Abstract
We show that groove recognition of nucleic acid triple helices can be achieved with aminosugars. Among these aminosugars, neomycin is the most effective aminoglycoside (groove binder) for stabilizing a DNA triple helix. It stabilizes both the T·A·T triplex and mixed-base DNA triplexes better than known DNA minor groove binders (which usually destabilize the triplex) and polyamines. Neomycin selectively stabilizes the triplex (T·A·T and mixed base) without any effect on the DNA duplex. The selectivity of neomycin likely originates from its potential and shape complementarity to the triplex Watson–Hoogsteen groove, making it the first molecule that selectively recognizes a triplex groove over a duplex groove. The groove recognition of aminoglycosides is not limited to DNA triplexes, but also extends to RNA and hybrid triple helical structures.
Intercalator–neomycin conjugates are shown to simultaneously probe the base stacking and groove surface in the DNA triplex. Calorimetric and spectrosocopic studies allow the quantification of the effect of surface area of the intercalating moiety on binding to the triplex. These studies outline a novel approach to the recognition of DNA triplexes that incorporates the use of non-competing binding sites. These principles of dual recognition should be applicable to the design of ligands that can bind any given nucleic acid target with nanomolar affinities and with high selectivity.
1. Introduction
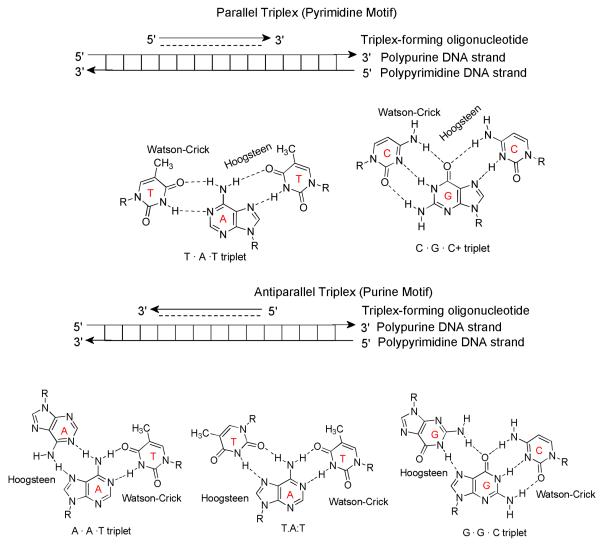
Duplex DNA can be sequence-specifically recognized by small molecules such as minor groove binders and larger molecules such as oligodeoxynucleotides (ODNs, major groove binders).1, 2 Major groove recognition of the duplex DNA by ODNs results in a triple helix in a parallel or antiparallel form (Figure 1). Triple helix formation was first reported in 1957 by Felsenfeld and Rich. 3, 4 The elucidation of the DNA duplex structure at the same time kept the limelight away from the three stranded DNA structures until the 1980s. Eventually, work by Dervan, Helene and others showed the broad potential of triplex forming ODNs in targeting duplex and single stranded DNA and in inhibiting sequence-specific DNA-protein interactions.5-9DNA triplex formation has continued to garner much interest in the scientific community because of the possible applications in developing new molecular biology tools as well as therapeutic agents.10-13 Specific inhibition of transcription has been induced via triplex formation at poly (purine/pyrimidine) sites in promoter sequences. These, and numerous other findings that support the feasibility of an antigene approach for therapeutically regulating specific gene expression have been discussed in a number of reports.14-16 A recent report has postulated that DNA triplexes may even be present at the active site of the DNA polymerases.17
Figure 1.
Base interactions in parallel (pyrimidine motif-Top) and antiparallel (Purine motif-Bottom) triple helices. These are defined with respect to the orientation of the TFO and homopurine Watson-Crick (W-C) strand.
Several intercalators as well as various DNA minor groove ligands have previously been shown to bind to DNA triple helices.18, 19, 19-21 Intercalators usually stabilize to a greater extent triple helices containing TAT triplets, whereas minor groove binders usually destabilize triplexes.22 At the beginning of this millennium, when we began our work on recognition of nucleic acids, one surprising fact stared at us: While there were a number of intercalators that stabilized DNA duplex and triplex structures (selectively and non-selectively), there were no examples of groove-binding ligands that selectively recognized the triple helices (spermine,23 with its flexible amines as the possible weak exception). This was in stark contrast to the numerous sequence-specific groove binders known to bind in the minor groove of duplex DNA.2, 24, 25 Time and again, one has had to look to natural products to deal with such problems in recognition of biomolecules. Examples of such natural products include crescent shaped netropsin and distamycin26-28 which were key leads in the design principles that are used to target the DNA minor groove today. In such a quest for ligands for triple helix specific stabilization, we decided to investigate aminoglycoside antibiotics, the antibacterial scaffolds first discovered by Selman Waksman in 1944 for activity against tuberculosis (Figure 2).29-32 In this account, I will present neomycin as one of the first examples that bridge the gap in molecular recognition of triplex structures and increase our understanding of the recognition principle(s) involved in selective targeting of nucleic acid triplex grooves.
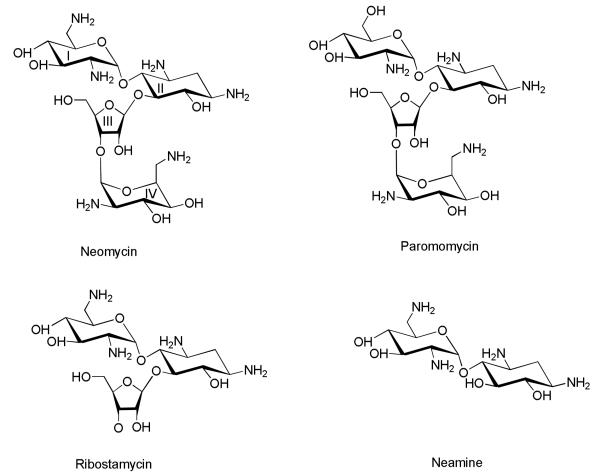
Figure 2.
Structures of some aminoglycoside antibiotics. Ring numbering scheme is shown for neomycin.
2. Thermal denaturation studies and aminoglycoside structure activity relationships
Thermal denaturation studies of triplexes formed from ODNs as well as polynucleotides were carried out in the presence of aminoglycosides, using UV spectroscopy at 260/280 nm. These studies show the remarkable effectiveness of neomycin in stabilizing the triplex without affecting the duplex Tm.33
In the thermal denaturation analysis of poly(dA)•2poly(dT) bound to neomycin, plots of absorbance at 260 and 284 nm (A260, A284) vs. temperature exhibit two distinct inflections {Tm3→2 (triplex melting point) = 34°C and Tm2→1 (duplex melting point) = 71°C, rdb=0.15 (ratio-drug(neomycin)/base triplet)}. Triplex stabilization was found to be dependent on neomycin concentration. Figure 3 shows that by increasing the molar ratios of neomycin from 0-25 μM, rdb = 0-1.67, the triplex melting point is increased by close to 25°C, whereas the duplex is virtually unaffected.
Figure 3.
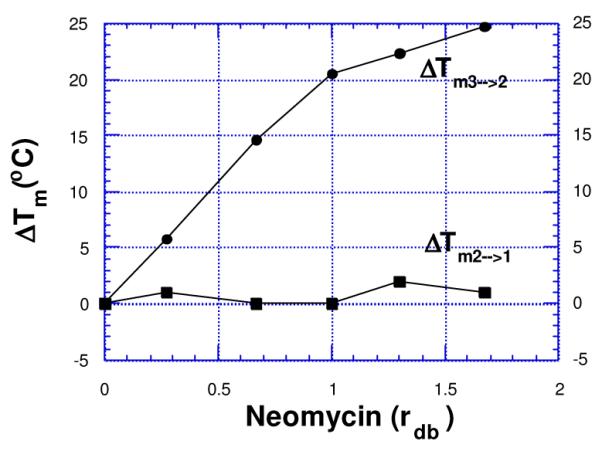
Plots of variation of triplex melting (Tm3→2) and duplex melting (Tm2→1) of poly(dA)•2poly(dT) as a function of increasing neomycin concentration {rdb = drug(neomycin) /base triplet ratio}. 10 mM sodium cacodylate, 150 mM KCl, pH 6.8, [poly(dA)•2poly(dT)] = 15 μM/base triplet.. Reprinted with permission from Bioorg. Med. Chem. Letts 2000,10, 1897-1899.
Nature has provided us antibiotics to allow for a simplified version of aminoglycoside-triplex structure activity relationship (Figure 4). A few of these can be viewed as simple variations of neomycin pharmacophore with functional group deletions. Most aminoglycosides with 5 or more amines are able to stabilize the triple helix (increasing ΔTm3→2, without significantly affecting the ΔTm2→1values, Figure 4). The structural difference between paromomycin and neomycin is a positively charged amino group (present in neomycin), replacing a neutral hydroxyl (present in paromomycin). This leads to a difference of 16°C in Tm3→2 values (rdb = 1.67) at 150 mM K+concentration. As also seen from Figure 4, neomycin is far more effective than paromomycin or ribostamycin in stabilizing triple helices.34 Since both paromomycin and ribostamycin can be derived by deletion of specific groups/rings in neomycin, these two aminoglycosides offer preliminary evidence for specific functional group (ring I amine) or ring (ring IV) participation in DNA triplex binding by neomycin.
Figure 4.
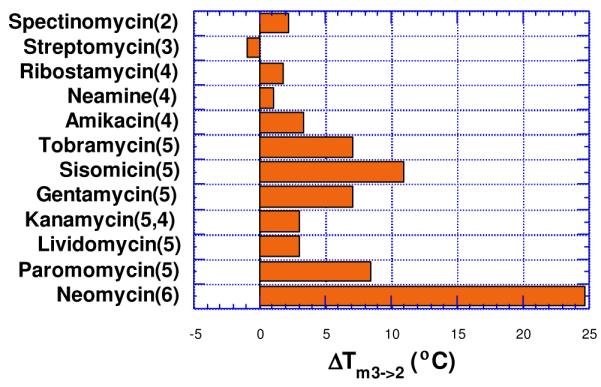
Effect of aminoglycoside antibiotics on the melting of poly (dA)•2Poly(dT) triplex (rdb = 1.67). Solution conditions: 150 mM KCl, 10 mM sodium cacodylate, pH 6.8, [poly(dA)•2poly(dT)] = 15 μM/base triplet. Number of amines in each antibiotic is shown in parenthesis. Reprinted with permission from J. Amer. Chem. Soc. 2001, 123, 5385-5395.
A fluorescent intercalator displacement (FID) assay was then used to determine the AC50 values for the three aminoglycosides with poly(dA)•2poly(dT) and an intramolecular 5′-dA12-x-dT12-x-dT12-3′ (x=hexaethyleneglycol linker) triplex. These studies have been recently published and the results are summarized below.35 The AC50 values reported in Table 1 are the aminoglycoside concentrations required to displace 50% of thiazole orange from the triplex, as measured by a decrease in 50% of the fluorescence. At pH 6.8, the neomycin AC50 (AC50 = 35.5 μM) is 6-fold lower than paromomycin (AC50 = 179 μM) and approximately 14 fold lower than ribostamycin (AC50 = 486 μM). A similar trend, with neomycin (AC50 = 13 μM) <paromomycin (AC50 = 157 μM)<ribostamycin (AC50 = 459 μM) is observed at pH 5.5
Table 1.
35 Fluorescence derived AC50 values for aminoglycoside binding to 5′-dA12-x-dT12-x-dT12-3′ triple helix at pH 5.5, 6.8 in 10 mM sodium cacodylate, 0.5 mM EDTA and 150 mM KCl. T=10 °C, [DNA triplex] = 100 nM/strand, [Thiazole Orange] = 700 nM.
| Aminoglycoside | AC50 (pH 6.8) (μM) | AC50 (pH 5.5) (μM) |
|---|---|---|
| Neomycin | 35.5 | 13.3 |
| Paromomycin | 179.0 | 157.9 |
| Ribostamycin | 486.0 | 459.0 |
Studies with the poly(dA)•2poly(dT) triplex showed the same trends in AC50 values. When the FID experiments were carried out with poly(dA)•poly(dT) duplex, thiazole orange could not be displaced by up to 10 mM neomycin, suggesting that the affinity of the aminoglycoside is 2-3 orders of magnitude lower for the AT rich DNA duplexes, when compared to its affinity for the AT rich DNA triplex.
These results suggest that: (i) paromomycin is much weaker in stabilizing the triplex (amino group in ring I is a key element in DNA triplex recognition), (ii) ribostamycin and neamine are much weaker in stabilizing the triplex, and both have similar effect on triplex stabilization (ring IV amines are involved in recognition; and ring III likely provides the neomycin conformer necessary for successful binding).
3. Stabilization of DNA triple helix poly(dA)•2poly(dT) by other ligands34
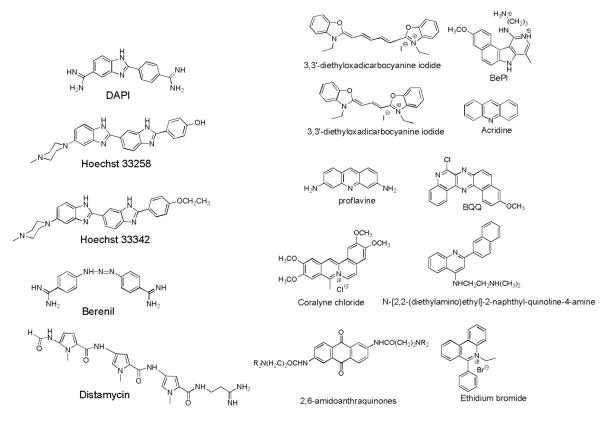
A comparison with DNA groove binders indicates that neomycin is much more active than the minor groove binders (Figure 5) in stabilizing the triplex. The minor groove binders, in general, are not triplex-specific (berenil, distamycin, Hoechst dyes) and some are known to even destabilize the triplex (berenil, distamycin) because of their preference for the DNA duplex.34 The minor groove binders such as Hoechst 33258 stabilize the DNA duplex much better than the DNA triplex, suggesting that the significant perturbations take place in the minor groove upon ODN binding to the duplex major groove. Binding studies suggest that neomycin, as opposed to minor groove DNA duplex binders, can preferentially bind the DNA triplex grooves over those present in AT rich DNA duplex. Structural data identifying specific groove-drug interactions would further clarify which of the three triplex grooves are being targeted. The intercalating ligands, on the other hand, are equally or more effective in stabilizing the triple helix at low concentrations.34 At higher concentrations, the intercalating ligands begin to stabilize the duplex as well though significant advances have recently been reported in the design of triplex selective intercalators.19, 20, 36, 37
Figure 5a.
Structures of groove binders (left) and intercalators (right), known to bind to DNA triplexes.
The planar moieties do offer an advantage over the aminoglycosides in terms of cellular permeability and nuclear localization. Based on the limited data that is available, aminoglycosides do not enter mammalian cells readily, whereas dyes such as Hoechst 33342 are known to rapidly enter the nucleus. Models for bacterial cell permeability by aminoglycosides have been proposed and discussed in a recent review.29 However, minor modifications in aminoglycosides can dramatically alter their permeability38 and should therefore not detract from the development of aminoglycosides in targeting mammalian cells.
As previously discussed, the minor groove (W-C groove) of the triplex is similar in width and depth to that of B-type duplex DNA, yet the two grooves are not identical.39-41 The perturbation upon major groove binding of the third strand leads to a difference in overall geometry. The B-form duplex minor groove has a U-shaped cross section, whereas the triplex minor groove adopts a V-shaped cross section. Duplex minor-groove binding ligands such as netropsin and distamycin can bind to triple helices, but they destabilize them as they preferentially bind to the duplex minor groove generated on loss of the triplex-forming strand.22, 34
The triplex major groove, on the other hand, is quite different from that in standard B-type DNA, since the presence of the third strand widens the major groove and divides it into two asymmetric parts: the smaller Crick-Hoogsteen (C-H) groove), and the wider Watson-Hoogsteen (W-H) groove (Figure 6). The W-H groove is wide enough to interact with such molecules as neomycin and in contrast to the smaller grooves, is more flexible. This flexibility is likely used by the DNA triplex to adapt its shape to an aminosugar such as neomycin. These results are consistent with W-H groove being the likely site for selective triplex stabilizers, as opposed to the DNA duplex selective minor groove binders, although structural evidence is still needed to verify this hypothesis.
Figure 6.
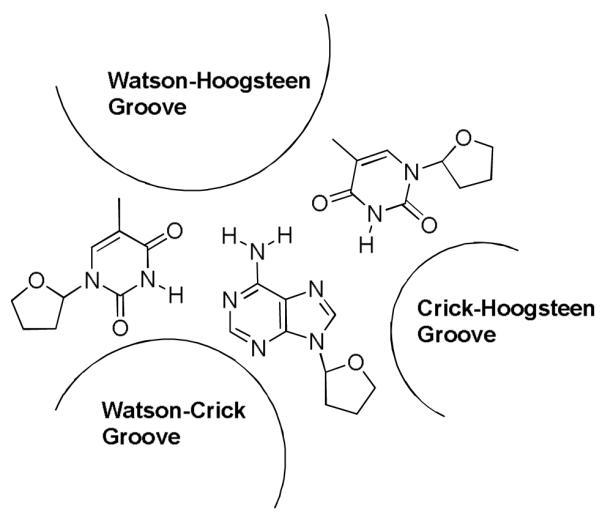
Structure of a TAT DNA triplex showing different grooves. W-H groove is formed between two pyrimidine strands.42-44
4. A proposed model for neomycin binding to the DNA Triplex Watson-Hoogsteen Groove21, 22
To build a model for neomycin binding to the major groove of the DNA triplex, CD spectroscopic studies, pH dependence, salt dependence and viscosity measurements were performed. A full thermodynamic profile of aminoglycoside complexation with DNA triplexes detailing these studies has been recently published.35
Key results from these studies are mentioned below. a] The binding constant (105-106M−1) decreases with increasing salt concentration, and a plot of log K vs. log (K+) shows that ~three ion pairs are formed between neomycin and the DNA triplex (Figure 7).
Figure 7.
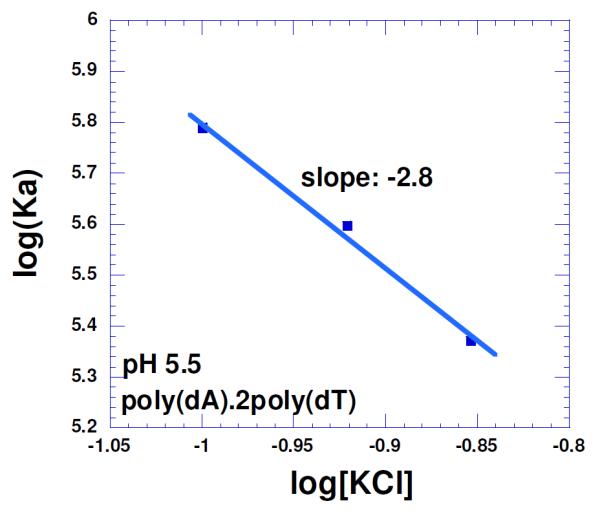
Salt dependence of the neomycin binding with poly(dA)•2poly(dT) triplex in 10 mM sodium cacodylate, 0.5 mM EDTA and pH 5.5. T= 10 °C. The experimental data were fit with linear regression and the solid line reflects the resulting curve fit. Reprinted with permission from Biochimie, 2010, 92, 5, 514-529.
b] The viscosity of poly(dA)•2poly(dT) triplex with increasing concentrations of neomycin was also investigated to understand the mode of drug interaction. Intercalating compounds, upon binding to the nucleic acid, lead to an increased solution viscosity as the length of the rod (DNA polymer) increases. Neomycin, like other groove binding drugs, on the other hand, leads to a decrease in viscosity upon its interaction with the poly(dA)•2poly(dT) triplex, 45 suggesting groove binding.
Modeling studies suggest that the shape and potential complementarity of neomycin to the W-H groove is largely responsible for its selectivity in triplex recognition (Figure 8).45 The selective binding of neomycin to the triplex is achieved in the presence of physiological salt (100-150 mM KCl). In the absence of salt, one observes indiscriminate binding to duplex/triplex backbones,34 suggesting the importance of shape and potential complementarity of neomycin to DNA triplex as a key factor in recognition (in the presence of KCl).
Figure 8.
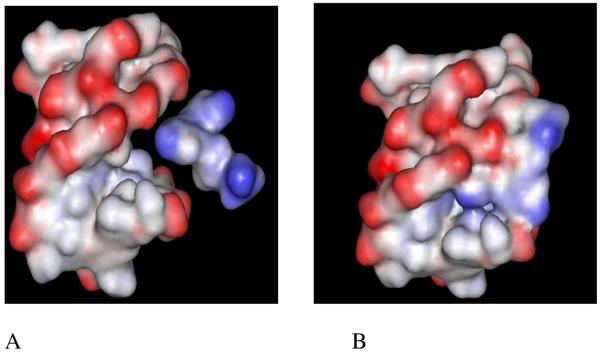
Electrostatic surface potential maps of (A) neomycin approaching the W-H groove of the triplex, and (B) neomycin buried in the triplex groove. Reprinted with permission from J. Amer. Chem. Soc. 2003, 125, 3733-3744.
5. Stabilization of oligomeric, mixed base and non-DNA triple helices
Neomycin also stabilizes oligomeric TAT triplexes that do not form spontaneously, in addition to mixed base sequences. As seen in the thermal denaturation studies of a dA16•2dT16 triplex45, in the absence of drug, a transition for this triplex is not observed (150 mM KCl, pH=6.8, 10 mM sodium cacodylate, 0.5 mM of EDTA, UV or CD melts), even when the dT16 and dA16 strands are mixed in a 2:1 ratio. Upon addition of small amounts of neomycin, a clear Tm3→2 transition becomes visible at 31°C, while the duplex transition is not affected. Thermal denaturation studies of mixed base triplexes such as the 22-mer triplex (below) in the presence of neomycin were found to stabilize the triplex without any effect on the duplex at a pH of 6.8.

Stabilization of RNA and DNA.RNA triple helices
Single-stranded DNA or RNA can be targeted by an ODN, which can form both Watson-Crick base pairing and Hoogsteen base pairing with the target sequence.1, 10, 46-48 Among all the aminoglycosides investigated, neomycin, paromomycin and gentamycin were found to be the most active in stabilizing poly(rA)•2poly(rU) triplex (rdb =0-1). and neomycin is the most active in stabilizing poly(rA)•2poly(rU) triplex as well.34 Similar to DNA triplex, the RNA triplex melting points increase as the number of amines in the aminoglycosides increase.34 These trends of higher affinity of aminoglycosides to RNA triplexes are also observed in FID and calorimetric assays (unpublished results).
Neomycin has also been shown to induce the formation of Poly (rA)•2Poly(dT) triplex (Tm3-2 = 53.2) much more effectively than previously reported ligands (berenil , Tm3-2 = 34.2). Without any ligand, only the duplex transition is seen (Figure 9). A similar effect has been reported with the 2Poly (rA)•Poly(dT) triplex.49 As mentioned with the RNA triplex, FID and ITC results show that for AT/U rich nucleic acids, affinity of neomycin follows the trend: DNA duplex< DNA triplex< DNA.RNA hybrids<RNA duplex~RNA triplex.
Figure 9.
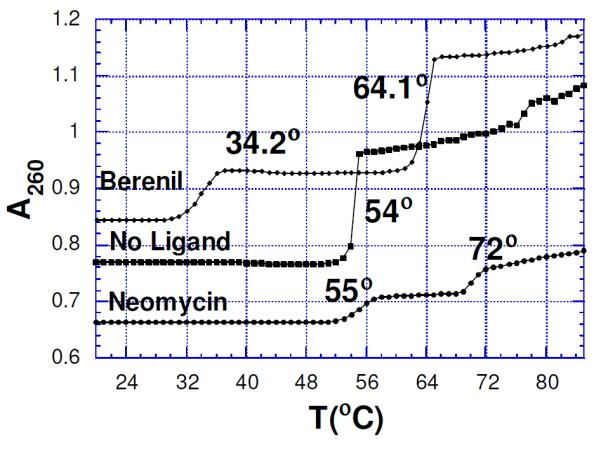
Melting curves for Poly (rA)• 2Poly(dT) complex showing ligand (20 μM) induced triplex formation in the presence of berenil and neomycin. Solution conditions: rA: 30 μM/base triplet; 18 mM NaCl; 10 mM sodium cacodylate; 0.1 mM EDTA, pH 6.8. Reprinted with permission from J. Amer. Chem. Soc. 2001, 123, 11093-11094.
6. Molecular recognition of DNA triple helices by dual recognition conjugates
With synthetic modifications, it is now possible to design ligands that can even compete with aminoglycoside binding to their natural targets-duplex RNA. The triplex-specific interactions (at physiological salt concentrations), of neomycin can be combined with intercalating agents to lead to an overall stabilization of the triplex over the parent duplex.
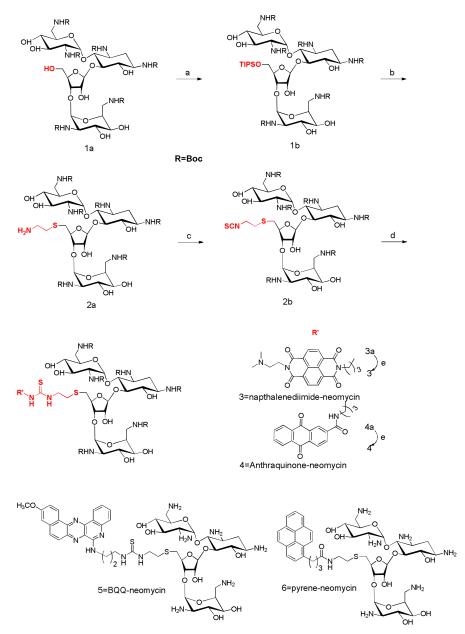
6a. Synthetic approach towards the development of neomycin-intercalator conjugates50
The synthesis of conjugates 3-6 adopts a uniform strategy, in which the 5′-OH position of neomycin (highlighted in red) was selected to tether the intercalators because conjugation at 5′-OH leaves available all aminoglycoside amines necessary for binding to the triplex. The successful approach to synthesize a neomycin derivative (1a) that was further used for conjugation with intercalators involved conversion of the 5′-OH group of 1a into a good leaving group (triisopropylbenzenesulfonyl, TPS) in 1b, followed by reaction with 1,2-aminothiolethane to yield 2a, which further reacted with 1,1′-thiocarbonyldi-2-(1H)-pyridone (TCDP) to convert the amino group into an electrophile (isothiocyanate) (Scheme 1). Subsequently reacting 1b with intercalator amines yielded 3a- 6a. Treatment of 3a-6a with trifluoroacetic acid (TFA) removed the acid labile Boc-protecting groups to produce 3-6 in quantitative yields. Intermediate 2b has broad applications in terms of conjugation of neomycin with other moieties containing amino groups and has been used to conjugate aminoglycosides to numerous nucleophiles (Scheme 1). 51-54, 54
Scheme 1.
a) Triisopropylbenzenesulfonyl chloride, pyridine, r.t. b) 1,2-aminothiolethane, Na, and EtOH c) TCDP, DCM, r.t. d) intercalator-amine, CH2Cl2. e) TFA/CH2Cl2. The site of aminoglycoside modification is shown in red.
6b. Thermodynamics of dual recognition-DNA triplex interactions
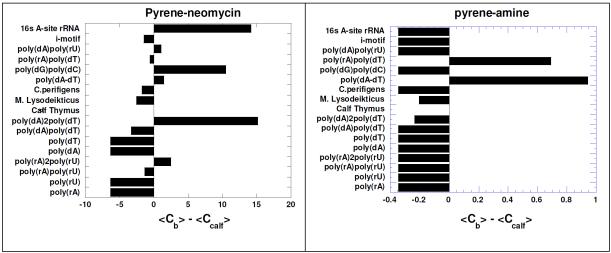
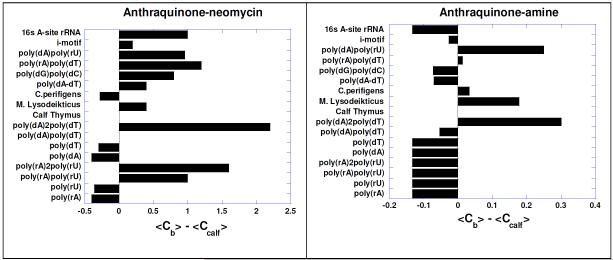
The binding preference of intercalator-neomycin (3-6) to poly(dA)•2poly(dT) was confirmed by the competition dialysis experiments (Figure 10 ). Results (Figure 10) showed that all conjugates 3-6 showed selectivity towards the DNA triplex. Binding of conjugates 3-6 was comparable or even higher than the natural aminoglycoside target, the RNA A-site. Pyrene-neomycin 6 and anthraquinone-neomycin 4 bind to the poly(dG).poly(dC) duplex while the isolated intercalators do not show any binding to this duplex. We have previously shown that neomycin binds to A-form nucleic acids,55 such as the poly(dG).poly(dC) duplex and the competition dialysis studies here confirm our previous findings.
Figure 10.
Competition dialysis results of pyrene-neomycin and anthraquinone-neomycin as well as their corresponding intercalators pyrene-amine and anthraquinone-amine (1 μM) with various types of nucleic acids (75 μM). Buffer: Na2HPO4 (6 mM), NaH2PO4 (2 mM), Na2EDTA (1 mM), NaCl (185 mM), and pH 7.0. Reprinted with permission from Biochemistry, 2010, 49, 5540-5552. X-axis shows the concentration of bound drug (Cb)-concentration of drug bound to calf thymus DNA (Ccalf).
Thermodynamic studies were then conducted at pH 5.5 to minimize the heats of binding induced protonation of amino groups in neomycin, allowing the estimation of intrinsic heats of binding. The binding affinity of all tested ligands with poly(dA)•2poly(dT) increased in the order neomycin < 6 < 3 < 4 < 5 (Table 2). Amongst them, the binding constant [(2.7 ± 0.3) × 108 M−1] of 5 with poly(dA)•2poly(dT) was the highest, almost 1000 fold more than that of neomycin. d] The binding of compounds 3-6 with poly(dA)•2poly(dT) was mostly enthalpy—driven and gave negative ΔCp values (Table 2). While discussing the small molecule-DNA interactions, the negative sign of ΔCp generally has been used to suggest removal of large amounts of nonpolar surface upon complex formation. ΔCp values can however be impacted by a number of other factors including the release of constrained water molecules from the hydration shell, binding induced changes in internal vibrational modes, and electrostatic interactions. Surprisingly, most small molecules that bind to nucleic acids have been shown to have negative ΔCp values56 and aminoglycoside-nucleic acid (RNA, DNA triplex, quadruplex) interactions universally show negative ΔCp values.
Table 2.
Thermodynamic profiles of neomycin and intercalator-neomycin conjugates (3-6) with poly(dA)•2poly(dT) at pH 5.5. Experimental condition: sodium cacodylate (10 mM), EDTA (0.5 mM), KCl (150 mM).50
| Ligand | ΔCpd (cal/mol.K) |
KT(20°C)e (M−1) |
|---|---|---|
| Neomycin | −87±4 | (2.4±0.1)×105 |
| 6 (Pyrene-neomycin) | −10±3 | (1.9±0.1)×106 |
| 3 (Naptheleneimide-neomycin) | −40±20 | (5.5±0.3)×106 |
| 4 (Anthraquinone-neomycin) | −110±15 | (3.7±0.1)×107 |
| 5 (BQQ-neomycin) | −220±20 | (2.7±0.3)×108 |
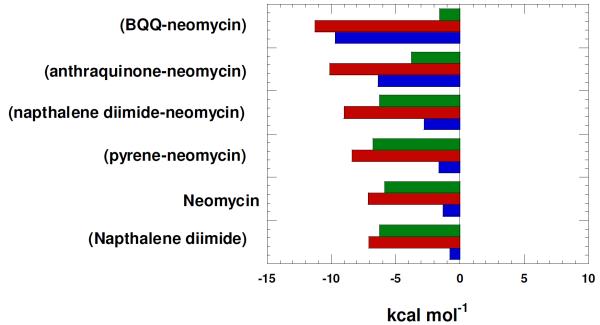
These results provided a measure of quantification of triplex binding when the ligand surface area is increased in the order pyrene < naphthalenediimide < anthraquinone < BQQ. It is not a surprise that 5 is the most potent DNA triplex stabilizing agent amongst all the intercalator-neomycin conjugates in this study because the BQQ moiety (5) was developed by Helene as a rationally designed DNA triplex specific binding ligand. 37
Figure 11 shows the breakdown of thermodynamic properties of ligand-triplex interactions which have been discussed, in detail, in a recent report36 and are summarized below. Neomycin is a major groove binder,45 naphthalene diimide is a well-known intercalator,57 and neomycin conjugates 3-6 bind through dual recognition mode (major groove and intercalation). [a] The binding of neomycin (groove binder) as well as the naphthalene diimide (intercalator) are largely driven by enthalpic contributions. [b]As one goes from from pyrene-neomycin to BQQ-neomycin, an increase in intercalator surface area leads to an increase in the enthalpy of interaction, which clearly leads to a more negative free energy of binding. [c] The free energy ΔG napthalenediimide-neomycin:triplex is not a sum of free energy of ΔGneomycin:triplex and ΔG napthalenediimide-:triplex , but leads to a ΔGcoupling of ~5kcal/mol. While the ΔH napthalenediimide-neomycin:triplex increases substantially over ΔGneomycin:triplex, the corresponding increase in the ΔS is not observed, suggesting that the conjugate, as linked, pays an entropic cost of covalently bridging the groove binder to the intercalator. [d] For the higher affinity conjugates, BQQ-neomycin and Anthraquinone-neomycin, the entropic contribution to the ΔGcomplexation drop substantially. Different linker length variations will lead to even better design of triplex specific ligands and are currently being investigated. This approach utilizing dual recognition of DNA triplexes should be widely applicable to development of structure-specific ligands for a host of nucleic acids where such groove binding and intercalating ligands with independent binding sites can be identified.
Figure 11.
Thermodynamics of binding interactions of ligands with poly(dA)•2poly(dT) triplex at pH 5.5.; red bars represent ΔG, blue bars represent ΔH, and green bars represent TΔS. Experimental condition: sodium cacodylate (10 mM), EDTA (0.5 mM), KCl (150 mM), T=20°C, pH 5.5. Reprinted with permission from Biochemistry, 2010, 49, 5540-5552.
7. Conclusions and Future Directions
a. A large number of DNA triplex intercalators (selective and nonselective) have been used to stabilize triplex structures but the selective targeting of DNA/RNA triplex grooves has been difficult to achieve. Data presented here shows that neomycin is unique in targeting nucleic acid triplex grooves. Molecular recognition of DNA triplex by neomycin offers a different recognition motif when compared to other known groove binders which overwhelmingly prefer the W-C duplex minor groove.
b. RNA and hybrid duplexes and triplexes are much harder to stabilize. DNA•RNA triplexes only form at multimolar (2-4 M) salt concentrations. These findings show great promise for neomycin induced hybrid duplex and triplex stabilization under physiologically relevant salt conditions.
c. A number of X-ray and NMR structures are available for complexes of compounds that bind to both DNA and RNA by intercalation, and on compounds that bind in the DNA minor groove. Similar success has been attained in solving the structures of proteins and peptides that bind in the major groove of DNA and RNA. There is, however, little information available for small molecules that selectively bind DNA triplex grooves or RNA triplex grooves. Infact, no triplex X-ray structures have ever been solved (with the exception of some triplex duplex junctions). Recently, structures of drug-quadruplex have been solved.58 It is the author’s hope that ligand (neomycin)-triplex complexation will allow ligand-triplex X-ray structures to be solved in the near future, even though his personal efforts in crystallization of DNA triplex-neomycin complexes have been unsuccessful. NMR studies could also shed important light on details of aminoglycoside binding to the DNA triplex and are currently being pursued.
d. Repeating copolymers of polypurine and polypyrmidine sequences can assume a hinged DNA structure (H-DNA) which consists of triple-stranded and single-stranded regions. The H-DNA prone sequences are abundant in eukaryotes,59-61 suggesting that binding to the bacterial ribosome may not be the only site of action for these compounds and modified aminoglycosides that can permeate nucleus and mitochondria should be investigated for their binding to non RNA A-form structures present in the cell. While aminoglycosides on their own do not internalize into eukaryotic cells,38 conjugated aminoglycosides such as benzimidazole-neomycin, BQQ-neomycin and antraquinone-neomycin, rapidly internalize into DU145 cells as seen using confocal laser scanning microscopy (unpublished results). These findings have prompted the exploration of these conjugates in targeting Friederich’s ataxia (GAA)n repeats and in triplex forming sequences in malaria.21, 62 Since the intercalator-aminosugar conjugates bind numerous nucleic acids (Figure 10), their benefits in targeting the small percent of TAT triplex forming sites will require an assessment of their triplex binding specificity, but an equally important aspect that should also be explored is their permeability and localization in the cells. Conjugates that show preference for nuclear or mitochondrial localization, in addition to triplex over duplex specificity would be promising candidates for triplex targeting, since their transport will allow them to bypass the large excess of RNA targets on the way to the DNA.
e. Data presented here, in addition to previous work, clearly suggests that aminoglycoside binding is not simply RNA selective but selective for nucleic acids that can adopt the A-conformation, such as RNA duplex, A-form DNA duplex, DNA-RNA hybrid duplex, DNA triplex and RNA triplex.29, 30, 33, 34, 36, 49, 51, 54, 55, 63-66 When studying new aminosugar modifications, it would therefore be important to know their effects on different nucleic acids to ascertain their selectivities.
f. These findings, in addition to the fact that neomycin also helps deliver ODNs to cells,67 has led to the development of covalent aminoglycoside-ODN conjugates to target hybrid duplex and triplex forming sites in the mammalian and bacterial genome.51-53 Previous work in targeting DNA duplex has included the use of intercalator, polyamine, and groove binder-ODN conjugates that have been shown to target multiple sites in the mammalian genome. These approaches have shown success in developing high affinity TFOs that have been able to sequence-specifically recognize duplex DNA, act as artificial nucleases, and been able to target a number of genes to regulate diseases such as HIV and cancer.68, 69, 69-71 Previous studies with TFOs linked to spermine,72 acridine,73 hoechst 33258 74, cyclopropapyrroloindole 75, and psoralen76 have shown remarkable increases in triplex stabilization. However, their selectivity for triplex stabilization, as compared to duplex stabilization, is much lower than the selectivity of neomycin.34 The duplex selectivity has limited intercalator conjugation to the end of the TFOs where intercalation is restricted to duplex-triplex junctions.77 Our approach, where neomycin can be coupled every 5-6 triplets,51 is thus unattainable with intercalator conjugates. Alternative and unpredictable mechanisms of gene repression where the intercalating agent might induce bending or distortion of the DNA, alter chromatin structure, or recruit DNA topoisomerase II and DNA repair enzymes are a concern. The intercalator’s lack of specificity has also been noted 15 in potential clinical applications of TFO. For this reason, intercalators have largely been restricted to one attachment per ODN, so that the conjugate retains the binding properties of the ODN and not that of the intercalator.
Improvements in a) ODN binding to the duplex, and b) ODN transfection, are needed to allow ODN directed triple helix therapies to advance to the clinic. Previous approaches in TFO-small molecule conjugates have been designed to improve only one of the two limitations mentioned above. Neomycin considerably enhances the binding affinities of TFOs to their duplex DNA target (vide infra). Additionally, neomycin, when combined with a cationic lipid preparation, like DOTAP, enhances transfection efficiency of both reporter plasmids and ODNs in cancer cells.67 As more aminoglycoside specific triplex and duplex sequences are identified, highly specific ODN-aminoglycoside conjugates can then be developed for targeting DNA and RNA, leading to novel approaches in antisense or antigene therapies.
CONSPECTUS.
A DNA duplex can be recognized sequence-specifically in the major groove by an oligodeoxynucleotide (ODN). The resulting structure is a DNA triple helix, or triplex. The scientific community has invested significant research capital in the study of DNA triplexes because of their robust potential for providing new applications, including molecular biology tools and therapeutic agents. The triplex structures have inherent instabilities, however, and the recognition of DNA triplexes by small molecules has been attempted as a means of strengthening the three-stranded complex. Over the decades, the majority of work in the field has focused on heterocycles that intercalate between the triplex bases. In this Account, we present an alternate approach to recognition and stabilization of DNA triplexes.
Figure 5b.
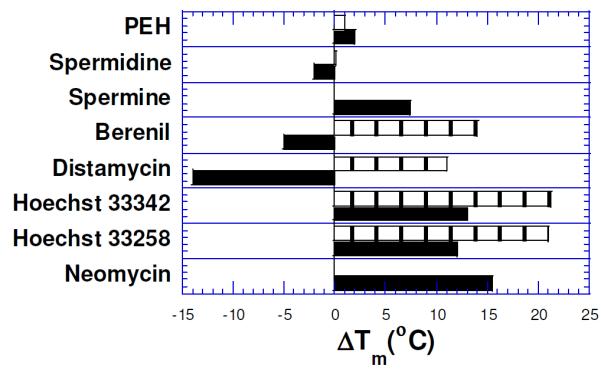
Effect of 10 μM (rdb=0.66) groove binders on the DNA triplex melt{poly(dA)•2poly(dT)}(black) and the duplex melt {poly(dA)•poly(dT)}(boxed). Distamycin does not show Tm3→2 transition(<20°C). PEH= pentaethylene hexamine. Solution conditions: 150 mM KCl, 10 mM sodium cacodylate, pH 6.8, DNA = 15 μM/base triplet. Reprinted with permission from J. Amer. Chem. Soc. 2001, 123, 5385-5395.
ACKNOWLEDGMENTS
This study was made possible by funds from NSF (CHE/MCB-0134932) and NIH (R15CA125724). I am thankful to the numerous students and postdoctoral fellows who contributed to this work.
Footnotes
“Out of the earth shall come thy salvation”
Selman Waksman
REFERENCES
- (1).Kool ET. Design of Triplex-forming Oligonucleotides for Binding DNA and RNA: Optimizing Affinity and Selectivity. New J. Chem. 1997;21:33–45. [Google Scholar]
- (2).Dervan PB. Molecular Recognition of DNA by Small Molecules. Bioorg. Med. Chem. 2001;9:2215–2235. doi: 10.1016/s0968-0896(01)00262-0. [DOI] [PubMed] [Google Scholar]
- (3).Felsenfeld G, Davies D, Rich A. Formation of a Three-Stranded Polynucleotide Molecule. J. Amer. Chem. Soc. 1957;79:2023–2024. [Google Scholar]
- (4).Felsenfeld G, Rich A. Studies on the Formation of Two and Three Stranded Polyribonucleotides. Biochim. Biophys. Acta. 1957;26:457–468. doi: 10.1016/0006-3002(57)90091-4. [DOI] [PubMed] [Google Scholar]
- (5).Maher LJ, III, Wold B, Dervan PB. Inhibition of DNA Binding Proteins by Oligonucleotide-Directed Triple Helix Formation. Science. 1989;245:725–730. doi: 10.1126/science.2549631. [DOI] [PubMed] [Google Scholar]
- (6).Moser HE, Dervan PB. Sequence-Specific Cleavage of Double Helical DNA by Triple Helix Formation. Science. 1987;238:645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- (7).Beal PA, Dervan PB. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991;251:1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- (8).Giovannangeli C, Montenay-Garestier T, Rougee M, Chassignol M, Thuong NT, Helene C. Single-Stranded DNA as a Target for Triple-Helix Formation. J. Amer. Chem. Soc. 1991;113:7775–7777. [Google Scholar]
- (9).Helene C, Toulme JJ. Specific Regulation of Gene Expression by Antisense, Sense, and Antigene Nucleic Acids. Biochim. Biophys. Acta. 1990;1049:99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- (10).Kool ET. Recognition of DNA, RNA, and Proteins by Circular Oligonucleotides. Acc. Chem. Res. 1998;31:502–510. doi: 10.1021/ar9602462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Duca M, Vekhoff P, Oussedik K, Halby L, Arimondo PB. The triple helix: 50 years later, the outcome. Nucleic Acids Res. 2008;36:5123–5138. doi: 10.1093/nar/gkn493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Schleifman EB, Chin JY, Glazer PM. Triplex-mediated gene modification. Methods Mol. Biol. 2008;435:175–190. doi: 10.1007/978-1-59745-232-8_13. [DOI] [PubMed] [Google Scholar]
- (13).Jain A, Wang G, Vasquez KM. DNA triple helices: biological consequences and therapeutic potential. Biochimie. 2008;90:1117–1130. doi: 10.1016/j.biochi.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Jain A, Magistri M, Napoli S, Carbone GM, Catapano CV. Mechanisms of triplex DNA-mediated inhibition of transcription initiation in cells. Biochimie. 2010 doi: 10.1016/j.biochi.2009.12.012. [DOI] [PubMed] [Google Scholar]
- (15).Seidman MM, Glazer PM. The potential for gene repair via triple helix formation. Journal of Clinical Investigation. 2003;112:487–494. doi: 10.1172/JCI19552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Vazquez KM, Glazer PM. Triplex Forming Oligonucleotides: priniciples and applications. Quarterly Reviews of Biophysics. 2002;35:89–107. doi: 10.1017/s0033583502003773. [DOI] [PubMed] [Google Scholar]
- (17).Lestienne PP. Are there three polynucleotide strands in the catalytic centre of DNA polymerases? Biochimie. 2009;91:1523–1530. doi: 10.1016/j.biochi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- (18).Strekowski L, Hojjat M, Wolinska E, Parker AN, Paliakov E, Gorecki T, Tanious FA, Wilson WD. New triple-helix DNA stabilizing agents. Bioorg. Med. Chem. Lett. 2005;15:1097–1100. doi: 10.1016/j.bmcl.2004.12.019. [DOI] [PubMed] [Google Scholar]
- (19).Holt PA, Ragazzon P, Strekowski L, Chaires JB, Trent JO. Discovery of novel triple helical DNA intercalators by an integrated virtual and actual screening platform. Nucleic Acids Res. 2009;37:1280–1287. doi: 10.1093/nar/gkn1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chaires JB, Ren J, Henary M, Zegrocka O, Bishop GR, Strekowski L. Triplex selective 2-(2-naphthyl)quinoline compounds: origins of affinity and new design principles. J. Amer. Chem. Soc. 2003;125:7272–7283. doi: 10.1021/ja034181r. [DOI] [PubMed] [Google Scholar]
- (21).Bergquist H, Nikravesh A, Fernandez RD, Larsson V, Nguyen CH, Good L, Zain R. Structure-specific recognition of Friedreich’s ataxia (GAA)n repeats by benzoquinoquinoxaline derivatives. Chembiochem. 2009;10:2629–2637. doi: 10.1002/cbic.200900263. [DOI] [PubMed] [Google Scholar]
- (22).Lane A, Jenkins T. Structures and properties of multi-stranded nucleic acids. Curr. Org. Chem. 2001;5:845–869. [Google Scholar]
- (23).Antony T, Thomas T, Shirahata A, Sigal LH, Thomas TJ. Selectivity of Spermine Homologs on Triplex DNA Stabilization. Antisense Nucleic Acid Drug Dev. 1999;9:221–231. doi: 10.1089/oli.1.1999.9.221. [DOI] [PubMed] [Google Scholar]
- (24).Neidle S. DNA minor-groove recognition by small molecules. Natural Product Reports. 2001;18:291–309. doi: 10.1039/a705982e. [DOI] [PubMed] [Google Scholar]
- (25).Dervan PB, Edelson BS. Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr Opin Struct Biol. 2003;13:284–99. doi: 10.1016/s0959-440x(03)00081-2. [DOI] [PubMed] [Google Scholar]
- (26).Kopka ML, Yoon C, Goodsell D, Pjura P, Dickerson RE. The Molecular Origin of DNA Drug Specificity in Netropsin and Distamycin. Proc. Natl. Acad. Sci. USA. 1985;82:1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Dervan PB. Design of Sequence-Specific DNA-Binding Molecules. Science. 1986;232:464–71. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- (28).Wartell RM, Larson JE, Wells R. D. Netropsin. A specific probe for A-T regions of duplex deoxyribonucleic acid. J. Biol. Chem. 1974;249:6719–6731. [PubMed] [Google Scholar]
- (29).Willis B, Arya DP. An Expanding View of Aminoglycoside-Nucleic Acid Recognition. In: Horton D, editor. Advances in Carbohydrate Chemistry and Biochemistry. Vol. 60. Elsevier; 2006. pp. 251–302. [DOI] [PubMed] [Google Scholar]
- (30).Arya DP. Aminoglycoside-nucleic acid interactions: the case for neomycin. Top. Curr. Chem. 2005;253:149–178. [Google Scholar]
- (31).Waksman SA, Lechevalier HA. Neomycin, a New Antibiotic Active against Streptomycin-Resistant bacteria, Including Tuberculosis Organisms. Journal of Antibiotics. 1949;109:305–309. doi: 10.1126/science.109.2830.305. [DOI] [PubMed] [Google Scholar]
- (32).Schatz A, Bugie E, Waksman SA. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Proc. Soc. Exptl. Biol. Med. 1944:55. doi: 10.1097/01.blo.0000175887.98112.fe. [DOI] [PubMed] [Google Scholar]
- (33).Arya DP, Coffee RL. DNA triple helix stabilization by aminoglycoside antibiotics. Bioorg. Med. Chem. Lett. 2000;10:1897–9. doi: 10.1016/s0960-894x(00)00372-3. [DOI] [PubMed] [Google Scholar]
- (34).Arya DP, Coffee RL, Willis B, Abramovitch AI. Aminoglycoside-Nucleic Acid Interactions: Remarkable Stabilization of DNA and RNA Triple Helices by Neomycin. J. Amer. Chem. Soc. 2001;123:5385–5395. doi: 10.1021/ja003052x. [DOI] [PubMed] [Google Scholar]
- (35).Xi H, Kumar S, Dosen-Micovic L, Arya DP. Calorimetric and spectroscopic studies of aminoglycoside binding to AT-rich DNA triple helices. Biochimie. 2010;92:514–529. doi: 10.1016/j.biochi.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Arya DP, Xue L, Tennant P. Combining the Best in Triplex Recognition: Synthesis and Nucleic Acid Binding of a BQQ-Neomycin Conjugate. J. Amer. Chem. Soc. 2003;125:8070–8071. doi: 10.1021/ja034241t. [DOI] [PubMed] [Google Scholar]
- (37).Zain R, Marchand C, Sun J, Nguyen CH, Bisagni E, Garestier T, Helene C. Design of a Triple-Helix-Specific Cleaving Reagent. Chem Biol. 1999;6:771–7. doi: 10.1016/s1074-5521(99)80124-0. [DOI] [PubMed] [Google Scholar]
- (38).Luedtke NW, Carmichael P, Tor Y. Cellular uptake of aminoglycosides, guanidinoglycosides, and poly-arginine. J. Am. Chem. Soc. 2003;125:12374–12375. doi: 10.1021/ja0360135. [DOI] [PubMed] [Google Scholar]
- (39).Radhakrishnan I, Patel DJ. DNA Triplexes: Solution Structures, Hydration Sites, Energetics, Interactions, and Function. Biochemistry. 1994;33:11405–11416. doi: 10.1021/bi00204a001. [DOI] [PubMed] [Google Scholar]
- (40).Radhakrishnan I, Patel DJ. Solution structure of a purine.purine.pyrimidine DNA triplex containing G.GC and T.AT triples. Structure. 1993;1:135–152. doi: 10.1016/0969-2126(93)90028-f. [DOI] [PubMed] [Google Scholar]
- (41).Shields GC, Laughton CA, Orozco M. Molecular dynamics simulations of the d(T.A.T) Triple Helix. J. Amer. Chem. Soc. 1997;119:7463–7469. [Google Scholar]
- (42).Feigon J, Wang E. Structures of nucleic acid triplexes. In: Neidle S, editor. Nucleic Acid Structure. Vol. 1. Oxford University Press; Oxford: 1999. pp. 355–388. [Google Scholar]
- (43).Phipps AK, Tarkoy M, Schultze P, Feigon J. Solution Structure of an Intramolecular DNA Triplex Containing 5-(1-propynyl)-2′-deoxyuridine Residues in the Third Strand. Biochemistry. 1998;37:5820–30. doi: 10.1021/bi972811u. [DOI] [PubMed] [Google Scholar]
- (44).Tarkoy M, Phipps AK, Schultze P, Feigon J. Solution Structure of an Intramolecular DNA Triplex Linked by Hexakis(ethylene glycol) Units: d(AGAGAGAA-(EG)6-TTCTCTCT-(EG)6TCTCTCTT) Biochemistry. 1998;37:5810–5819. doi: 10.1021/bi9728102. [DOI] [PubMed] [Google Scholar]
- (45).Arya DP, Micovic L, Charles I, Coffee RL, Willis B, Xue L. Neomycin Binding to DNA Triplex Watson-Hoogsteen (W-H) Groove: A Model. J. Amer. Chem. Soc. 2003;125:3733–3744. doi: 10.1021/ja027765m. [DOI] [PubMed] [Google Scholar]
- (46).Kool ET. Preorganization of DNA: Design Principles for Improving Nucleic Acid Recognition by Synthetic Oligonucleotides. Chem. Rev. 1997;97:1473–1487. doi: 10.1021/cr9603791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Kool ET. Circular oligonucleotides: new concepts in oligonucleotide design. Annu. Rev. Biophys. Biomol. Struct. 1996;25:1–28. doi: 10.1146/annurev.bb.25.060196.000245. [DOI] [PubMed] [Google Scholar]
- (48).Prakash G, kool ET. Molecular Recognition by Circular Oligonucleotides. Strong Binding of single-stranded DNA and RNA. Chem. Commun. 1991:1161–1163. doi: 10.1039/C39910001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Arya DP, Coffee RL, Charles I. Neomycin-Induced Hybrid Triplex Formation. J. Amer. Chem. Soc. 2001;123:11093–11094. doi: 10.1021/ja016481j. [DOI] [PubMed] [Google Scholar]
- (50).Xue L, Xi H, Kumar S, Gray D, Davis E, Hamilton P, Skriba M, Arya DP. Probing the recognition surface of a DNA triplex: binding studies with intercalator-neomycin conjugates. Biochemistry. 2010;49:5540–5552. doi: 10.1021/bi100071j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Charles I, Xi H, Arya DP. Sequence-specific targeting of RNA with a neomycin-DNA conjugate. Bioconjugate Chemistry. 2007;18:160–169. doi: 10.1021/bc060249r. [DOI] [PubMed] [Google Scholar]
- (52).Charles I, Arya DP. Synthesis of Neomycin-DNA/Peptide Nucleic Acid Conjugates. J. Carb. Chem. 2005;24:143–157. [Google Scholar]
- (53).Charles I, Xue L, Arya DP. Synthesis of Aminoglycoside-DNA Conjugates. Bioorg. Med. Chem. Lett. 2002;12:1259–1262. doi: 10.1016/s0960-894x(02)00157-9. [DOI] [PubMed] [Google Scholar]
- (54).Shaw NN, Xi H, Arya DP. Molecular recognition of a DNA:RNA hybrid: sub-nanomolar binding by a neomycin-methidium conjugate. Bioorg. Med. Chem. Lett. 2008;18:4142–4145. doi: 10.1016/j.bmcl.2008.05.090. [DOI] [PubMed] [Google Scholar]
- (55).Arya DP, Xue L, Willis B. Aminoglycoside (neomycin) preference is for A-form nucleic acids, not just RNA: results from a competition dialysis study. J Am Chem Soc. 2003;125:10148–9. doi: 10.1021/ja035117c. [DOI] [PubMed] [Google Scholar]
- (56).Chaires JB. Energetics of Drug-DNA Interactions. Biopolymers. 1998;44:201–215. doi: 10.1002/(SICI)1097-0282(1997)44:3<201::AID-BIP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- (57).Gianolio DA, Segismundo JM, McLaughlin LW. Tethered naphthalene diimide-based intercalators for DNA triplex stabilization. Nucl. Acids Res. 2000;28:2128–2134. doi: 10.1093/nar/28.10.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Neidle S. The structures of quadruplex nucleic acids and their drug complexes. Curr. Opin. Struct. Biol. 2009;19:239–250. doi: 10.1016/j.sbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- (59).Schroth GP, Ho PS. Occurrence of potential cruciform and H-DNA forming sequences in genomic DNA. Nucleic Acids Res. 1995;23:1977–1983. doi: 10.1093/nar/23.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Mirkin SM, Frank-Kamenetskii MD. H-DNA and Related Structures. Annu. Rev. Biophys. Biomol. Struct. 1994;23:541–576. doi: 10.1146/annurev.bb.23.060194.002545. [DOI] [PubMed] [Google Scholar]
- (61).Htun H, Dahlberg JE. Topology and Formation of Triple-Stranded H-DNA. Science. 1989;243:1571–1576. doi: 10.1126/science.2648571. [DOI] [PubMed] [Google Scholar]
- (62).Horrocks P, Wong E, Russell K, Emes RD. Control of gene expression in Plasmodium falciparum - ten years on. Mol. Biochem. Parasitol. 2009;164:9–25. doi: 10.1016/j.molbiopara.2008.11.010. [DOI] [PubMed] [Google Scholar]
- (63).Xi H, Gray D, Kumar S, Arya DP. Molecular recognition of single-stranded RNA: neomycin binding to poly(A) FEBS Lett. 2009;583:2269–2275. doi: 10.1016/j.febslet.2009.06.007. [DOI] [PubMed] [Google Scholar]
- (64).Shaw NN, Arya DP. Recognition of the unique structure of DNA:RNA hybrids. Biochimie. 2008;90:1026–1039. doi: 10.1016/j.biochi.2008.04.011. [DOI] [PubMed] [Google Scholar]
- (65).Xi H, Arya DP. Recognition of triple helical nucleic acids by aminoglycosides. Curr Med Chem Anticancer Agents. 2005;5:327–38. doi: 10.2174/1568011054222328. [DOI] [PubMed] [Google Scholar]
- (66).Xue L, Charles I, Arya DP. Pyrene-neomycin conjugate: dual recognition of a DNA triple helix. Chem.Commun. 2002;1:70–71. doi: 10.1039/b108171c. [DOI] [PubMed] [Google Scholar]
- (67).Napoli S, Carbone GM, Catapano C, Shaw N, Arya DP. Neomycin improves cationic lipid-mediated transfection of DNA in human cells. Bioorganic and Medicinal Chemistry Letters. 2005;15:3467–3469. doi: 10.1016/j.bmcl.2005.04.038. [DOI] [PubMed] [Google Scholar]
- (68).Benfield AP, Macleod MC, Liu Y, Wu Q, Wensel TG, Vasquez KM. Targeted generation of DNA strand breaks using pyrene-conjugated triplex-forming oligonucleotides. Biochemistry. 2008;47:6279–6288. doi: 10.1021/bi7024029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Oh DH, Hanawalt PC. Triple helix-forming oligonucleotides target psoralen adducts to specific chromosomal sequences in human cells. Nucleic Acids Res. 1999;27:4734–4742. doi: 10.1093/nar/27.24.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Giovannangeli C, Perrouault L, Escude C, Thuong N, Helene C. Specific Inhibition of in Vitro Transcription Elongation by Triplex-Forming Oligonucleotide-Intercalator Conjugates Targeted to HIV Proviral DNA. Biochemistry. 1996;35:10539–10548. doi: 10.1021/bi952993x. [DOI] [PubMed] [Google Scholar]
- (71).Francois J, Saison-Behmoaras T, Barbier C, Chassignol M, Thuong NT, Helene C. Sequence-specific recognition and cleavage of duplex DNA via triple-helix formation by oligonucleotides covalently linked to a phenanthroline-copper chelate. Proc. Natl. Acad. Sci. USA. 1989;86:9702–9706. doi: 10.1073/pnas.86.24.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Rajeev KG, Jadhav VR, Ganesh KN. Triplex formation at physiological pH: comparative studies on DNA triplexes containing 5-Me-dC tethered at N4 with spermine and tetraethyleneoxyamine. Nucleic Acids Res. 1997;25:4187–4193. doi: 10.1093/nar/25.21.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Stonehouse TJ, Fox KR. DNase I footprinting of triple helix formation at polypurine tracts by acridine-linked oligopyrimidines: stringency, structural changes and interaction with minor groove binding ligands. Biochim. Biophys. Acta-Gene Struct. Expression. 1994;1218:322–330. doi: 10.1016/0167-4781(94)90184-8. [DOI] [PubMed] [Google Scholar]
- (74).Robles J, McLaughlin LW. DNA Triplex Stabilization Using a Tethered Minor Groove Binding Hoechst 33258 Analogue. J. Amer. Chem. Soc. 1997;119:6014–6021. [Google Scholar]
- (75).Dempcy RO, Kutyavin IV, Mills AG, Lukhtanov EA, Meyer RB. Linkers designed to intercalate the double helix greatly facilitate DNA alkylation by triplex-forming oligonucleotides carrying a cyclopropapyrroloindole reactive moiety. Nucleic Acids Res. 1999;27:2931–2937. doi: 10.1093/nar/27.14.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Liu Y, Nairn RS, Vasquez KM. Targeted gene conversion induced by triplex-directed psoralen interstrand crosslinks in mammalian cells. Nucleic Acids Res. 2009;37:6378–6388. doi: 10.1093/nar/gkp678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Zhou B, Puga E, Sun J, Garestier T, Helene C. Stable Triple Helices Formed by Acridine-Containing Oligonucleotides with Oligopurine Tracts of DNA Interrupted by One or Two Pyrimidines. J. Amer. Chem. Soc. 1995;117:10425–10428. [Google Scholar]