Abstract
Social animals establish flexible behaviors and integrated decision-making processes to adapt to social environments. Such behaviors are impaired in all major neuropsychiatric disorders and depend on the prefrontal cortex (PFC). We previously showed that nicotinic acetylcholine receptors (nAChRs) and norepinephrine (NE) in the PFC are necessary for mice to show adapted social cognition. Here, we investigated how the cholinergic and NE systems converge within the PFC to modulate social behavior. We used a social interaction task (SIT) in C57BL/6 mice and mice lacking β2*nAChRs (β2−/− mice), making use of dedicated software to analyze >20 social sequences and pinpoint social decisions. We performed specific PFC NE depletions before SIT and measured monoamines and acetylcholine (ACh) levels in limbic corticostriatal circuitry. After PFC-NE depletion, C57BL/6 mice exhibited impoverished and more rigid social behavior and were 6-fold more aggressive than sham-lesioned animals, whereas β2−/− mice showed unimpaired social behavior. Our biochemical measures suggest a critical involvement of DA in SIT. In addition, we show that the balance between basal levels of monoamines and of ACh modulates aggressiveness and this modulation requires functional β2*nAChRs. These findings demonstrate the critical interplay between prefrontal NE and nAChRs for the development of adapted and nonaggressive social cognition.—Coura, R. S., Cressant, A., Xia, J., de Chaumont, F., Olivo-Marin, J. C., Pelloux, Y., Dalley, J. W., Granon, S. Nonaggressive and adapted social cognition is controlled by the interplay between noradrenergic and nicotinic receptor mechanisms in the prefrontal cortex.
Keywords: norepinephine, decision making, dopamine, 5-HT
Humans, akin to all social animals, develop flexible behaviors and integrated decision-making processes to adapt to social environments. Understanding the neural mechanisms of social decisions is important because impaired social cognition is prevalent in a number of psychiatric disorders, such as schizophrenia and depression (1–4). Work in animals indicates that the salience of an external stimulus and introceptive cues collectively influence flexible decision-making during novel social encounters (5).
The prefrontal cortex (PFC) is a brain structure involved in planning complex cognitive behaviors, personality, working memory, response inhibitory control, attention, decision making, and moderating social behavior (6, 7). Such behaviors are strongly modulated by acetylcholine (ACh) and norepinephrine (NE) in this region (6, 8, 9).
ACh is a broadly tuned modulator of cortical activity playing crucial roles in attentional processes and conscious awareness (9, 10). Neuronal nicotinic acetylcholine receptors (nAChRs) bind nicotinic ligands with high affinity (11), are widely located throughout the central nervous system (12), and play an important role in mediating higher cognitive functions dependent on cholinergic transmission (13). Presynaptic nAChRs play a major role in modulating neurotransmitter release (12, 14–17), especially β2-containing heteromeric nAChRs (β2*nAChRs) and α7-homomeric nAChRs (a7*nAChRs), which are widely present in the PFC (12, 17, 18). In particular, β2*nAChRs have an ubiquitous role in modulating neurotransmitter release in the PFC and thereby the behaviors and cognitive processes modulated by distinct neurotransmitter systems in this region, including attentional processing (19), working memory (20, 21), reward-related processing and learning (22), as well as flexible decision-making (23, 24).
Norepinephrinergic projections to the PFC are similarly implicated in cognitive functions, including attentional processes, working memory, and learning (25), thereby having relevance to the pathophysiology of schizophrenia and depression. Activation of nAChRs is known to increase NE release in the hippocampus (26), amygdala (27), cerebellum (28), hypothalamus (29), and spinal cord (30). However, there are limited data on the effects of nAChR stimulation in the PFC on NE function and consequently on the profile of behavioral and cognitive processes modulated by NE levels in this region. Elucidating the functional significance of nAChR-NE interactions is important for understanding the basis of cognitive and executive processes dependent on the PFC (31, 32).
The present study investigates the functional interaction between β2*nAChRs and the NE system in the context of social behavioral interactions. To address this question, we carried out global NE brain depletion or local NE deafferentiation in the prelimbic cortex (PrL) area of the PFC in mice lacking the β2-subunit of nAChRs (β2−/− mice) and C57BL/6 control mice. Subjects were assessed on a social interaction task (SIT) previously shown to depend on the functional integrity of the PrL of the PFC and β2*nAChRs (24, 33).
We previously demonstrated that C57BL/6 mice, but not β2−/− mice, exhibit a complex repertoire of social behaviors that evolves with time and favors the progressive emergence of adapted risk-prone or risk-tolerance postures (33). We also demonstrated that reexpressing the β2-subunit of the nAChRs specifically into the PFC of β2−/− mice was sufficient to restore normal social patterns in these animals, thus showing that β2−/− social defects were not due to gross compensatory mechanisms (24, 34, 35).
Here, we found that C57BL/6 mice exhibited impoverished social repertoire after PFC-NE depletion and marked aggressiveness compared with their nonlesioned controls. By contrast, β2−/− mice showed restored social behaviors after NE depletion compared with control β2−/− mice. Our biochemical measures suggest a critical involvement of dopamine (DA) in SIT. In addition, we show a functional dissociation between aggressiveness and social dominance, the first potentiated by prefrontal NE and the latter by ACh. Collectively, these findings show the critical interplay between prefrontal NE and nicotinic systems for development of social and flexible behavior.
MATERIALS AND METHODS
Animals
All mice were bred in the Charles River facility (Charles River, L'Arbresle, France) and transported to the laboratory on request. Male C57BL/6J mice and knockouts for the β2-subunit of nicotinic receptors (β2−/−), aged 7–8 wk on the arrival were used in this study. Both C57BL/6J and β2−/− mice arrived at the same time from the breeding facility in two distinct transportation boxes. Both strains were treated similarly: they were initially group housed (4 mice/cage) for 1 wk to acclimatize to the animal facility under a 12/12-h light-dark cycle (lights on at 7:30 AM). Some C57BL/6J and all β2−/− mice were then separated and housed individually for 4 wk, while some C57BL/6J mice remained group housed (4 mice/cage). The β2−/− mice were originally generated from a 129/Sv ES cell line as described previously (36) and backcrossed onto the C57BL/6J strain for 20 generations, which is twice the number of backcrosses suggested by the Bandburry conference guidelines (37). They were shown to be at >99.99% C57BL/6J by a genomic analysis using 400 markers. For these reasons, and because littermates are not available in the breeding facilities, we used C57BL/6J mice as controls.
Groups consisting of 8–12 mice were formed for the 3 experimental procedures: global NE depletion and behavior (experiment 1); ex vivo assessment of brain monoamine levels (experiment 2); and PrL NE depletion and behavior (experiment 3). Each experimental procedure consisted of 4 groups: sham-treated (C57BL/6 and β2−/−) and lesioned (C57BL/6 and β2−/−) mice. In total, 95 animals were used. However, a small number of animals were not included in the final analysis due to the presence of gliosis (10 mice) or due to outliers (4 mice), as confirmed by the Dixon test (38). Supplemental Table S1 summarizes the final number of mice in each experimental group. Control groups used for behavioral experiments 1 (10 C57BL/6 and 7 β2−/− mice) and 3 (15 C57BL/6 and 14 β2−/− mice) were pooled since there were no statistical differences between them (ANOVA main group effect, P=0.66, NS). Animals were returned to their individual home cage for 1 wk after surgery and before behavioral testing.
All experimental procedures were carried out in accordance with the ethical standard defined by the French Centre National de la Recherche Scientifique and European Community guidelines for care of laboratory animals (no. 86/609). All procedures were conducted to reduce the number of mice used when possible and to reduce their level of pain and discomfort as much as possible.
Drugs
The selective dopaminergic-noradrenergic neurotoxin 6-hydroxydopamine (6-OHDA; Sigma, St. Louis, MO, USA) was dissolved in 0.9% saline containing 0.1% ascorbic acid. GBR 12909 dihydrochloride (Sigma) was dissolved in DMSO (25 mg/ml). The selective noradrenergic neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4); Tocris Cookson, Ellisville, MO, USA) was dissolved in distilled water (50 mg/kg).
Global depletion of NE
Effects of global NE depletion on behavior and brain monoamine content
Global NE depletion was achieved by systemic administration of DSP-4. At 8 d before the behavioral experiment, adult male C57BL/6 mice and β2−/− mice were injected intraperitoneallly with DSP-4 (50 mg/kg) or vehicle (sham-treatment group). At 30 min before the DSP-4 or vehicle injections, animals were pretreated with GBR 12909 dihydrochloride (25 mg/ml) to protect dopaminergic neurons (39).
Effects of NE depletion in the PrL cortex on behavior
Mice were anesthetized with a mix of ketamine/xylazine [1000:20 mg/ml, 300:50 μl, respectively, in phosphate-buffered saline (PBS), qs 2–0.3 ml/mouse] and positioned in a stereotactic instrument (Stoelting, Wood Dale, IL USA). Bilateral infusions were made into the PrL of the PFC under stereotactic guidance (40) (from bregma: +2.2 mm anteroposterior, ±0.3 mm mediolateral, −2.2 mm dorsoventral). Animals were infused with 6-OHDA (10 μg in 0.5 μl/site of injection) or vehicle (0.5 μl of 0.9% saline containing 0.1% ascorbic acid/site of injection). Infusions were made through a fine needle with a flat tip (36 gauge; Coopers Needle Works, Birmingham, UK) connected via PEG tubing to a 5-μl syringe (Hamilton) held by a microliter infusion pump. The delivery rate of injection was 0.2 μl/min. To allow sufficient time for diffusion, the needle was left in place for 10 min after each infusion. Mice were given 7 d to recover from surgery. To minimize nonspecific damage, mice lesioned with 6-OHDA were pretreated with GBR 12909 (15 mg/kg i.p.) to protect dopaminergic neurons. Pretreatments were carried out 30 min before the injection. The magnitude and neurochemical selectivity of the lesion were verified afterward by immunofluorescence for norepinephrine transporter (NET; see Materials and Methods and Supplemental Data).
Behavioral testing
Social interaction task
At 8 d after surgery, male mice were tested in the social interaction paradigm to evaluate the effects of local or global NE depletion on social behavior. Mice were isolated and maintained in individual cages for 4 wk before the test. Mice socially isolated were shown to display a higher motivation for social contact than mice reared in groups (24, 41). As described previously (23, 33, 42), each mouse previously isolated was placed individually in a large transparent testing cage for a 30-min habituation period. This mouse was called the isolated-host (IH) mouse. At the end of the habituation period, a drug/behavior-naive, group-housed C57BL/6 mouse [called the social-visitor (SV) mouse] of same weight, age, and sex was introduced into the testing cage. Each SV mouse was gently placed in a corner opposite to the IH mouse for an 8-min test session recorded via a camera on a computer for offline scoring. Behavioral features of affiliative social behavior, i.e., social contact duration and follow behavior (24, 33, 43), were first manually measured and compared between C57BL/6 and β2−/− mice, as well as between lesioned and sham-treated mice. Furthermore, a more detailed analysis was carried out using MiceProfiler software (33). Briefly, with this semiautomatic analysis, we distinguished contact events, defined as any position in which mice were at whiskers distance or less apart. Within contact events, we discriminated oral-oral, oral-genital, and side-by-side contacts. Apart from contact events, we scored different postures in each mouse of the dyad. Among them, we distinguished stop behaviors, back-to-back postures, and follow behaviors. Several previous studies have defined stop behaviors, that is short bouts of stopping in between locomotor sequences, as being important for the organization of actions in different animals (44–47). Stop behaviors may be very brief and last only one image frame (1/15 s). In addition, animals may not stop completely (speed <1.75 cm/s) but can scan the environment, rear, sniff, and make head movements (47). Therefore, as stop behaviors have been shown to be important choice points in various animal models during novelty exploration and locomotion, we included them in the repertoire we defined in our social interaction task that allows large movements and novelty seeking (33). Back-to-back postures were scored when both mice were looking in opposite directions, were relatively static, not touching each other, and had their heads at an angle that did not allow them to see each other. Follow behaviors were defined by mice moving at a speed >1.75 cm/s with one mouse behind the other and a distance between them of <1.5 cm (33). These complex events that have been shown to be indicative of behavioral flexibility were virtually impossible to analyze manually in such a detail.
We also assessed dominance and aggressiveness during the social task. Dominance was characterized as a threat behavior that may be made to reinforce a dominant position or to territory protection. This behavior may progress to aggression, depending on the flight/submissive or defensive behavior of the congener (48–50). Here, like in our previous studies (51, 52), we defined the index of social dominance as the combination of two types of behaviors: “paw control” and “aggressive grooming.” Paw control events were scored each time the IH mouse put at least one of its forepaws on the back or head of the SV mouse. Aggressive grooming was scored when the IH mouse excessively groomed the SV mouse. We also calculated an index of aggressiveness, which included the number of times the IH mouse circled around, bit, and rattled its tail in front of the SV mouse.
Anxiety in dark-light task
At 24 h after the social interaction test, animals were assessed for 10 min in a light/dark box to evaluate anxiety. The apparatus consisted of a rectangular acrylic box, divided into two compartments: one dark that was covered by a dark opaque top, and one white that was open and brightly illuminated (400 lux; ref. 33). The mouse was gently placed in the corner of the light box and could move freely from one compartment to the other through a dark corridor. A video camera was fixed above the apparatus to record the animal's behavior for offline analyses, so that the test was conducted in absence of an experimenter. The criteria used to assess an animal's level of anxiety were the initial latency to escape the light box, the number of entries into the dark box, and the total time spent in the light compartment. An entry into a compartment was registered if the mouse placed both forepaws into the box.
Anxiety in the elevated plus maze (EPM)
The standard EPM test is commonly used to assess anxiety-like behavior in rodents. This task relies on conflict between the innate fear that rodents exhibit toward elevated and open areas vs. natural exploration of novel environments. The maze was a cross-shaped elevated maze with two open arms facing each other and two walled arms. In such a maze, the natural tendency of rodents is to prefer enclosed dark spaces to open brightly lit spaces. The day following the dark-light test, animals were tested for 10 min in EPM. Light intensity was set to 100 lux on the central platform. A video camera fixed on the ceiling of the experimental room right above the central platform allowed the experimenter to record and track offline the mouse position within the maze. Total time spent in open and closed arms and numbers of entries into the open arms were measured. Entrances were counted when subjects put both forepaws on an arm or on the platform. From these measures, we derived the percentage of time spent on the open arms, expressed as a percentage of time spent on the open and closed arms.
Neurochemical imaging
At the completion of the last behavioral test, mice were deeply anesthetized with intraperitoneal pentobarbital and transcardially perfused with 20 ml of ice-cold 0.1 M PBS (pH 7.4), followed by 50 ml of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were rapidly removed and postfixed overnight in the same solution at 4°C. Then, 60-μm coronal sections were cut on a vibratome. Sections were collected in 0.1 M PBS. One section in 4 was used for Cresyl Violet histology, tyrosine hydroxylase (TH) and serotonin [5-hydroxytryptamine (5-HT)] immunohistochemistry, and NET immunofluorescence.
Histology
Cresyl Violet staining was used to verify the most ventral position of injectors in the PFC as well as the integrity of the adjacent tissue. Immunohistochemistry was used to demonstrate the preservation of both dopaminergic and serotoninergic systems (i.e., to ensure of the selectivity of our procedure with DSP4 or 6-OHDA injection coupled to GBR 12909 injection).
Analysis of brain monoamines and ACh
In experiment 2, we determined and compared constitutive brain levels of monoamines and ACh in C57BL/6 and β2−/− mice. Twelve animals of each group (C57BL/6 and β2−/−) were killed by exposure to a rising concentration of carbon dioxide and cervical dislocation (53). Brains were rapidly removed and frozen in dry ice. Coronal sections (150 μm) were cut on a cryostat. Frozen sections were dissected using a punch from 6 regions of interest (40): rostral prelimbic cortex (rPrL), caudal prelimbic cortex (cPrL), orbitofrontal cortex (OFC), hippocampus (H), nucleus accumbens (Acb), and amygdala (A). Tissue aliquots were derived from both hemispheres and homogenized in 200 ml of 0.2M perchloric acid by an ultrasonic cell disruptor (Microson, Barcelona, Spain). Levels of NE, DA, and 5-HT and their metabolites (DOPAC, 5HIAA, and MHPG) were determined in the supernatant by reversed-phase, high-performance liquid chromatography (HPLC), as described previously (53). The analysis of ACh was also performed by HPLC, with the mobile phase containing 75 mM Na2HPO4 and 5 μl/ml ProClin reagent (BAS, Congleton, UK), pH 8.0 (adjusted with 46/48% NaOH), and a flow rate of 120 L/min (582 solvent delivery module; ESA Inc., Chelmsford, MA, USA). Samples were injected manually (UniJet microbore valve; BAS) onto a microbore analytical column (530×1 mm, 10 μm; BAS), as described previously (54).
To verify possible differences in the balance between these neurotransmitters after global noradrenergic lesion, 12 C57BL/6 and 12 β2−/− mice were pretreated with the selective noradrenergic neurotoxin DSP-4 (50 mg/kg i.p.). Control animals received 0.9% saline on the same schedule as treated groups. These animals were assessed postmortem for brain tissue levels of monoamines and ACh, as described above.
Statistical analysis
The main effects of genotype (2 levels: C57BL/6 and β2−/−) and of lesion (2 levels: sham treatment and global NE lesion, or NE PrL depletion), as well as statistical interactions between these factors, were assessed with ANOVA and Statview 4.57 (SAS Institute, Cary, NC, USA). Post hoc analyses were performed on significant main effects or significant interactions using Bonferonni's test. Dixon's Q test (38) was used before any other statistical treatment to objectively detect a single outlier within a group of data for which a gaussian distribution applies. The criterion for statistical significance was set at P < 0.05.
RESULTS
Effect of global NE depletion on social behavior
As flexible social behaviors are strongly modulated by NE and ACh systems, we first investigated the effect of global NE depletion on social behavior in C57BL/6 and β2−/− mice. For that purpose, we used a social interaction task in which a previously isolated male mouse was confronted with a novel male C57BL/6 mouse in a novel, large environment. This behavioral setting triggers frequent switching between social and nonsocial actions in both animals, thereby generating frequent and varied behavioral choices. We quantified various social behavioral sequences reflecting flexible behavior (33) such as physical contact, dynamic approaches and escape behaviors, and relative position events. We also quantified dominance behavior and aggressiveness.
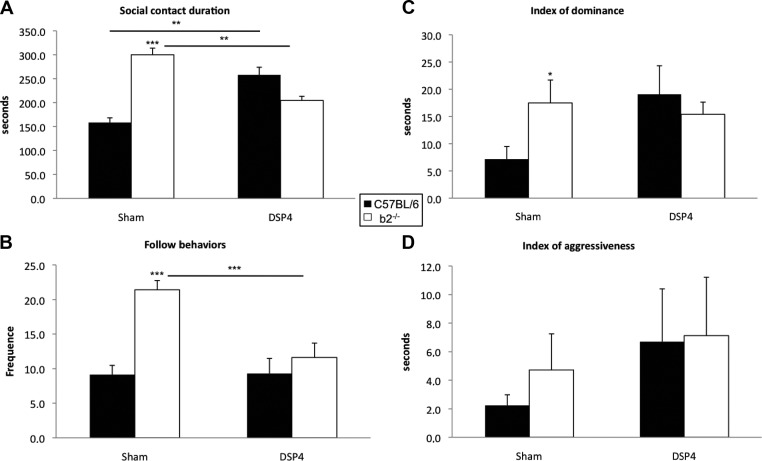
In agreement with our previous results (23, 24, 33, 43), C57BL/6 mice spent less time in social contact than β2−/− mice (P<0.0001) and made fewer follow behavioral sequences (P<0.0001; Fig. 1A, B).
Figure 1.
Effects of global NE lesion by injection of DSP-4 on social interaction and on social aggressive-like behavior in C57BL/6 mice (sham treated n=25, lesioned n=10) and β2−/− mice (sham treated n=21; lesioned n=8). A) Contact duration, showing the total time spent in social contact, which increases for C57BL/6 mice and decreases for β2−/− mice, after the lesion. B) Total number of follow behaviors. DSP-4 lesions produced a decrease of this behavior in β2−/− mice but had no effect in C57BL/6 mice. C) β2−/− mice show a higher index of dominance (paw control and aggressive grooming). D) Lack of effect of DSP-4 on aggressiveness (circling, bite, and tail rattling) in C57BL/6 and β2−/− mice. Histograms show mean ± se values. *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005.
In this social interaction paradigm, the time spent in social contact and the frequency of follow sequences are the first obvious features that are indicative of social behavior flexibility. A flexible behavior will thus be reflected by a reduction of frequency with time of some behavioral sequences, postures, or contact and by the emergence of novel behavioral features. Thus, these results corroborate the rigid social phenotype of β2−/− mice described previously (23, 24, 33, 43).
Global depletion of brain NE led to opposite effects in C57BL/6 and β2−/− mice during social testing. Specifically, the total time spent in social contact and the total number of follow sequences were significantly affected by the lesion (P<0.0001 and P=0.001, respectively) but differently according to genotype: in C57BL/6 mice, global NE depletion led to a 40% increase in the duration of social contact (P=0.003). By contrast, in β2−/− mice, NE depletion led to a 33% decrease in the duration of social contact (P=0.0007) and a 43% decrease in the number of follow sequences (P=0.0002; Fig. 1A, B).
β2−/− mice exhibited higher dominance scores compared with C57BL/6 mice (P=0.02) but a similar low level of aggressiveness (P=0.3317). Global NE depletion increased dominance behavior only in C57BL/6 mice (P<0.005) and had no significant effect on aggressive behavior in any group of mice (Fig. 1C, D).
Postmortem neurochemical evaluation
It is known that monoamine and ACh contribute to flexible behaviors mainly via prefrontal modulation. However, no information as to the levels of any of the major NT was available in β2−/− mice. We thus measured basal levels of DA, NE, 5-HT, and ACh, as well as their metabolites, in both groups of mice and tested the hypothesis that the opposite behavioral effects after global NE depletion could be explained by constitutive biochemical differences. The biochemical quantification was performed by HPLC on frozen punches of PFC, OFC, Acb, H, and A regions.
Basal levels of NE, DA, 5-HT, and ACh were increased in β2−/− mice compared with C57BL/6 mice in the PFC (P=0.0014, P=0.0075, P=0.0022, and P<0.0001, respectively; Fig. 2). Moreover, in the OFC and A, basal levels of NE and 5-HT were also increased. In the Acb, only NE levels were significantly higher, while in the H, only the DA levels were increased (Supplemental Fig. S1). Overall, the 5-HT activity was significantly increased in β2−/− mice (P=0.02), while the activity of NE and DA remained unchanged, as we could assess by measuring their respective metabolites (P=0.9866 and P=0.0991, respectively).
Figure 2.
Levels of monoamines and ACh in the PrL of C57BL/6 (n=12) and β2−/− mice (n=12). Tissue content of all monoamines and ACh increased in β2−/− mice compared with C57BL/6 mice. Histograms show mean ± se values. **P ≤ 0.005, ***P ≤ 0.0005.
Neuroanatomical evaluation
Neurochemical analysis revealed that basal levels of monoamines and ACh were higher in β2−/− mice. To elucidate whether this effect was associated with an increase in monoaminergic fiber density, we measured the density of TH+, 5-HT+, and NET+ fibers in the PFC of C57BL/6 and β2−/− mice. The β2−/− mice showed a significantly lower density of TH+, 5-HT+, and NET+ fibers (P=0.048, P=0.0001, and P=0.0047, respectively) compared with C57BL/6 mice (Supplemental Fig. S2). Therefore, increased basal levels of monoamines in the PrL was unlikely to be due to an increase in the density of monoaminergic fibers in this region. There was no effect of NE depletion on TH fiber density (P=0.201). By contrast, 5-HT fiber density was reduced in C57BL/6 mice after NE depletion (P=0.001).
Neurochemical and neuroanatomical analyses therefore indicated that β2−/− mice exhibited increased basal levels of monoamines, although the density of monoaminergic innervations was decreased. Moreover, the increase in basal neurotransmitter levels was not always followed by an increase in monoaminergic activity. Collectively, these results suggest that vesicular storage of monoamines may be increased in β2−/− mice.
Effect of NE depletion in the PrL on anxiety
NE depletion of the PrL had no significant effect on anxiety-related behavior in the dark-light paradigm in C57BL/6 or β2−/− genotypes. However, in the EPM, the percentage of time spent in open arms was altered differently in C57BL/6 and in β2−/− mice by PFC NE depletion (genotype X treatment F(1,43)=6.89, P=0.012). Post hoc t tests revealed that NE depletion of the PFC led to a significant anxiolytic effect in β2−/− mice (sham treatment: 19 ± 2%; NE depletion: 28 ± 2.76%; t=2.7, df=20, P=0.013) but had no effect in C57BL/6 mice (t=0.88, df=23, P=0.39).
Effect of NE depletion in the PrL on social behavior
In previous experiments, we showed that the profound alteration in social interaction exhibited by β2−/− mice was similar to that obtained after damage to the PrL of the PFC. Notably, we showed that expression of functional β2*nAChRs in the PrL of β2−/− mice was necessary and sufficient for restoring flexible social interaction (24). Here, we observed that global NE depletion produced opposite effects in social behavior of β2−/− and C57BL/6 mice. To further investigate the putative role of the NE transmission in the PrL, we carried out specific PrL NE depletion and tested its effect in the same social paradigm.
Dominant-like behaviors associated with β2−/− mice were not affected by PrL NE depletion (P=0.40, NS; Fig. 3). Dominant behavior was also not altered in C57BL/6 mice (P=0.1, NS; Fig. 3). By contrast, in C57BL/6 mice, the index of aggressiveness increased dramatically by 6-fold following the selective depletion of NE from the PrL (P=0.0002; Fig. 3). This was unlikely to be due to anxiogenic effects, as anxiety was not altered by NE PrL depletion in C57BL/6 mice (see above).
Figure 3.
Effects of NE PrL depletion on dominance and aggressive-like behavior in C57BL/6 (sham treated n=25; lesioned n=10) and β2−/− mice (sham treated n=21; lesioned n=8). β2−/− mice show a higher index of dominance (paw control and aggressive grooming, A) but not of aggressiveness (B) as compared with C57BL/6 mice. After lesion, there was a significant increase in aggressiveness in C57BL/6 mice but not in β2−/− mice. Histograms show mean ± se values. *P ≤ 0.05, ***P ≤ 0.0005.
The same results obtained after global NE lesion were found in β2−/− mice after specific NE PrL depletion. To investigate whether these effects were due to fine changes in the social repertoire, we assessed the key behavioral sequences of the social repertoire that were specifically altered by NE depletion. For this purpose, we analyzed social data with MiceProfiler (33) software that we designed for underpinning decision points during complex behavioral chains.
NE PrL depletion produced a similar effect than that of the global lesion in β2−/− mice, i.e., a 44% decrease in social contact duration (P<0.0001; Fig. 4) and a 36% decrease in the number of follow behaviors (P=0.007; Supplemental Fig. S3) compared with β2−/− sham-treated mice. A more detailed temporal analysis of some behavioral parameters indicated that the duration of social contact decreased steadily from the beginning of the experiment and followed a normal pattern in β2−/− mice after the PrL NE lesion (Fig. 4).
Figure 4.
Global effects of NE PrL depletion on social interaction in C57BL/6 (sham treated n=25; lesioned n=10) and β2−/− mice (sham treated n=21; lesioned n=8). Contact duration (top panel) decreased for β2−/− mice following the lesion but not for C57BL/6 mice. Detailed analysis (bottom panel) illustrates the restoration, in lesioned β2−/− mice, of the temporal evolution of the contact events throughout the 8-min experiment, as compared with β2−/− sham-treated mice. Histograms show mean ± se values. ***P ≤ 0.0005.
While the duration of social contact was not altered in C57BL/6 mice after NE PrL depletion, the global organization of behavior and the richness of repertoire were drastically modified (Fig. 5). These parameters were assessed by the construction of graphs representing the probability of transition from an event to another (the thicker the arrow, the more probable the event). The symbols representing both mice and their respective postures are provided in Supplemental Table S2. Arrows represent the string of events: preceding event to succeeding event. Transitions are computed for the first 4 min and the 4 last min of the experiment. Black arrows represent events that occur steadily (with the same probability) for the first and the last 4 min. Red and green arrows represent events that occur only in the 4 first and the 4 last minutes respectively. Transitional graphs showed an increased proportion of unchanged behavioral sequences (black arrows) in β2−/− mice as compared with C57BL/6 mice. After NE-PrL depletion, behaviors conserved with time (black arrows) were increased in C57BL/6 mice and decreased in β2−/− mice.
Figure 5.
Transitional behavioral graphs after prefrontal NE depletion in C57BL/6 (sham treated n=25; lesioned n=10) and β2−/− mice (sham treated n=21; lesioned n=8). These graphs represent the probability of transition from an event to another (the thicker the arrow, the more probable the event). The symbols representing both mice and their respective postures are provided in Supplemental Table S2. Arrows represent the string of events: preceding event to succeeding event. The transition is computed for the first 4 min and the 4 last min of the experiment. Black arrows represent events that occur steadily (with the same probability with an overlap of 1 σ of their respective sd) for the first and the last 4 min. Red and green arrows represent events that occur only in the 4 first and the 4 last minutes, respectively. Transitional graphs showed an impoverishment of the social repertoire in C57BL/6 mice after NE prefrontal depletion, with less various behavioral events than in sham-treated mice. Temporal evolution of behavioral sequences, illustrated by the number of red and green arrows, by comparison with events linked by black arrows, was also drastically lower in C57BL/6 mice after NE prefrontal depletion. Therefore, C57BL/6 mice exhibited more rigid social behavior after NE prefrontal depletion. By contrast, in β2−/− mice, NE PrL depletion favored a recovery of the temporal evolution of contact and follow events, thus reducing significantly rigid behaviors. Stop behaviors, which were significantly less frequent in β2−/− mice than in C57BL/6 mice, were restored after NE lesion.
Detailed social behavioral analyses allowed us to assess other key behavioral markers of flexibility beyond social contact duration and the frequency of follow sequences. Within the behavioral repertoire, we pinpointed two important markers of social flexibility (33): stop behaviors, which constitutes behavioral choice points. This parameter indicates a capability to stop moving and disengage from an ongoing action to make a novel movement based on environmental cues. The other marker is reflected by a relative position of both mice. In this posture, both mice are almost immobile, looking in opposite directions and are not touching or seeing each other. This back-to-back behavior is a risk-prone posture that provides the dyad with a full panoramic view of the environment, reflecting a form of cooperative social behavior. Both types of behaviors were previously shown to occur much less frequently in β2−/− mice compared with control mice (33).
Stop behaviors, which were significantly decreased in β2−/− mice compared with C57BL/6 mice (P<0.0001) were restored after PrL NE depletion (lesion effect in β2−/− mice P<0.0001; Fig. 6). The number of back-to-back sequences that was decreased in β2−/− mice compared with C57BL/6 mice (P=0.001) was also restored in β2−/− mice after NE PrL depletion (lesion effect in β2−/− mice, P=0.002; Fig. 6). These results show that specific NE PrL depletion normalized flexible social behavior in β2−/− mice by restoring social contact duration, follow sequences, immobility periods and back-to-back postures. By contrast, stop behaviors and back-to-back sequences, like contact duration, were not affected by NE depletion in C57BL/6 mice (depletion effect in C57BL/6 mice all P>0.4, NS).
Figure 6.
Detailed effects of NE PrL depletion on key elements of social interaction in C57BL/6 (sham treated n=25, lesioned n=10) and β2−/− mice (sham treated n=21, lesioned n=8). NE depletion of the PFC restored both immobility (A) and back-to-back (B) sequences in β2−/− mice, both behaviors drastically impaired before lesion. Histograms show mean ± se values. ***P ≤ 0.0005.
Furthermore, dominance and aggressiveness were shown to be functionally dissociated, with the former being modulated by the cholinergic nicotinic system and the latter potentiated by prefrontal NE but only in presence of functional β2*nAChRs.
DISCUSSION
In a social context, mice make multiple decisions that take into account their own motivations and choices, as well as the unpredictable actions of a conspecific. Such encounters maximize uncertainty, generating high attentional states (55) that bias behavior toward socially rewarding stimuli (56, 57). The social paradigm used in the present study requires flexible decision-making processes that depend on the functional integrity of the PFC and β2*nAChRs in this region (24). Here we demonstrated that global depletion of NE in the brain produced strongly divergent effects on social behavior in C57BL/6 and β2−/− mice. While it caused rigid social behavior in C57BL/6 mice, it restored behavioral flexibility in β2−/− mice.
It has been shown that prefrontal NE is necessary for attentional processing, especially when the task is novel and challenging, and stimuli are unpredictable (25, 58–60). Individuals, whether animals or humans, naturally orient to novel stimuli with high sensory salience, particularly when attentional demands are low (60, 61). NE signaling has been postulated to provide an “interrupt” (62) or “reset” signal (58) thereby allowing the flexible detection of an unexpected target in the environment. According to the hypothesis that NE modulates the salience of external stimuli (58, 60–62) and that an optimal level of NE activity is necessary to optimize attention and decision-making performance (31), our results show that β2−/− mice were more susceptible than C57BL/6 control mice to external stimuli during a social encounter. This susceptibility may be driven by disrupted NE transmission in the PrL and by the salience of social stimuli being augmented by prior social isolation (24). The prosocial behavior observed in β2−/− mice is likely due to a hypersalience of external stimuli, which in the social context is mainly generated by the congener. Thus, NE depletion appears to have normalized stimulus salience in β2−/− mice.
The loci of this effect appears to be the PFC and functional nAChRs, as specific NE PFC depletion normalized major social flexible behaviors (e.g., stop behaviors, contact duration and follow behaviors) in β2−/− mice. In addition, PrL-NE depletion restored back-to-back sequences that were virtually absent in these mice. Finally, we built transitional graphs (see Fig. 5) that illustrate the probability of transition from 1 behavioral state to another, confirming the more rigid behavior in β2−/− mice (33, 43), with a majority of behavioral sequences that remain unchanged throughout the experiment. Moreover, transitional graphs unraveled an impoverishment of the social repertoire in C57BL/6 mice after NE prefrontal depletion, with less various behavioral events than in sham-treated mice. These events were also more conserved and evolved less with time. Therefore, C57BL/6 mice exhibited more rigid social behavior after NE prefrontal depletion. By contrast, in β2−/− mice, NE PrL depletion favored a recovery of the temporal evolution of contact and follow events, thus reducing significantly rigid behaviors.
We have previously shown that β2−/− mice are hyperactive (63) and exhibit increased c-fos expression in the PFC, specifically when exposed to a novel environment (64). These findings indicate an increased arousal/vigilance state when mice are confronted with highly arousing stimuli, such as a novel social interaction (23), shown to trigger PFC activation (24). This increased arousal/vigilance state can be, in part, explained by the high basal levels of monoamines and ACh, observed especially in the PFC and that is likely to be due to an increased storage of these neurotransmitters.
In a previous work, we showed that whole-brain NE depletion increased global aggressiveness in C57BL/6 mice (52). Here, distinguishing aggressive and dominance behaviors, we found that global NE depletion affected neither aggressiveness nor dominance. By contrast, neurochemically selective NE depletion of the PrL led to a 6-fold increase in C57BL/6 control mouse aggressiveness, while it had no effect on β2−/− mice. Increased aggressiveness was associated with an impoverishment of the social repertoire. Thus, our data show that a lack of prefrontal NE transmission promotes aggressive-like behaviors. Furthermore, we report a functional dissociation between aggressiveness and dominance. β2−/− mice exhibited more dominant behavior than C57BL/6 mice, which was not perturbed by NE-PrL depletion, suggesting therefore that the dominant behavior we observed was related to the absence of β2*nAChRs. By contrast, the balance between basal levels of monoamines and of ACh appears to modulate aggressiveness, and this modulation requires functional β2*nAChRs. In β2−/− mice, which exhibited constitutively increased levels of monoamines and ACh in the PFC, NE depletion did not favor aggressiveness. By contrast, in C57BL/6 mice, NE-depletion led to a profound increase in aggressive-like behavior.
Our biochemical analysis showed that, in C57BL/6 mice, an increase in DA levels after global NE depletion is sufficient to cause the β2−/−-behavioral phenotype (see Fig. 1). Indeed, after NE global depletion in C57BL/6 mice, DA levels increased in the PFC (data not shown), suggesting an involvement of DA in prosocial behaviors. This may be relevant to an analogous behavioral profile in DA transporter-knockout (DAT−/−) mice, which shows increased levels of DA, hyperactivity, impaired decision-making, and behavioral rigidity that includes an inability to shift and adjust behaviors in a new context (65, 66).
Our current results may also be relevant to understanding how cholinergic and monoaminergic systems interact in the PFC to modulate social cognition in schizophrenia. The cholinergic system has been implicated in schizophrenia and mood disorders (67). One of the proposed contributions of the cholinergic system to the pathophysiology of schizophrenia is through an imbalance between the cholinergic and dopaminergic systems (68). Recent data suggest that cholinergic perturbations in schizophrenia are not simply due to changes in ACh levels but rather to the balance between activation of both nicotinic and muscarinic receptors, which collectively determine the functional outcome of central cholinergic stimulation (67, 69). While the putative role of α7* nAChRs in schizophrenia is controversial and not well established, β2*nAChRs have been shown to be up-regulated in this disorder (70), control epigenetic modifications (71), and also mediate the effects of cholinergic transmission on NE, 5-HT, and DA function in patients with schizophrenia (72). In particular, cholinergic modulation of DA release by β2*nAChR stimulation has been proposed to contribute specifically to schizophrenia symptoms (73–75). Furthermore, NE has been suggested to underlie the pathophysiogical mechanisms of schizophrenia, depression, and Parkinson's disease-associated depression through its regulatory interactions with the DA and serotonergic systems (76–81).
Our findings indicate constitutive hypermonoaminergia and hypercholinergia in β2−/− mice. Hyperdopaminergia was also observed in C57BL/6 mice after NE depletion and is likely to be sufficient to cause the β2−/−-prosocial phenotype in these mice. By contrast, in the absence of β2*nAChRs, there was no change in the basal level of DA or 5-HT after NE depletion, which corroborates the role of these receptors in modulating DA or 5-HT release. Therefore, these data are compatible with the notion that the hypervigilant states associated with the positive symptoms of schizophrenia are associated with an overactivity of brain NE function whereas the negative symptoms of this disorder are related to an underactivity of brain NE (76). In addition, our results suggest that β2*nAChRs may contribute to the pathophysiology of schizophrenia through complex and intricate underlying interactions between cholinergic, dopaminergic and noradrenergic systems in the PFC.
In summary, the present findings point to a clear and crucial role of β2*nAChRs in the modulation of monoamine and cholinergic ACh activity and their effect on social and flexible behavior. In particular, our findings indicate the NE innervation of the PFC has a pivotal role in inhibitory response control and adaptive behavior, as well as in the modulation of aggression, which depend on functional interactions with the cholinergic system in this region.
Supplementary Material
Acknowledgments
This work was partly supported by the Centre National de la Recherche Scientifique, an Agence pour la Recherche grant (to S.G. and J.C.O.-M.) and a chaire d'excellence from Paris Sud University (to S.G.). This work was partly supported by the Welcome Trust and Medical Research Council in support of the Behavioral and Clinical Neuroscience Institute at Cambridge University.
The authors thank Jean-Antoine Girault, Denis Hervé, and David Belin for the revision and for enriching discussions. The authors declare no conflicts of interest. Author contributions: R.C. and A.C. performed and analyzed experiments; J.X. performed neurochemical analysis; R.C. conducted statistical analyses and wrote the manuscript; Y.P. participated in the biochemical analyses; F.D.C and J.C.O.-M. created the tracking and analysis methods for automatical behavior analysis; J.D. and J.C.O.-M. wrote the manuscript; S.G. conceived the project, conducted statistical analysis and wrote the manuscript.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 5-HT
- 5-hydroxytryptamine (serotonin)
- 6-OHDA
- 6-hydroxydopamine
- α7*nAChR
- α7 homomeric neuronal nicotinic acetylcholine receptor
- β2−/−
- β2 knockout
- β2*nAChR
- β2-containing heteromeric neuronal nicotinic acetylcholine receptor
- A
- amygdala
- Acb
- nucleus accumbens
- ACh
- acetylcholine
- cPrL
- caudal prelimbic cortex
- DA
- dopamine
- DSP-4
- N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride
- EPM
- elevated plus maze
- H
- hippocampus
- HPLC
- high-performance liquid chromatography
- IH
- isolated host
- nAChR
- neuronal nicotinic acetylcholine receptor
- NE
- norepinephrine
- NET
- norepinephrine transporter
- OFC
- orbitofrontal cortex
- PB
- phosphate buffer
- PBS
- phosphate-buffered saline
- PFC
- prefrontal cortex
- PrL
- prelimbic cortex
- rPrL
- rostral prelimbic cortex
- SIT
- social interaction task
- SV
- social visitor
- TH
- tyrosine hydroxylase
REFERENCES
- 1. Siever L. J., Davis K. L. (2004) The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am. J. Psychiatry 161, 398–413 [DOI] [PubMed] [Google Scholar]
- 2. Fett A. K., Viechtbauer W., Dominguez M. D., Penn D. L., van O. S. J., Krabbendam L. (2011) The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev.35, 573–588 [DOI] [PubMed] [Google Scholar]
- 3. Cusi A. M., Nazarov A., Holshausen K., Macqueen G. M., McKinnon M. C. (2012) Systematic review of the neural basis of social cognition in patients with mood disorders. J. Psychiatry Neurosci. 37, 154–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasler G. (2010) Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry 9, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Connell L. A., Hofmann H. A. (2012) Evolution of a vertebrate social decision-making network. Science 336, 1154–1157 [DOI] [PubMed] [Google Scholar]
- 6. Robbins T. W., Granon S., Muir J. L., Durantou F., Harrison A., Everitt B. J. (1998) Neural systems underlying arousal and attention. Implications for drug abuse. Ann. N. Y. Acad. Sci. 21, 222–237 [PubMed] [Google Scholar]
- 7. Granon S., Floresco S. (2009) Functional neuroanatomy of flexible behaviors in mice and rats. In Endophenotypes of Psychiatric and Neurodegenerative Disorders in Rodent Models (Granon S., ed.) pp. 83–103, Transworld Research Network, Kerala, India [Google Scholar]
- 8. Dalley J. W., Cardinal R. N., Robbins T. W. (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 28, 771–784 [DOI] [PubMed] [Google Scholar]
- 9. Arnsten A. F., Li B. M. (2005) Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry 57, 1377–1384 [DOI] [PubMed] [Google Scholar]
- 10. Schliebs R., Arendt T. (2011) The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 221, 555–563 [DOI] [PubMed] [Google Scholar]
- 11. Briley M. S., Changeux J. P. (1977) Isolation and purification of the nicotinic acetylcholine receptor and its functional reconstitution into a membrane environment. Int. Rev. Neurobiol. 20, 31–63 [DOI] [PubMed] [Google Scholar]
- 12. Gotti C., Zoli M., Clementi F. (2006) Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci. 27, 482–491 [DOI] [PubMed] [Google Scholar]
- 13. Changeux J. P. (2010) Allosteric receptors: from electric organ to cognition. Annu. Rev. Pharmacol. Toxicol. 50, 1–38 [DOI] [PubMed] [Google Scholar]
- 14. Vizi E. S., Lendvai B. (1999) Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res. Rev. 30, 219–235 [DOI] [PubMed] [Google Scholar]
- 15. Mansvelder H. D., van Aerde K. I., Couey J. J., Brussaard A. B. (2006) Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology 184, 292–305 [DOI] [PubMed] [Google Scholar]
- 16. Livingstone P. D., Wonnacott S. (2009) Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochem. Pharmacol. 78, 744–755 [DOI] [PubMed] [Google Scholar]
- 17. Coura R. S., Granon S. (2012) Prefrontal neuromodulation by nicotinic receptors for cognitive processes. Psychopharmacology 221, 1–18 [DOI] [PubMed] [Google Scholar]
- 18. Taly A., Corringer P. J., Guedin D., Lestage P., Changeux J. P. (2009) Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 8, 733–750 [DOI] [PubMed] [Google Scholar]
- 19. Guillem K, Bloem B., Poorthuis R. B., Loos M., Smit A.B., Maskos U., Spijker S., Mansvelder H. D. (2011) Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science 333, 888–891 [DOI] [PubMed] [Google Scholar]
- 20. Granon S., Poucet B., Thinus-Blanc C., Changeux J. P., Vidal C. (1995) Nicotinic and muscarinic receptors in the rat prefrontal cortex: differential roles in working memory, response selection and effortful processing. Psychopharmacology 119, 139–144 [DOI] [PubMed] [Google Scholar]
- 21. Chan W. K., Wong P. T., Sheu F. S. (2007) Frontal cortical alpha7 and alpha4beta2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology 52, 1641–1649 [DOI] [PubMed] [Google Scholar]
- 22. Picciotto M. R., Zoli M., Rimondini R., Léna C., Marubio L. M., Pich E. M., Fuxe K., Changeux J. P. (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177 [DOI] [PubMed] [Google Scholar]
- 23. Granon S., Faure P., Changeux J. P. (2003) Executive and social behaviors under nicotinic receptor regulation. Proc. Natl. Acad. Sci. U. S. A. 100, 9596–9601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avale M. E, Chabout J., Pons S., Serreau P., De Chaumont F., Olivo-Marin J. C., Bourgeois J. P., Maskos U., Changeux J. P., Granon S. (2011) Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J. 25, 2145–2155 [DOI] [PubMed] [Google Scholar]
- 25. Aston-Jones G., Rajkowski J., Cohen J. (1999) Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 46, 1309–1320 [DOI] [PubMed] [Google Scholar]
- 26. Azam L., McIntosh J. M. (2006) Characterization of nicotinic acetylcholine receptors that modulate nicotine-evoked [3H]norepinephrine release from mouse hippocampal synaptosomes. Mol. Pharmacol. 70, 967–976 [DOI] [PubMed] [Google Scholar]
- 27. Le Novère N., Corringer P. J., Changeux J. P. (2002) The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 53, 447–456 [DOI] [PubMed] [Google Scholar]
- 28. O'Leary K. T., Leslie F. M. (2003) Developmental regulation of nicotinic acetylcholine receptor-mediated [3H]norepinephrine release from rat cerebellum. J. Neurochem. 84, 952–959 [DOI] [PubMed] [Google Scholar]
- 29. O'Leary K. T., Leslie F. M. (2006) Enhanced nicotinic acetylcholine receptor-mediated [3H]norepinephrine release from neonatal rat hypothalamus. Neuropharmacology 50, 81–88 [DOI] [PubMed] [Google Scholar]
- 30. Li X., Eisenach J. C. (2002) Nicotinic acetylcholine receptor regulation of spinal norepinephrine release. Anesthesiology 96, 1450–1456 [DOI] [PubMed] [Google Scholar]
- 31. Aston-Jones G., Cohen J. D. (2005) Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 493, 99–110 [DOI] [PubMed] [Google Scholar]
- 32. Tait D. S., Brown V. J., Farovik A., Theobald D. E., Dalley J. W., Robbins T. W. (2007) Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur. J. Neurosci. 25, 3719–3724 [DOI] [PubMed] [Google Scholar]
- 33. De Chaumont F., Coura R. D., Serreau P., Cressant A., Chabout J., Granon S., Olivo-Marin J C. (2012) Computerized video analysis of social interactions in mice. Nat. Meth. 9, 410–417 [DOI] [PubMed] [Google Scholar]
- 34. Maskos U., Molles B. E., Pons S., Besson M., Guiard B. P., Guilloux J. P., Evrard A., Cazala P., Cormier A., Mameli-Engvall M., Dufour N., Cloëz-Tayarani I., Bemelmans A. P., Mallet J., Gardier A. M., David V., Faure P., Granon S., Changeux J. P. (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107 [DOI] [PubMed] [Google Scholar]
- 35. Avale M. E., Faure P., Pons S., Robledo P., Deltheil T., David D. J., Gardier A. M., Maldonado R., Granon S., Changeux J. P., Maskos U. (2008) Interplay of beta2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proc. Natl. Acad. Sci. U. S. A. 105, 15991–15996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Picciotto M. R., Zoli M., Léna C., Bessis A., Lallemand Y., Le Novère N., Vincent P., Pich E. M., Brûlet P., Changeux J. P. (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374, 65–67 [DOI] [PubMed] [Google Scholar]
- 37. Anonymous (1997) Mutant mice and neuroscience: recommendations concerning genetic background. Banbury conference on genetic background in mice. Neuron 19, 755–759 [DOI] [PubMed] [Google Scholar]
- 38. Rorabacher D. B. (1991) Statistical treatment for rejection of deviant values: critical values of dixon q parameter and related subrange ratios at the 95 percent confidence level. Anal. Chem. 63, 139–146 [Google Scholar]
- 39. O'Leary O. F., Bechtholt A. J., Crowley J. J., Valentino R. J., Lucki I. (2007) The role of noradrenergic tone in the dorsal raphe nucleus of the mouse in the acute behavioral effects of antidepressant drugs. Eur. Neuropsychopharmacol. 17, 215–226 [DOI] [PubMed] [Google Scholar]
- 40. Paxinos G., Franklin K. B. (2001) The Mouse Brain in Stereotaxic Coordinates, Academic Press, New York [Google Scholar]
- 41. Chabout J., Serreau P., Ey E., Bellier L., Aubin T., Bourgeron T., Granon S. (2012) Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS One 7, e29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jamain S., Radyushkin K., Hammerschmidt K., Granon S., Boretius S., Varoqueaux F., Ramanantsoa N., Gallego J., Ronnenberg A., Winter D., Frahm J., Fischer J., Bourgeron T., Ehrenreich H., Brose N. (2008) Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. U. S. A. 105, 1710–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Serreau P., Chabout J., Suarez S. V., Naudé J., Granon S. (2011) Beta2-containing neuronal nicotinic receptors as major actors in the flexible choice between conflicting motivations. Behav. Brain Res. 225, 151–159 [DOI] [PubMed] [Google Scholar]
- 44. Golani I., Benjamini Y., Eilam D. (1993) Stopping behavior: constraints on exploration in rats (Rattus norvegicus). Behav. Brain Res. 53, 21–33 [DOI] [PubMed] [Google Scholar]
- 45. Gallistel C. R., King A., McDonald R. (2004) Sources of variability and systematic error in mouse timing behavior. J. Exp. Psychol. Anim. Behav. Process. 30, 3–16 [DOI] [PubMed] [Google Scholar]
- 46. Layne J. E., Barnes W. J., Duncan L. M. (2003) Mechanisms of homing in the fiddler crab Uca rapax. 2. Information sources and frame of reference for a path integration system. J. Exp. Biol. 206, 4425–4442 [DOI] [PubMed] [Google Scholar]
- 47. Maubourguet N., Lesne A., Changeux J. P., Maskos U., Faure P. (2008) Behavioral sequence analysis reveals a novel role for beta2* nicotinic receptors in exploration. PLoS Compt. Biol. 4, e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Audet M.-C., Anisman H. (2010) Neuroendocrine and neurochemical impact of aggressive social interactions in submissive and dominant mice: implications for stress-related disorders. Int. J. Neuropsychopharmacol. 13, 361–372 [DOI] [PubMed] [Google Scholar]
- 49. Giammanco M., Tabacchi G., Giammanco S., Di Majo D, Guardia M. L. (2005). Testosterone and aggressiveness. Med. Sci. Monit. 11, RA136–RA145 [PubMed] [Google Scholar]
- 50. Crowcroft P., Rowe F. P. (1963) Social organization and territorial behaviour in the wild house mouse. Proc. Zool. Soc. Lond. 140, 517–531 [Google Scholar]
- 51. Crawley J. N. (2000) What's Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice, John Wiley & Sons, New York [Google Scholar]
- 52. Cambon K., Dos-Santos Coura R., Groc L., Carbon A., Weissmann D., Changeux J. P., Pujol J. F., Granon S. (2010) Aggressive behavior during social interaction in mice is controlled by the modulation of tyrosine hydroxylase expression in the prefrontal cortex. Neuroscience 171, 840–885 [DOI] [PubMed] [Google Scholar]
- 53. Dalley J. W., Theobald D. E., Eagle D. M., Passeti F., Robbins T. W. (2002) Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacolgy 26, 716–728 [DOI] [PubMed] [Google Scholar]
- 54. Dalley J. W., McGaughy J., O'Connell M. T., Cardinal R. N., Levita L., Robbins T. W. (2001) Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J. Neurosci. 21, 4908–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu A. J., Dayan P. (2005) Uncertainty, neuromodulation, and attention. Neuron 46, 681–692 [DOI] [PubMed] [Google Scholar]
- 56. Kohls G., Peltzer J., Herpertz-Dahlmann B., Konrad K. (2009) Differential effects of social and non-social reward on response inhibition in children and adolescents. Dev. Sci. 12, 614–625 [DOI] [PubMed] [Google Scholar]
- 57. King H. M., Kurdziel L. B., Meyer J. S., Lacreuse A. (2012) Effects of testosterone on attention and memory for emotional stimuli in male rhesus monkeys. Psychoneuroendocrinology 37, 396–409 [DOI] [PubMed] [Google Scholar]
- 58. Bouret S., Sara S. J. (2005) Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582 [DOI] [PubMed] [Google Scholar]
- 59. McGaughy J., Ross R. S., Eichenbaum H. (2008) Noradrenergic, but not cholinergic deafferentation of prefrontal cortex impairs atentional set-shifting. Neuroscience 153, 53–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ventura R., Latagliata E. C., Morrone C., La Mela I., Puglisi-Allegra S. (2008) Prefrontal norepinephrine determines attribution of “high” motivational salience. PLoS One 3, e3044–e3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Corbetta M., Patel G., Shulman G. L. (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dayan P., Yu A. J. (2006) Phasic norepinephrine: a neural interrupt signal for unexpected events. Network 17, 335–350 [DOI] [PubMed] [Google Scholar]
- 63. Granon S., Changeux J. P. (2006) Attention-deficit/hyperactivity disorder: a plausible mouse model? Acta Paediatr. 95, 645–649 [DOI] [PubMed] [Google Scholar]
- 64. Bourgeois J. P., Meas-Yeadid V., Lesourd A. M., Faure P., Pons S., Maskos U., Changeux J. P., Olivo-Marin J. C., Granon S. (2012) Modulation of the mouse prefrontal cortex activation by neuronal nicotinic receptors during novelty exploration but not by exploration of a familiar environment. Cereb. Cortex 22, 1007–1015 [DOI] [PubMed] [Google Scholar]
- 65. Morice E., Billard J. M., Denis C., Mathieu F., Betancur C., Epelbaum J., Giros B., Nosten-Bertrand M. (2007) Parallel loss of hippocampal LTD and cognitive flexibility in a genetic model of hyperdopaminergia. Neuropsychopharmacology 32, 2108–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dreher J. C., Kohn P., Kolachana B., Weinberger D. R., Berman K. F. (2009) Variation in dopamine genes influences responsivity of the human reward system. Proc. Nat. Accad. Sci. 106, 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scarr E., Billard J. M., Denis C., Mathieu F., Betancur C., Epelbaum J., Giros B., Nosten-Bertrand M. (2013) Cholinergic connectivity: it's implications for psychiatric disorders. Front. Cell. Neurosci. 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tandon R., Greden J. F. (1989) Cholinergic hyperactivity and negative schizophrenic symptoms. A model of cholinergic/dopaminergic interactions in schizophrenia. Arch. Gen. Psychiatry. 46, 745–753 [DOI] [PubMed] [Google Scholar]
- 69. Lucas-Meunier E, Fossier P., Baux G., Amar M. (2003) Cholinergic modulation of the cortical neuronal network. Pflügers Arch. 446, 17–29 [DOI] [PubMed] [Google Scholar]
- 70. Martin-Ruiz C. M., Haroutunian V. H., Long P., Young A. H., Davis K. L., Perry E. K., Court J. A. (2003) Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol. Psychiatry 54, 1222–1233 [DOI] [PubMed] [Google Scholar]
- 71. Maloku E., Kadriu B., Zhubi A., Dong E., Pibiri F., Satta R., Guidotti A. (2011) Selective α4β2 nicotinic acetylcholine receptor agonists target epigenetic mechanisms in cortical GABAergic neurons. Neuropsychopharmacology 36, 1366–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Briand L. A., Gritton H., Howe W. M., Young D. A., Sarter M. (2007) Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog. Neurobiol. 83, 69–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou F. M., Liang Y., Dani J. A. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 4, 1224–1229 [DOI] [PubMed] [Google Scholar]
- 74. Toth E., Sershen H., Hashim A., Vizi E. S., Lajtha A. (1992) Effect of nicotine on extracellular levels of neurotransmitters assessed by microdialysis in various brain regions: role of glutamic acid. Neurochem. Res. 17, 265–271 [DOI] [PubMed] [Google Scholar]
- 75. Marshall D. L., Redfern P. H., Wonnacott S. (1997) Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J. Neurochem. 68, 1511–1519 [DOI] [PubMed] [Google Scholar]
- 76. Yamamoto K., Hornykiewicz O. (2004) Proposal for a noradrenaline hypothesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 28, 913–922 [DOI] [PubMed] [Google Scholar]
- 77. Siuta M. A., Robertson S. D., Kocalis H., Saunders C., Gresch P. J., Khatri V., Shiota C., Kennedy J. P., Lindsley C. W., Daws L. C., Polley D. B., Veenstra-Vanderweele J., Stanwood G. D., Magnuson M. A., Niswender K. D., Galli A. (2010) Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 8, e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Frånberg O., Marcus M. M., Svensson T. H. (2012) Involvement of 5-HT(2A) receptor and α(2)-adrenoceptor blockade in the asenapine-induced elevation of prefrontal cortical monoamine outflow. Synapse 66, 650–660 [DOI] [PubMed] [Google Scholar]
- 79. Gamo N. J., Arnsten A. F. (2011). Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav. Neurosci. 125, 282–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nutt D., Demyttenaere K., Janka Z., Aarre T., Bourin M., Canonico P. L., Carrasco J. L., Stahl S. (2007) The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J. Psychopharmacol. 21, 461–471 [DOI] [PubMed] [Google Scholar]
- 81. Moret C., Briley M. (2011) The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 7(Suppl. 1), 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.