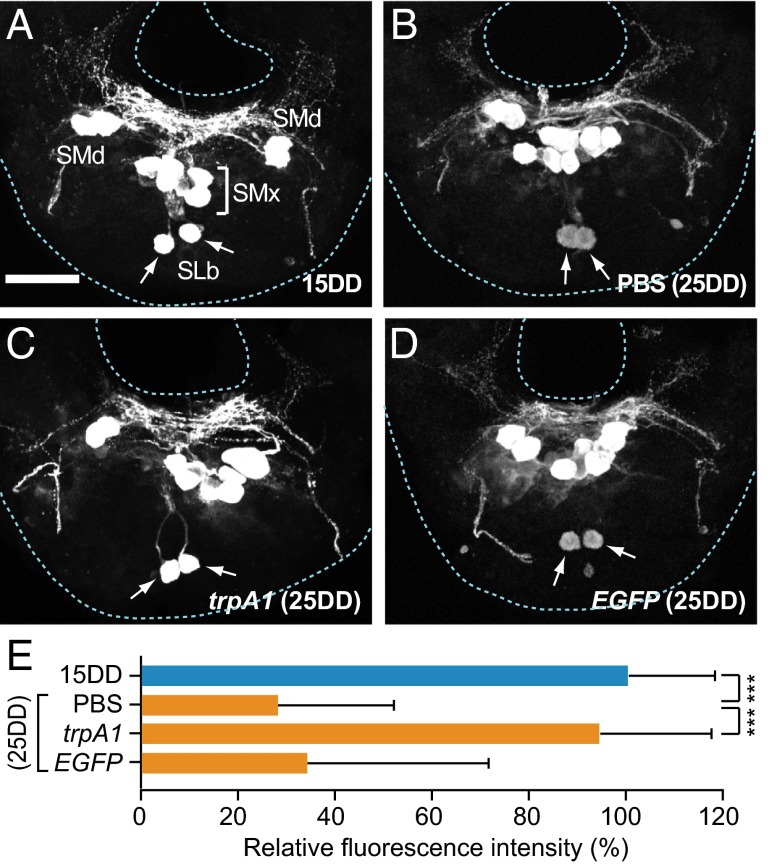
Immunohistochemistry using anti-DH antibody on pupae after 4 d of pupation indicated that, in a pair of SLb somata, one of the DHPCs of the SG in pupae (25DD) injected with PBS had lower DH immunoreactivity (30.2 ± 22.2%) than those of SLb somata from 15DD pupae (Fig. 3 A, B, and E). As described previously (16), this finding indicates that DH is actively released from these neurosecretory cells in 25DD pupae and that its activity is closely correlated with the determination of diapause type. If BmTRPA1 indeed affects diapause status in progeny through control of DH release, it is predicted that the immunoreactive intensity of SLb cells would be altered by RNAi. DH immunoreactivity was similar in BmTrpA1 RNAi of 25DD and 15DD pupae (93.7 ± 24.4%) (Fig. 3 C and E). On the other hand, injection of EGFP dsRNA resulted in immunoreactive intensity that was similar to that of PBS-injected (25DD) pupae (35.3 ± 36.6%) (Fig. 3 D and E). These results suggest that embryonic BmTrpA1 RNAi affects DH release during pupal-adult development.
Fig. 3.
BmTrpA1 knockdown affects immunoreactive intensity of SLb somata in DHPCs. (A–D) Representative images of immunohistochemistry of pupal SG using anti-DH antibody. Images are of 15DD (A), and 25DD (B). Also shown are results from pupae injected with BmTrpA1 dsRNA (C) and EGFP dsRNA (D). The dsRNA was injected into newly laid eggs, and the pupal SG was dissected out 4 d after pupation. The arrow indicates the SLb somata. (E) The relative fluorescence intensity in the SLb somata is reported as a percentage of that for 15DD. Each bar represents the mean value of 30 samples ± SD; ***P < 0.001 vs. PBS (25DD). (Scale bar, 100 μm.)
BmTRPA1 Is a Thermosensitive TRP Channel Activated Above 21 °C.
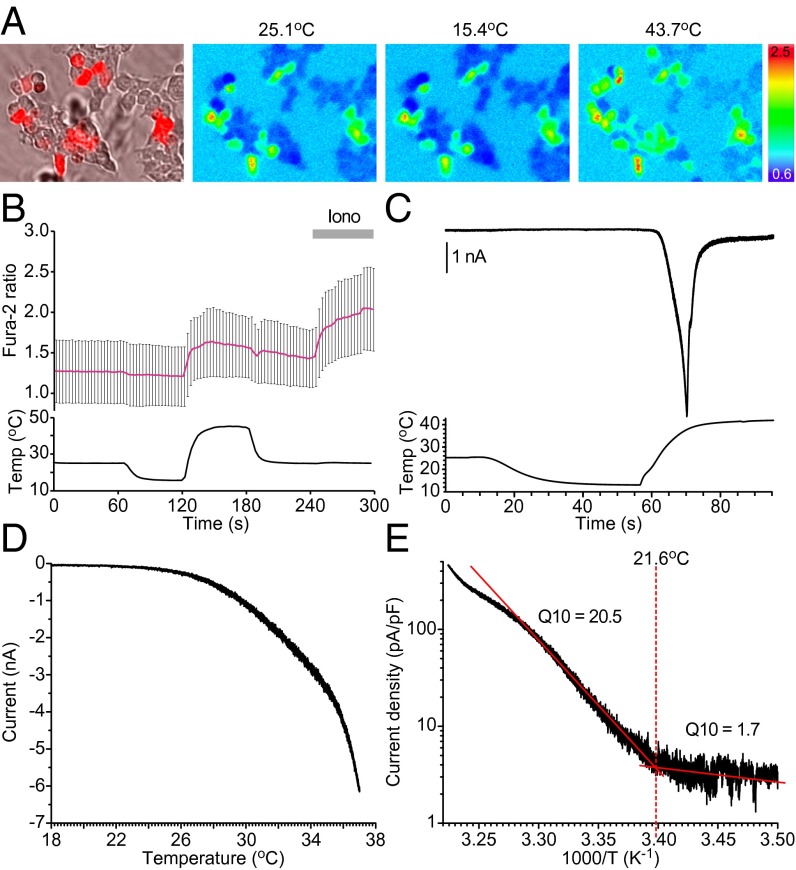
To examine whether BmTRPA1 is a thermosensitive TRP channel, we first performed Ca2+-imaging experiments using fura-2 to determine whether BmTRPA1-expressing cells increased intracellular Ca2+ concentration ([Ca2+]i) upon temperature fluctuation (Fig. 4A). There was no change in [Ca2+]i when temperature was reduced from 25 °C to 15 °C, but a clear increase in [Ca2+]i was observed in BmTRPA1-expressing cells when the temperature was raised to >25 °C (Fig. 4 A and B). However, the [Ca2+]i increase was abolished in the absence of extracellular Ca2+ (Fig. S4A), indicating that it was caused by an influx of Ca2+ from the extracellular space through BmTRPA1. Next, we conducted patch-clamp experiments to clarify the electrophysiological properties of BmTRPA1. In the whole-cell configuration, BmTRPA1-expressing cells showed transient inward currents at membrane potential of –60 mV when the temperature was increased from 15 °C to >20 °C, but not when it was decreased from 25 °C (Fig. 4C). Mock-transfected HEK293 cells did not display heat-induced [Ca2+]i increases in a Ca2+-imaging experiment (Fig. S4B), suggesting that the currents are specifically mediated through BmTRPA1. A temperature profile of BmTRPA1 activation showed that the current gradually began to develop at temperatures >21 °C, followed by large transient currents toward 40 °C (Fig. 4 C and D). An Arrhenius plot showed a flex point at 21.6 ± 1.8 °C, and the mean Q10 value was markedly shifted at this temperature, from 1.7 ± 0.3–20.5 ± 5.6 (Fig. 4E). These values are comparable to those of known thermosensitive TRP channels (30). We therefore conclude that BmTRPA1 is a temperature-sensitive ion channel activated at temperatures >21 °C.
Fig. 4.
BmTRPA1 is a thermosensitive TRP channel. (A) BmTRPA1-expressing cells were identified using coexpressed DsRed protein (Left). Representative results are shown for Fura-2 Ca2+ imaging at 25.1 °C, 15.4 °C, and 43.7 °C. The pseudocolor indicates intensity of the 340/380-nm fluorescence ratio. (B) Temporal changes in the Fura-2 ratio in BmTRPA1-expressing cells (Upper) and temperature (Lower) in the presence of 2 mM extracellular Ca2+. The average trace (red line) ± SD is shown at the top. Ionomycin was added at 240 s (Iono, gray bar), n = 85. (C) Temporal changes in the current (Upper trace) in a BmTRPA1-expressing cell and temperature (Lower trace) during temperature-fluctuation voltage-clamp whole-cell in patch-clamp analysis. Holding potential was −60 mV throughout the experiment. (D) Representative temperature-response profile for heat-induced BmTRPA1 current, as observed in C. (E) An Arrhenius plot for the heat-induced BmTRPA1 current shows a clear flex point in temperature dependency (data in D were converted). The temperature threshold for BmTRPA1 activation was defined as the point at which the two linear-fitted lines crossed (a flex point). The Q10 value was calculated for each line.
BmTRPA1 Is Activated by Chemical Compounds.
It is well known that the TRPA subfamily channels in insects and vertebrates are activated by a variety of pungent natural compounds and environmental irritants, including electrophiles (28, 31–34). We therefore attempted to identify chemical compounds that either activate or inhibit BmTRPA1. Candidate activators were screened using Ca2+-imaging experiments; we found that formalin, H2O2, cinnamaldehyde (CA), and camphor obviously increased intracellular Ca2+ levels of BmTRPA1-expressing cells. The effects of these compounds on BmTRPA1 were further tested using a patch-clamp method (Fig. S5). Currents developed slowly after addition of formalin, H2O2, CA, and camphor, followed by slow inactivation after washout in BmTRPA1-expressing HEK293 cells. The dose-dependent activation was observed in formalin (0.0003∼1%), H2O2 (0.05∼500 mM), and CA (0.1∼3 mM), but not in mock-transfected HEK293 cells (Fig. S6). The current–voltage relationship during heat activation showed outward rectification with the reversal potential close to zero, which is strikingly similar to TRPA1 properties in other species. Currents evoked by formalin, H2O2, CA, and camphor were BmTRPA1-dependent: the same cells responded to heat stimulation and the chemical-evoked currents displayed the same current-voltage relationship as that of heat currents. These results suggest that BmTRPA1 is a receptor for these chemical compounds.
Treatment with BmTRPA1 Chemical Activators Affects Diapause Induction, Pupal Body Weight, and Weight of Cocoon Shells.
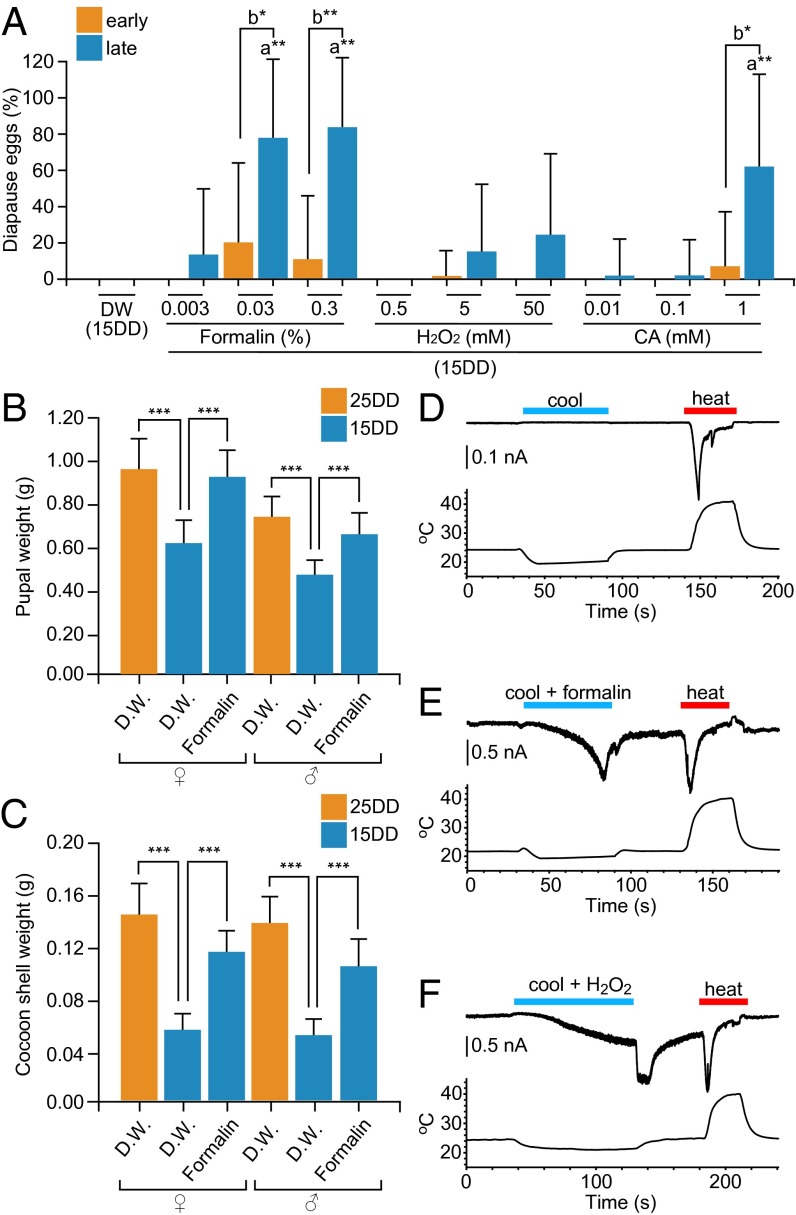
We tested whether the BmTRPA1 chemical activators artificially induced progeny diapause (Fig. 5A). Throughout embryogenesis, either formalin, H2O2, or CA was floated as a volatile substance around 15DD eggs. Although diapause eggs were never obtained from distilled water (DW)-treated moths, diapause eggs were oviposited by moths that were treated with formalin, H2O2, or CA at the embryo stages in a dose-dependent manner (Fig. 5A). Larvae that initiated spinning late (>50% of all larvae) oviposited relatively high proportions of diapause eggs. Because it is known that 25DD pupae have extended developmental periods and heavier bodies and cocoon shells (10), we tested whether pupal and cocoon-shell weights were affected in formalin-treated pupae. Both male and female formalin-treated pupae had bodies (Fig. 5B) and cocoon shells (Fig. 5C) that were heavier than those of 15DD control pupae and that were similar in weight to those of 25DD pupae. Next, we examined the effects of BmTrpA1 RNAi on formalin treatment. When formalin treatment was performed throughout embryogenesis after injection of PBS or dsRNA of BmTrpA1, most eggs never hatched, and those that did died during larval stages. Therefore, limited formalin treatment was performed, from stage 23 until hatching. If BmTRPA1 and its chemical activators act during the sensitive period (stages 20–23), it is expected that even limited formalin treatment in the sensitive period would cause partial induction of diapause eggs in batches and would increase pupal and cocoon shell weights, because those effects were knocked down by BmTrpA1 RNAi. Although we were able to obtain only a few pupae in this condition, the bodies and cocoon shells (Fig. S7 A and B) of female and male pupae injected with dsRNA of BmTrpA1 tended to be lighter, despite formalin treatment, than those of formalin-treated control pupae, and were similar in weight to those of pupae treated with DW and injected with either PBS or dsRNA of BmTrpA1. Thus, BmTrpA1 RNAi suppressed the increase in weight of the pupal body and cocoon shell obtained by formalin treatment. However, all eggs became nondiapause eggs in this condition and we could not judge the effect of RNAi on diapause induction. Collectively, BmTRPA1 chemical activators artificially induce progeny diapause and increase pupal body and cocoon shell weights at nondiapause-inducing temperatures. To confirm whether the chemical activators of BmTRPA1 efficiently activate the channel regardless of thermal conditions, we tested the chemical activation under cool conditions (<21 °C) (Fig. 5 D–F). In thermal gradients colder than 20 °C, currents developed slowly after addition of formalin, H2O2 (Fig. 5 E and F), and CA. When chemicals were not added, BmTRPA1 was not activated at <20 °C, as described above (Fig. 5D). Therefore, BmTRPA1 is responsive to formalin, H2O2, and CA even at temperatures <21 °C.
Fig. 5.
Chemical compounds activate BmTRPA1 independently of heat, induce diapause, and increase body and cocoon-shell weight. (A) Effect of treatment with chemical compounds on diapause-egg–inducing activity. Eggs were incubated at 15DD with formalin, H2O2, and CA, and the percentage of diapause eggs produced by their progeny was calculated. The moths were divided into two groups: larvae that initiated spinning earlier (<50% of total larvae; early), and larvae that initiated spinning later (>50% of total larvae; late). (B and C) Effects on pupal and cocoon-shell weights. Eggs were incubated at 25 °C or 15 °C in the dark with or without 0.3% formalin. Pupae (B) and cocoon-shell (C) weights after 3 d of pupation are shown. In A–C, 15DD (n = 54), 25DD (n = 141), formalin (0.003%; n = 23, 0.03%; n = 46, 0.3%, n = 87), H2O2 (0.5 mM; n = 64, 5 mM; n = 68, 50 mM; n = 72), CA (0.01 mM; n = 52, 0.1 mM; n = 53, 1 mM; n = 44). Data represent means ± SD; ***P < 0.001, **P < 0.01, *P < 0.05. In A, lowercase letters indicate statistically significant differences in DW-treated vs. chemical-compound-treated animals (a), and early vs. late animals (b). (D–F) Temporal changes in current (Upper trace) in a BmTRPA1-expressing cell, and temperature (Lower trace) during temperature fluctuation without additional chemical compounds (D), with 0.01% formalin (E), and 10 mM H2O2 (F) during cooling in a whole-cell patch-clamp experiment. Membrane potential was held at −60 mV. Note that a sudden increase in current level was observed after H2O2 washout, probably because currents were still gradually developing at that time and were enhanced by the temperature increase.
Discussion
In general, the extent of the thermo- or photoperiod during which insects are sensitive to diapause-inducing stimuli varies considerably among species, ranging from a few days to almost an entire year, but is more restricted within species (6). In B. mori, temperature during the thermosensitive period must remain continuously above the threshold for long periods during embryogenesis to induce diapause. This thermosensitive property differs from processes used to detect and respond quickly to extreme variability in temperature, such as thermal nociception and thermotaxis, which provide immediate survival advantages. This aspect suggests that the thermosensitive properties and activation process of BmTRPA1 differ from those of other known TRPA1 channels, such that BmTRPA1 may require constant or intermittent activation during the sensitive period to induce diapause. High temperature during the sensitive period is an essential and sufficient condition for induction of progeny diapause in 25DD embryos. Embryonic BmTRPA1 could be thermally activated at >21 °C during embryogenesis, and an unknown signaling pathway linked to the release of DH may then be activated. Therefore, BmTRPA1 is a thermosensitive TRP channel that acts as a molecular switch for diapause induction and for the development of an alternative phenotype in an animal with seasonal polyphenism. However, the effect of BmTrpA1 RNAi on both diapause induction and treatment with BmTRPA1 chemical activator was weak and variable. Therefore, BmTRPA1 may play a partial role in the signaling, and other unidentified factors (e.g., cold-sensitive thermosensors activated at ≤15 °C) may also be involved in diapause induction.
It has been speculated that in cases of seasonal polyphenism, environmental information is stored and later transduced to neuroendocrine signals via neural remodeling to induce the alternative phenotype. However, identification of the molecular signals involved has not yet been accomplished (6). Consistent with this notion, we revealed that BmTrpA1 knockdown affects DH accumulation in SLb neurosecretory cells in the SG during pupal-adult development. The reception of thermal information in 25DD probably activates the unknown signal transduction pathway, thereby causing neural and brain remodeling. This process coordinates the operation of neural circuits, including regulation of dendritic growth, neural activity, and neuromodulator release. The release of DH is a critical step for the induction of diapause eggs, which was suppressed by BmTrpA1 knockdown. Therefore, to our knowledge, we have identified the first molecular pathway participating in a long-term predictive-adaptive response in seasonal developmental plasticity processes. In addition, BmTrpA1 knockdown affects the duration of growth in the larval period. Because insulin and insulin-like peptides can stimulate the production of ecdysteroids and juvenile hormone in Drosophila, and because this signaling system ultimately regulates growth rate and is essentially identical in insects and vertebrates (35), BmTRPA1 signaling may help to modify the action of factors, such as insulin-like peptides and their signaling pathways, which would affect growth rate in addition to DH release. Furthermore, high expression of the BmTrpA1 mRNA was observed in various tissues during larval-pupal development and after the sensitive period during embryogenesis (Fig. 2A), suggesting that BmTRPA1 may participate in other unknown physiological processes.
We observed that BmTRPA1 is ubiquitously expressed in nonneural epidermal cells in various embryonic tissues. This aspect is different from the expression of other known insect thermosensitive TRPA subfamily members, which are primarily localized in peripheral sensory neurons and the CNS in all life stages. These TRPAs are part of the system of transduction, integration, and rapid response to heat and chemical stimuli (23, 27, 28, 36–38). Recent in vitro and in vivo studies have provided evidence that both TRPA1 and TRPV3 channels are expressed in nonneural cells, including skin epithelial cells in vertebrates (39–41). Furthermore, it has been proposed that TRPV3 in the skin may release diffusible molecules, such as adenosine triphosphate, which would then mediate thermotransduction in skin (41). This situation may apply to BmTRPA1. We speculate that the epidermal cells receive environmental temperature cues using the broadest and largest available interface with the external environment when these signals are not necessary for rapid and immediate responses through neuronal integration and behavior. Our findings suggest that nonneural insect embryonic cells may participate in thermosensitive events through TRP channel activation. Further study of the molecular mechanism is required to determine how thermosensation in nonneural cells causes DH release, which functions via transduction mechanisms, neural plasticity, and storage mechanisms.
Because 25DD pupae have extended developmental periods, heavier bodies, and cocoon shells, and later lay a larger quantity of eggs than 15DD pupae, research into embryonic diapause as it relates to the prosperity of the sericulture industry in Japan began in the early 20th century (10). We showed that chemical treatment can result in moths with diapause-type characteristics, such as oviposition of diapause eggs and heavy pupal and cocoon-shell weight. These results indicate that BmTRPA1 may be a good target for controlling seasonal polyphenism in silk moths to maximize the efficiency of silk production. It has been suggested that insect TRPAs represent potential targets for disrupting thermal preferences and other thermosensory behaviors in agricultural pests and disease vectors (34). Our findings suggest that chemical ligands of insect TRPA1 and TRPA subfamily members may be used as a new class of compounds for pest control through perturbation of seasonal polyphenism.
Materials and Methods
Silkworms.
Bivoltine (Kosetsu) and polyvoltine (N4) strains of B. mori were used in these experiments. Insects were reared as described previously (16). During the larval stages, animals were reared at 25–27 °C under a 12-h light/dark cycle and relative humidity of 50–60%. Kosetsu eggs were incubated under two different conditions throughout embryogenesis: at 25 °C (25DD) or at 15 °C (15DD) under continuous darkness, to obtain diapause or nondiapause eggs, respectively. The embryos were staged using a light microscope and stages were numbered from 1 to 30 based on morphological markers, as described previously (9, 42). The eclosed moths were allowed to oviposit overnight and these eggs were incubated at 26–27 °C. Diapause eggs were identified as those that were colored dark brown on day 3 and remained unhatched at 2 wk after oviposition, at which time all nondiapause eggs had hatched. Both diapause and nondiapause egg-inducing activity were estimated by counting the numbers of eggs in diapause and those not in diapause after the nondiapause eggs hatched. The results are expressed as percentages of diapause or nondiapause eggs in each egg batch (43).
cDNA Cloning.
We found highly homologous sequences from Bombyx whole-genome shotgun and KAIKO cDNA databases by searching the tblastx program of KAIKOBLAST (http://kaikoblast.dna.affrc.go.jp) against Drosophila melanogaster TrpA1 (AY302598), Painless (AY268106), Pyrexia (NM_138171), and Wtrw (NM_169200). Each partial cDNA sequence was determined by RT-PCR using the first-strand DNA derived from eggs at stage 22 and pupal brain-SG complexes as templates using the specific primer sets shown in Table S1. The full-length cDNA sequences were determined using a SMART RACE cDNA amplification kit (Clontech). Finally, the ORF sequences of BmTrpA1 (AB243445, AB703646), BmPainless (AB264789), BmPyrexia (AB437368), BmWtrw (AB437369), and BmTrpA4 (AB703647) were amplified by RT-PCR.
Embryonic RNA Interference (RNAi).
RNAi procedures were adapted from Masumoto et al. (44) with some modifications. The synthesis of dsRNA was performed by a T7 RiboMAX Express RNAi System (Promega) using the primers shown in Table S1. The concentration of dsRNAs was adjusted to 3 µg/µL. For injection into eggs, nondiapause eggs of the bivoltine strain (Kosetsu) were collected within 2 h of oviposition during the syncytial blastoderm stage. Then, dsRNA was injected into eggs using a glass needle (uMPm-02; Daiwa Union) attached to a manipulator (kaikopuchu-STDU1; Daiwa Union) and FemtoJet (Eppendorf). Eggs injected with dsRNA were incubated at 25 °C in moist conditions until hatching.
RT-PCR Analysis.
Eggs were collected from 0.2 d after oviposition until day 9.0 (25DD) or day 34 (15DD) of eclosion at each developmental stage and stored at –80 °C until use. Poly (A)+ RNAs were prepared according to Shiomi et al. (45) using Dynabeads Oligo (dT)25 (Dynal Biotech). First-strand DNA was synthesized using a SMART RACE amplification kit (Clontech); mRNA PCR amplification was carried out for BmTrpA1 and ActA3. We amplified the +1,053 to +1,930 region of a BmTrpA1 cDNA (AB703646), and region +70 to +498 of an ActA3 cDNA (AB701689) using the primers shown in Table S1. The PCR products were subjected to electrophoresis and then visualized with ethidium bromide staining.
Histochemical Analysis.
Standard protocols were used for immunohistochemistry as previously described (16, 29). For anti-BmTRPA1 immunohistochemistry, we used an anti-BmTRPA1 mouse antibody prepared from mice immunized with BSA-conjugated 14-aa coding sequences from R1134 to A1147 of the BmTRPA1. The anti-BmTRPA1 IgG was purified by NAbProtein A/G Spin Columns (Thermo). BmTrpA1-transfected HEK293 cells were processed for immunostaining as described in Matsuura et al. (29). Anti-BmTRPA1 antibody was used at 500-fold dilution. The Alexa Fluor 488-labeled anti-mouse IgG (Hypromatrix) was used for the second antibody. For immunostaining of embryos, both the anti-BmTRPA1 (100-fold dilution) and Cy3-labeled anti-mouse F(ab′)2 antibodies (Jackson ImmunoResearch) were incubated with embryos overnight. The fluorescence signals were detected using an Olympus FV1000-D confocal microscope (Olympus). Images were adjusted and assembled using Adobe Photoshop CS3 (Adobe). The immunoreaction procedure for the anti-DH antibody was adapted from Hagino et al. (16). Images were acquired by an Olympus FV1000-D confocal microscope and analyzed with National Institutes of Health Image 1.62 (http://rsb.info.nih.gov/nih-image), as previously described (16). To determine the relative fluorescence intensity of the SLb cells, we compared the intensity of the mean pixel fluorescence for individual somata. Images to be compared were processed identically and image capture was set to avoid fluorescence saturation. Analysis was performed as described previously (16). Data were presented as the percentage intensity relative to the mean intensity of the control. Bombyx CNS terminology followed Sato and colleagues (46). For scanning electron microscope observation, dissected embryos were fixed and dehydrated as described in Hagino et al. (16). The embryos were dried in liquid nitrogen and observed using a JSM-6010LA scanning electron microscope (JEOL).
Western Blot Analysis.
Embryos were dissected from eggs 4.0, 5.5, and 9.0 d after oviposition and homogenized in 1× SDS sample buffer. Eggs at 1.0 d after oviposition were directly homogenized in 1× SDS sample buffer. After separation by 7.7% (wt/vol) SDS/PAGE, proteins were transferred to an Immobilon-P PVDF membrane (Millipore), which was then incubated with anti-BmTRPA1 antibody at 1,000-fold dilution at 4 °C overnight. The signals were detected using horseradish peroxidase-linked goat anti-mouse IgG (Invitrogen) and ECL-Plus Western blotting detection reagents (GE Healthcare).
Ca2+ Imaging and Patch-Clamp Analyses.
For construction of the BmTRPA1-expressing vector for mammalian cells (HEK 293), the ORF sequence of BmTrpA1 was amplified by PCR using the primers shown in Table S1. The ORF sequence was then inserted into a pcDNA3 vector (Invitrogen). This recombinant vector was cotransfected with DsRed or pGreen Lantern-1 marker into HEK293 cells using Lipofectamine (Invitrogen) and the cells were cultured for 20–40 h at 33 °C. These transient-transfected cells were used for Ca2+ imaging and patch-clamp analyses. Procedures adapted from Kohno et al. (28) were used for Ca2+ imaging and patch-clamp recording.
Egg Treatment with Chemical Ligands of BmTRPA1.
Egg treatment with chemical ligands of BmTRPA1 was carried out in plastic Petri dishes (60-mm diameter). Each egg batch (200–300 eggs) oviposited on drafting paper by newly eclosed females was sterilized with 3% formalin for 5 min, dried, and mounted onto the underside of a Petri dish lid using double-stick tape. Formalin, H2O2, and CA were serially diluted and added to the bottom of the Petri dishes, which were then sealed with Parafilm and incubated at 15 °C in continuous darkness. Embryonic development was checked by observing eggs incubated with continuous illumination. The day before hatching, each egg batch was transferred to a 12-h light/dark cycle at 25 °C until hatching.
Statistical Analysis.
Data were compared using Student t tests. Multiple comparisons were performed using Tukey’s tests. The significance of differences presented in Figs. 1B and 5A and Fig. S2A was evaluated using the Steel–Dwass test. Statistical analyses were performed in Excel 2011 (Microsoft) with the software add-in Toukei-Kaiseki v2.0 (Esumi).
Supplementary Material
Acknowledgments
We thank Y. Kubota, D. Kumamoto, and H. Miyazawa for silkworm rearing; K. Kakegawa for scanning electron microscope observations; Dr. Y. Matsumura for useful discussion on statistical analysis; and the Division of Gene Research, Research Center for Human and Environmental Sciences, and VBL at Shinshu University for providing facilities for these studies. This research was funded by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan, and supported partly by the NAITO Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AB243445, AB703646, AB264789, AB437368, AB437369, and AB703647).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322134111/-/DCSupplemental.
References
- 1.West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford Univ Press; 2003. [Google Scholar]
- 2.Gilbert SF, Epel D. How agents in the environment effect molecular changes in development. In: Gilbert SF, Epel D, editors. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution. Sunderland, MA: Sinauer Associates; 2009. pp. 38–78. [Google Scholar]
- 3.Mousseau TA, Fox CW. Maternal Effects as Adaptations. New York: Oxford Univ Press; 1998. [Google Scholar]
- 4.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20(10):527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Kostál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52(2):113–127. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. New York: Oxford Univ Press; 1986. [Google Scholar]
- 7.Nijhout HF. Development and evolution of adaptive polyphenisms. Evol Dev. 2003;5(1):9–18. doi: 10.1046/j.1525-142x.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K. Studies on the voltinism of the silkworm, Bombyx mori. Bull Sericult Exp Sta (Tokyo) 1924;6:411–455. [Google Scholar]
- 9.Morita A, Niimi T, Yamashita O. Physiological differentiation of DH-PBAN-producing neurosecretory cells in the silkworm embryo. J Insect Physiol. 2003;49(12):1093–1102. doi: 10.1016/j.jinsphys.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita O, Yaginuma T. Silkworm eggs at low temperatures: Implication for sericulture. In: Lee JRE, Denlinger DL, editors. Insects at Low Temperature. New York: Chapman and Hall; 1991. pp. 424–445. [Google Scholar]
- 11.Xu WH, Sato Y, Ikeda M, Yamashita O. Stage-dependent and temperature-controlled expression of the gene encoding the precursor protein of diapause hormone and pheromone biosynthesis activating neuropeptide in the silkworm, Bombyx mori. J Biol Chem. 1995;270(8):3804–3808. doi: 10.1074/jbc.270.8.3804. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita O. Diapause hormone of the silkworm, Bombyx mori: Structure, gene expression and function. J Insect Physiol. 1996;42(7):669–679. [Google Scholar]
- 13.Homma T, et al. G protein-coupled receptor for diapause hormone, an inducer of Bombyx embryonic diapause. Biochem Biophys Res Commun. 2006;344(1):386–393. doi: 10.1016/j.bbrc.2006.03.085. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y, et al. Precursor polyprotein for multiple neuropeptides secreted from the suboesophageal ganglion of the silkworm Bombyx mori: Characterization of the cDNA encoding the diapause hormone precursor and identification of additional peptides. Proc Natl Acad Sci USA. 1993;90(8):3251–3255. doi: 10.1073/pnas.90.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato Y, Ikeda M, Yamashita O. Neurosecretory cells expressing the gene for common precursor for diapause hormone and pheromone biosynthesis-activating neuropeptide in the suboesophageal ganglion of the silkworm, Bombyx mori. Gen Comp Endocrinol. 1994;96(1):27–36. doi: 10.1006/gcen.1994.1156. [DOI] [PubMed] [Google Scholar]
- 16.Hagino A, Kitagawa N, Imai K, Yamashita O, Shiomi K. Immunoreactive intensity of FXPRL amide neuropeptides in response to environmental conditions in the silkworm, Bombyx mori. Cell Tissue Res. 2010;342(3):459–469. doi: 10.1007/s00441-010-1083-4. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa N, et al. Establishment of a sandwich ELISA system to detect diapause hormone, and developmental profile of hormone levels in egg and subesophageal ganglion of the silkworm, Bombyx mori. Zoolog Sci. 2005;22(2):213–221. doi: 10.2108/zsj.22.213. [DOI] [PubMed] [Google Scholar]
- 18.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 19.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 20.Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci. 2013;92(8-9):394–403. doi: 10.1016/j.lfs.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wetsel WC. Sensing hot and cold with TRP channels. North American Hyperthermia Group. 2011;27(4):388–398. doi: 10.3109/02656736.2011.554337. [DOI] [PubMed] [Google Scholar]
- 22.Viswanath V, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423(6942):822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 23.Rosenzweig M, et al. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19(4):419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454(7201):217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci USA. 2008;105(38):14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144(4):614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G, et al. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur J Neurosci. 2009;30(6):967–974. doi: 10.1111/j.1460-9568.2009.06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohno K, Sokabe T, Tominaga M, Kadowaki T. Honey bee thermal/chemical sensor, AmHsTRPA, reveals neofunctionalization and loss of transient receptor potential channel genes. J Neurosci. 2010;30(37):12219–12229. doi: 10.1523/JNEUROSCI.2001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuura H, Sokabe T, Kohno K, Tominaga M, Kadowaki T. Evolutionary conservation and changes in insect TRP channels. BMC Evol Biol. 2009;9:228. doi: 10.1186/1471-2148-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 31.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103(51):19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445(7127):541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 33.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28(40):9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang K, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464(7288):597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shingleton AW. Evolution and the regulation of growth and body size. In: Flatt T, Heyland A, editors. Mechanisms of Life History Evolution. The Genetics and Physiology of Life History Traits and Trade-Offs. New York: Oxford Univ Press; 2011. pp. 43–55. [Google Scholar]
- 36.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113(2):261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37(3):305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 38.Al-Anzi B, Tracey WD, Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16(10):1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Cao DS, et al. Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLoS ONE. 2012;7(5):e38005. doi: 10.1371/journal.pone.0038005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nozawa K, et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA. 2009;106(9):3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandadi S, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458(6):1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takami T, Kitazawa T. External observation of embryonic development in the silkworm. Bull Sericult Exp Sta (Tokyo) 1960;75:1–31. [Google Scholar]
- 43.Yamashita O, Hasegawa K. Embryonic diapause. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol 1. Oxford: Pergamon Press; 1985. pp. 407–434. [Google Scholar]
- 44.Masumoto M, Yaginuma T, Niimi T. Functional analysis of Ultrabithorax in the silkworm, Bombyx mori, using RNAi. Dev Genes Evol. 2009;219(9-10):437–444. doi: 10.1007/s00427-009-0305-9. [DOI] [PubMed] [Google Scholar]
- 45.Shiomi K, et al. Myocyte enhancer factor 2 (MEF2) is a key modulator of the expression of the prothoracicotropic hormone gene in the silkworm, Bombyx mori. FEBS J. 2005;272(15):3853–3862. doi: 10.1111/j.1742-4658.2005.04799.x. [DOI] [PubMed] [Google Scholar]
- 46.Sato Y, Shiomi K, Saito H, Imai K, Yamashita O. Phe-X-Pro-Arg-Leu-NH(2) peptide producing cells in the central nervous system of the silkworm, Bombyx mori. J Insect Physiol. 1998;44(3-4):333–342. doi: 10.1016/s0022-1910(97)00140-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.