Abstract
Background
A major challenge for randomized phase III oncology trials is the frequent low rates of patient enrollment, resulting in high rates of premature closure due to insufficient accrual.
Purpose
We conducted a pilot study to determine the extent of trial closure due to poor accrual, feasibility of identifying trial factors associated with sufficient accrual, impact of redesign strategies on trial accrual, and accrual benchmarks designating high failure risk in the clinical trials cooperative group (CTCG) setting.
Methods
A subset of phase III trials opened by five CTCGs between August 1991 and March 2004 was evaluated. Design elements, experimental agents, redesign strategies, and pretrial accrual assessment supporting accrual predictions were abstracted from CTCG documents. Percent actual/predicted accrual rate averaged per month was calculated. Trials were categorized as having sufficient or insufficient accrual based on reason for trial termination. Analyses included univariate and bivariate summaries to identify potential trial factors associated with accrual sufficiency.
Results
Among 40 trials from one CTCG, 21 (52.5%) trials closed due to insufficient accrual. In 82 trials from five CTCGs, therapeutic trials accrued sufficiently more often than nontherapeutic trials (59% vs 27%, p = 0.05). Trials including pretrial accrual assessment more often achieved sufficient accrual than those without (67% vs 47%, p = 0.08). Fewer exclusion criteria, shorter consent forms, other CTCG participation, and trial design simplicity were not associated with achieving sufficient accrual. Trials accruing at a rate much lower than predicted (<35% actual/predicted accrual rate) were consistently closed due to insufficient accrual.
Limitations
This trial subset under-represents certain experimental modalities. Data sources do not allow accounting for all factors potentially related to accrual success.
Conclusion
Trial closure due to insufficient accrual is common. Certain trial design factors appear associated with attaining sufficient accrual. Defining accrual benchmarks for early trial termination or redesign is feasible, but better accrual prediction methods are critically needed. Future studies should focus on identifying trial factors that allow more accurate accrual predictions and strategies that can salvage open trials experiencing slow accrual.
Introduction
The National Cancer Institute (NCI) funded Clinical Trials Cooperative Groups (CTCG) serve the critical role of performing many pivotal phase III trials that ultimately advance clinical cancer care and prevention. However, only 3–5% of adult cancer patients in the US participate in NCI – sponsored clinical research trials [1,2]. In addition to, or perhaps due to these low patient participation rates, a substantial number of phase III trials close due to insufficient patient accrual. Other trials are open longer than predicted to reach accrual goals. Both are very costly in terms of taxpayer dollars and missed opportunities to advance cancer care. In a time of limited research funds and tremendous pressure to translate laboratory science rapidly to human therapies, trial accrual has been cast to the forefront of problems facing clinical oncology research. Approaches to accurately predict which trials are likely to have sufficient recruitment are critical to our ability to effectively manage limited resources and advance clinical oncology.
Reasons for low accrual have been thoughtfully studied and are summarized in Table 1 as patient and physician barriers [1,3–8]. Despite prior efforts to improve accrual, the proportion of adult cancer patients enrolling in clinical trials has remained stagnant for decades [1,2,9]. Yet, studies show that a greater proportion of potential patients hold a positive view of clinical trials and would seriously consider trial participation [10,11]. New approaches to improve trial accrual and trial planning are clearly needed. The Institute of Medicine (IOM) report ‘A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program’ released in April 2010 emphasizes improving the means of prioritizing, selecting, supporting, and completing cancer clinical trials as one of its four main goals [12]. New research in trial design and prioritization pertinent to accrual may be of high utility in informing how best to interpret and apply these new IOM committee recommendations.
Table 1. Previously studied barriers to clinical trial accrual encountered after trial opening.
| Physician barriers | Patient barriers |
|---|---|
| Inadequate support staff; limited resources | Burdensome demands on time |
| Time constraints | Additional costs |
| Inadequate reimbursement or insurance denial | Preferences for certain treatment |
| Lack of an interesting research question | Unavailability of an applicable trial |
| Lack of specific clinical trial awareness | Lack of awareness about trials |
| Insufficient reward for participation | Stringent eligibility criteria |
| Bias regarding patient eligibility or approachability | Clinician's influence |
| Concerns about exposing patient to undue burdens | Concerns about uncertainty of treatment, randomization |
| Preference for certain treatment | Concerns about toxicity |
| Loss of professional autonomy | Concerns about understanding consent |
| Consent-related difficulties | Transportation and other logistical constraints |
We conducted a pilot study assessing the extent of premature phase III trial termination secondary to poor accrual, and determining the feasibility of identifying trial factors associated with sufficient accrual. Our study also evaluated benchmarks to identify trials at risk for failed accrual, in order to allow timely trial closure or redesign. Finally, our study sought to assess whether redesign strategies initiated in the setting of poor accrual were associated ultimately with sufficient trial accrual. We present these data and outline future directions for research.
Methods
Trial set assembly
Initially, all phase III trials coordinated by a single CTCG and open between January 1, 1998, and October 11, 2002, were evaluated for accrual sufficiency. The proportion of trials requiring closure due to insufficient accrual reported here is from this group of trials only. A preliminary evaluation of trial factors associated with sufficient accrual prompted a study expansion.
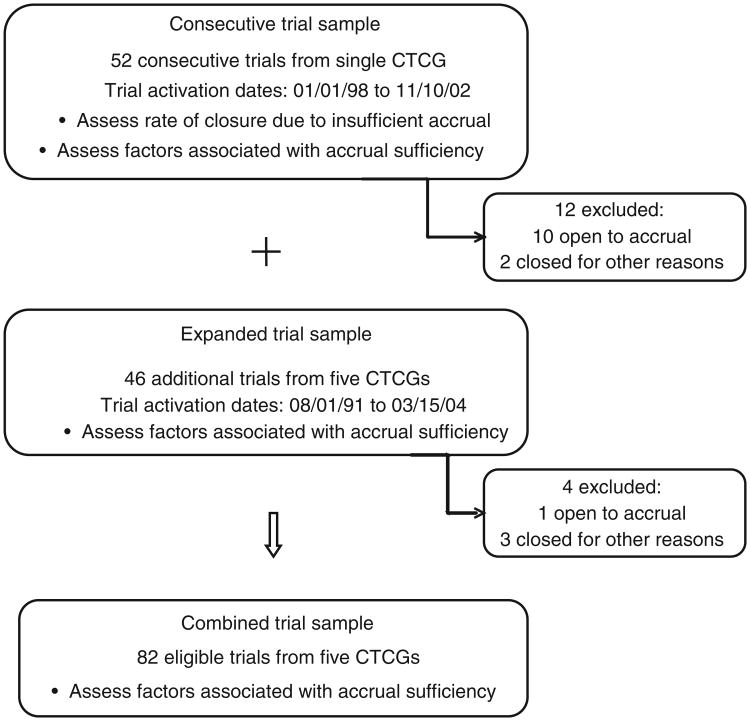
To evaluate the feasibility of identifying trial factors associated with sufficient accrual across several CTCGs, the trial sample was expanded to include a convenience sample of phase III trials coordinated by the original and four other CTCGs. These trials were open between January 8, 1991 and March 15, 2004 and offered accessible protocol documents. Eight to ten trials from each CTCG were selected to include a variety of disease sites, therapeutic and preventative interventions, and accrual sufficiency. Because the expanded trial set was selected in part based on accrual sufficiency, these additional trials could not be added to the consecutive trial set for purposes of calculating insufficient accrual closure rates. The combined sample of trials, however, could be evaluated for feasibility of identifying factors associated with sufficient accrual. The trial sample schema is represented in Figure 1. Participating CTCGs were Cancer and Leukemia Group B (CALGB), Eastern Cooperative Oncology Group (ECOG), North Central Cancer Treatment Group (NCCTG), National Surgical and Adjuvant Breast and Bowel Group (NSABP), and Southwest Oncology Group (SWOG).
Figure 1. Study sample schema.
Trial information was abstracted by the primary author and a research assistant from protocol documents, design schema, and other documents available through the CTCG, with permission from the CTCG leadership. The total predicted patient accrual number and predicted accrual duration were obtained. The actual number of patients enrolled and actual trial duration were used to calculate an average monthly accrual rate. Actual trial accrual duration was determined by activation and closure dates since no uniform run-in period could be attributed to all trials. A percent actual/predicted accrual rate averaged per month was calculated for each study.
Closed trials were identified either as having sufficient or insufficient accrual, based on the reason for termination documented by the CTCG. Trials with sufficient accrual included all trials which had met target accrual or had been closed based on results at an interim analysis, since these trials had attained sufficient accrual to address their respective scientific question. Trials with insufficient accrual were unable to address their scientific question and were documented as having closed due to inadequate accrual. Trials that remained active at the time of analysis were excluded. Trials closed due to circumstances unrelated to accrual or interim analyses were also excluded.
Additional trial data reflected a priori hypotheses about factors impacting accrual success as supported by the literature on accrual barriers [3]. These data included trial type (therapeutic vs nontherapeutic), study disease characteristics, experimental agent characteristics, trial design elements, trial participation complexity, redesign features, and pretrial accrual assessment. The presence of a pretrial accrual assessment was defined as any specific documentation in the protocol supporting the feasibility of predicted accrual goals. A redesign was considered significant if it substantially altered the applicable study population or resulted in a change in statistical considerations. Trials were examined for addenda broadening eligibility criteria, modifying sample size, altering endpoints, and changing the control or experimental arm(s). The addition of other CTCGs after trial opening was not included as a redesign, since the reasons for intergroup trials are varied and often not clearly documented. Redesigns instituted in the setting of poor accrual were examined for their association with accrual status at trial closure.
Initial summary analyses included univariate and bivariate summaries. Accrual status as sufficient or insufficient was evaluated by trial features using Pearson's chi-squared test. All reported probability values are two-sided. Given the exploratory nature of this study, multivariate analysis to identify independent predictors of accrual sufficiency was not carried out. Statistical analyses were performed with SPSS software (SPSS Inc., Chicago, IL) and STATA software (STATA Corp., College Station, TX). This study was approved by the University of Virginia Institutional Review Board (IRB-HSR #12582).
Results
Trial termination due to insufficient accrual
Trial termination related to accrual was evaluated in the 52 consecutive phase III trials coordinated by a single CTCG within a 5-year timeframe. Of these 52 trials, 12 were excluded from analysis: two trials closed for reasons unrelated to accrual and 10 were still open to accrual at the time of analysis. Of the remaining 40 trials, 19 (47.5%) closed having attained sufficient accrual and 21 (52.5%) closed due to insufficient accrual.
Study trials' profile and reasons for trial closure unrelated to accrual
The expanded sample of trials evaluated for factors related to accrual sufficiency included an additional 46 phase III trials from all participating CTCGs. Of these 46 trials, 4 were excluded from final analysis: 3 trials closed for reasons unrelated to accrual and 1 trial was still open to accrual at the time of analysis. Thus, of the 98 trials identified between the consecutive and expanded trial sets, 87 were closed to accrual at the time of analysis. An additional five trials (5%) were closed early for reasons unrelated to accrual or interim analysis results. Reasons for closure in both the consecutive and expanded samples included a manufacturing company electing to stop study agent development, expiration of antibody, or results from another study obviating the need for another clinical trial (Figure 1). The following results pertain to these 82 trials and are profiled in Table 2.
Table 2. Study variables, definitions, and profile.
| Trial feature | Definition of trial feature | Trials, % (n) |
|---|---|---|
| Trial type | ||
| Therapeutic | Study agent treated cancer specifically | 87 (71) |
| Nontherapeutic | Study agent treated cancer-related symptoms or tested in prevention trials | 13 (11) |
| Trial design | ||
| Simple | Single randomization point into ≥ 2 arms | 70 (57) |
| Complex | Not fitting definition of simple design | 30 (25) |
| Disease category | ||
| Hematologic | 17 (14) | |
| Solid organ | 79 (65) | |
| None or mixed | 4 (3) | |
| Disease site | ||
| Breast | 28 (23) | |
| GI | 21 (17) | |
| Leukemia/Lymphoma | 17 (14) | |
| Lung | 6 (5) | |
| GU | 6 (5) | |
| Head and neck | 6 (5) | |
| Others | 16 (13) | |
| Experimental study agent | ||
| Cytotoxic chemotherapy | 46 (38) | |
| Immunotherapy/BRM | 24 (20) | |
| Radiation | 7 (6) | |
| Surgery | 3 (2) | |
| Other | 7 (5) | |
| Nontherapeutic | 13 (11) | |
| Pretrial accrual assessment | Presence of specific documentation in protocol supporting the feasibility of predicted accrual goals | |
| Yes/No | Yes | 40 (33) |
| Placebo control arm | Presence of a placebo control arm | |
| Yes/No | Yes | 16 (13) |
| Observation control arm | Presence of an observation control arm | |
| Yes/No | Yes | 7 (6) |
| Other trial group participation | Participation of other cooperative trial group(s) | |
| Yes/No | Yes | 60 (49) |
| Entry criteria, number | All entry criteria separately counted | |
| ≤20 | 28 (23) | |
| 21–30 | 39 (32) | |
| >30 | 33 (27) | |
| Consent page length | ||
| ≤5 | 51 (42) | |
| >5 | 49 (40) | |
| Redesign of trial | Documentation of trial redesigned during courseof trial | |
| Yes/No | Yes | 32 (27) |
| Cooperative trial group | ||
| A | 52 (43) | |
| B | 12 (10) | |
| C | 13 (11) | |
| D | 11 (9) | |
| E | 11 (9) | |
Trial features associated with accrual sufficiency
This sample of 82 trials included 37 trials with insufficient accrual (45%) and 45 trials with sufficient accrual (55%). Among trials with sufficient accrual, four were closed at an interim analysis. Table 3 presents each trial feature present at trial inception and its association with sufficient or insufficient accrual. Trials testing immunotherapy or biologic response modifier agents and chemotherapy trials had sufficient accrual about 60% of the time. Trials using other types of study interventions not only met with mixed success but also represented a small subset of trials. Both the two surgical trials attained sufficient accrual. Of the six radiation therapy trials, only two had sufficient accrual. All four prevention trials ended with insufficient accrual.
Table 3. Association of trial features with accrual sufficiency.
| Trial feature | Sufficient accrual, % (n) | p-Value | |
|---|---|---|---|
| Trial type | Therapeutic (n = 71) | 59 (42) | |
| Nontherapeutic (n = 11) | 27 (3) | 0.05 | |
| Trial design | Simple (n = 57) | 53 (30) | |
| Complex (n = 25) | 60 (15) | 0.54 | |
| Disease category | Hematologic (n = 14) | 57 (8) | |
| Solid organ (n = 65) | 55 (36) | ||
| None (n =3) | 33 (1) | 0.90a | |
| Disease site | Breast (n = 23) | 65 (15) | |
| GI (n = 17) | 59 (10) | ||
| Leukemia/Lymphoma | 57 (8) | ||
| (n = 14) | 43 (12) | 0.43 | |
| Others (n = 28) | |||
| Study agent | Immunotherapy/BRM (n = 20) | 65 (13) | |
| Chemotherapy (n = 38) | 61 (23) | ||
| Others (n = 24) | 38 (9) | 0.12 | |
| Eligibility criteria | ≤20 (n = 23) | 52 (12) | |
| 21–30 (n = 32) | 47 (15) | ||
| >30 (n = 27) | 67 (18) | 0.30 | |
| Consent page | ≤5 (n = 42) | 48 (20) | |
| length | >5 (n = 40) | 63 (25) | 0.18 |
| Placebo arm | Yes (n = 13) | 38 (5) | |
| No (n = 69) | 58 (40) | 0.20 | |
| Observation arm | Yes (n = 6) | 50 (3) | |
| No (n = 76) | 55 (42) | 0.80 | |
| Other trial groups | Yes (n = 49) | 53 (26) | |
| participate | No (n = 33) | 58 (19) | 0.69 |
| Pretrial accrual | Yes (n = 33) | 67 (22) | |
| assessment | No (n = 49) | 47 (23) | 0.08 |
Solid organ vs hematologic.
Overall, only 40% of trials included an identifiable pretrial accrual assessment, defined as any specific documentation in the protocol supporting the feasibility of the predicted accrual goals. However, 49% of trials with sufficient accrual included a pretrial accrual assessment as compared to 30% of trials with insufficient accrual (p = 0.08). Placebo-controlled and observation-controlled trials resulted in insufficient accrual approximately 50–60% of the time. Fewer exclusion criteria, shorter consent forms, additional trial group participation, and trial design simplicity were not associated with sufficient accrual.
Benchmarks for accrual sufficiency
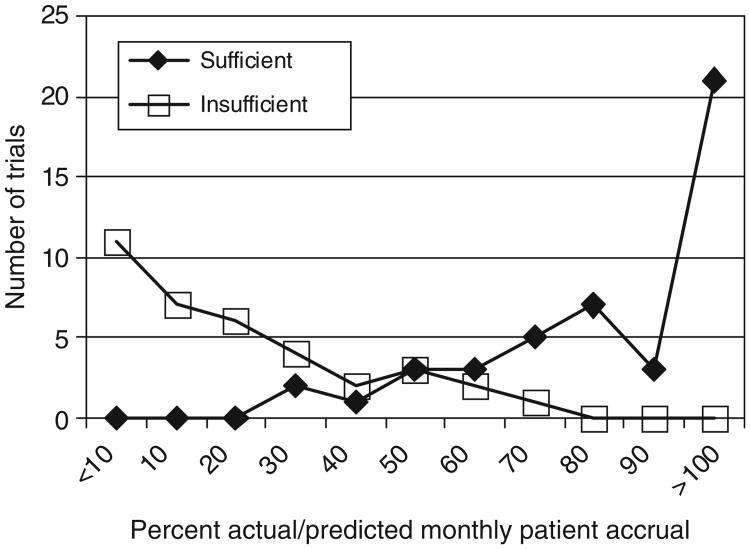
The percent actual versus predicted patient accrual ratio averaged on a monthly basis was calculated for each trial (Figure 2). Trials accruing at a rate less than 35% actual/predicted average monthly accrual rate were consistently closed due to poor accrual and only 10% of trials with <50% actual/predicted average monthly accrual rate ultimately attained sufficient accrual. Of three trials with <50% actual/predicted average monthly accrual rate that closed with sufficient accrual, two underwent redesign and one was closed early due to interim analysis results. In contrast, 88% of trials accruing at >50% actual/predicted average monthly accrual rate achieved sufficient accrual.
Figure 2. Accrual ratio for trials by accrual sufficiency at trial closure. All trials were plotted based on original accrual predictions.
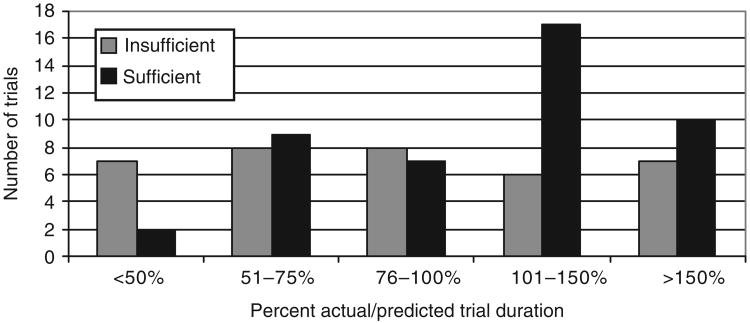
In terms of actual as compared to predicted duration for accrual completion, 50% of trials ran longer than predicted, with 21% running more than 150% of the predicted duration. Figure 3 shows the actual duration as a percent of the predicted duration for these trials. Among the trials that closed due to insufficient accrual, 36% ran beyond their predicted duration. Among the trials with sufficient accrual, 60% ran longer than anticipated and 22% required more than 150% of their predicted duration to complete accrual. There was no statistically significant difference between trials with sufficient and insufficient accrual in terms of percent of trials within each of the five time course categories presented in Figure 3.
Figure 3. Trial duration ratio for trials by accrual sufficiency at trial closure.
Redesign strategies
Among the 82 trials analyzed, 27 (33%) underwent a significant redesign. The frequency with which these redesign strategies were employed, their cited indications, and their associated accrual status at trial closure is presented in Table 4. Protocol changes most commonly involved removal of one or more study arms and occasionally involved the addition of a new study arm. One trial changed its randomization schema from 1:1 to 2:1 patients (treatment arm: placebo control arm) in order to improve accrual. This particular effort did not result in achieving sufficient accrual. Of the 49 trials with other CTCGs participating, 25 trials (51%) added these groups after trial opening. Within this subset, 10 (40%) of these trials did not attain sufficient accrual.
Table 4. Reasons and strategies for trial redesign.
| Characteristics of trials with major redesign (n = 27) | Frequency of redesigned trial characteristic by accrual status at trial closure | |
|---|---|---|
|
| ||
| Sufficient | Insufficient | |
| Primary reason for redesign | ||
| Slow accrual | 3 | 4 |
| Fast accrual | 3 | 0 |
| New data | 6 | 1 |
| Lower than predicted event rate | 3 | 1 |
| Interim analysis results | 1 | 0 |
| Different than anticipated trial coursea | 4 | 0 |
| Unknown | 0 | 1 |
| Accrual status at time of redesign | ||
| Slower than projected | 5 | 7 |
| On projected target | 10 | 0 |
| Faster than projected | 5 | 0 |
| Primary redesign strategyb | ||
| Decrease sample size | 2 | 1 |
| Increase sample size | 13 | 0 |
| Alter endpoint | 1 | 0 |
| Recalculate type 1 error from two- to one-sided | 1 | 0 |
| Modify eligibility criteria | 0 | 2 |
| Add/delete treatment arm | 3 | 1 |
| Modify treatment arm | 0 | 1 |
| Change randomization to 2:1 | 0 | 1 |
Includes different than anticipated treatment effects, treatment compliance, patient eligibility, or cooperative group participation.
One trial not included here closed and reopened as a different trial as a response to poor accrual.
Particular attention was focused on redesigns employed for slow accrual and in the setting of slow accrual even if a different primary reason for redesign was cited. Redesign was associated with insufficient accrual status at trial closure, primarily in settings where accrual was already problematic. Among trials undergoing redesign in the setting of slow accrual, seven (58%) did not ultimately result in attaining sufficient accrual. For trials experiencing slow accrual at redesign, the median actual/predicted average monthly accrual rate was 63% for trials that ultimately resulted in sufficient accrual as compared with 35% for trials which closed with insufficient accrual.
Discussion
Closure of phase III oncology trials because of insufficient accrual is a prevalent problem. Fully half of phase III trials conducted by one CTCG within a 5-year timeframe closed due to this reason. High closure rates due to poor accrual have been reported in a few other settings [7,13–16]. For instance, among 333 British oncology trials conducted between 1971 and 2000, 20% did not achieve even 25% of their target accrual, and just over 50% did not meet the planned target accrual [16]. A study of 41 randomized trials listed in the NIH inventory of 1979 showed that 41% of trials did not achieve at least 75% of their target accrual [15]. Published data on clinical trial closure rates due to poor accrual are scarce. The IOM report includes a verbal communication citing 40% of CTCG phase III trials open between 2000 and 2007 not meeting the minimum accrual goals [12]. Furthermore, these rates will be sensitive to the definition of unsuccessful accrual. Such definitions may entail reaching target accrual, some percentage of target accrual, or sufficient accrual to address the scientific question of the trial. To confirm or refine the closure rate due to poor accrual seen in this pilot study, our ongoing research systematically evaluates the phase III accrual experiences of five CTCGs over a 10-year time period and includes assessing closure rates using our accrual sufficiency definition.
This pilot study also demonstrated a need for more accurate accrual prediction practices and more reliable prioritization of trials with the highest scientific quality and clinical relevance. Accrual patterns fell into two distinct categories: sufficient or insufficient accrual. Trials with borderline accrual were infrequent. In many cases, trials either fell vastly short of target accrual or exceeded accrual rate predications. Both are indicative of limitations in accrual prediction capabilities. The importance of effective prioritization has been recognized both in the recent IOM report as well as the report of the Clinical Trials Working Group (CTWG) of the National Cancer Advisory Board entitled ‘Restructuring the National Cancer Clinical Trials Enterprise [12,17].’ However, identifying the best practices for successful, reliable trial concept prioritization remains challenging.
No trial factors were conclusively associated with accrual sufficiency in this study, reflecting the study's relatively small sample size and underlying complexity of reasons for accrual failure. Nonetheless, some of the a priori trial factors examined approached significance in association with accrual sufficiency and merit further evaluation. A trend toward accrual sufficiency was observed for trial protocols containing documentation supporting predicted accrual goals. This trend, if valid, suggests a potential benefit for greater attention for documenting the basis for accrual rate estimates. Currently, CTCG accrual predictions frequently reflect one or more of the following: (1) the group's previous experience with a certain disease type, stage, or treatment; (2) informal polls of physicians' accrual estimates; and (3) accrual estimates submitted by principal investigators at each institution [18]. Physicians are known to overestimate their ability to accrue patients by over fivefold [18]. In our study, trials evaluating novel therapies achieved accrual sufficiency more often, which may reflect greater uncertainty among experts about the superiority of an experimental versus an accepted treatment. Currently, there are no requirements for a proposed trial to demonstrate that the trial addresses clinically relevant uncertainty among a community of clinicians, though the importance of clinical equipoise is acknowledged [19]. Selecting an appropriate control intervention facilitates addressing a question of clinically relevant uncertainty [20]. Furthermore, equipoise and clinical relevance must be maintained throughout the trial duration. This requires knowledge of other research that could impact the trial's meaning, designing trials that can be conducted quickly, and shortening the time from trial concept to activation. Dilts and Sandler [21] have shown that the median time from study conception to activation is 784 days. Improving trial design and operational efficiencies are critical to retaining a clinically relevant scientific question.
In the event of poor accrual, strategies for early trial closure or redesign should exist. NCI's Cancer Therapy Evaluation Program (CTEP) recently implemented early stopping guidelines applicable to all CTEP-sponsored CTCG phase III treatment trials activated after April 1, 2004. They apply a uniform set of accrual benchmarks to trigger trial closure based on meeting accrual targets as a percent of projected accrual over time. Our results corroborate that defining benchmarks for termination or redesign based on accrual are feasible. Validation of the CTEP early stopping guidelines would prove useful in effectively managing trials with flagging accrual.
Among trials at risk for insufficient accrual, two approaches are often utilized to improve accrual: (1) trial redesign, or (2) opening the trial in additional CTCGs. Information about the utility of redesign in improving accrual is needed to inform decisions about pursuing redesign or closure. Our results suggest that it is feasible to distinguish major redesigns from common, minor adjustments. This supports efforts to evaluate redesign strategies critically and systematically. Furthermore, our data suggest that there likely is a cut-off point in actual versus projected accrual below which a redesign is highly unlikely to improve accrual. No literature was identified describing to what extent enlisting other CTCG participation results in successful trial completion. Historically, this strategy results in significant additional expense, duplication of effort, and postponement of other trials that may compete for eligible patients. In this study, trials with multiple participating CTCGs were not more likely to attain sufficient accrual than single CTCG trials. However, the reason for adding other CTCGs after trial opening was not consistently known.
Our study's strengths include its uncommon examination of the relationship between accrual success and trial design factors. Evaluation of 122 British trials in cancer and other diseases found that a few factors had marginally significant associations with accrual success, such as oncology trials and trials with dedicated trial managers [13]. Trial factors negatively influencing the trial recruitment, as summarized by Ellis [6], include protocol complexity and use of a no-treatment arm, both of which were not significant in our analysis. Additional trial factors, such as control arm deviation from standard practice, narrow eligibility criteria, and unimportant scientific question, could not be examined in our study but will be gauged in our ongoing evaluation of a larger sample of trials. Importantly, a systematic evaluation of phase III trial design and accrual prediction practices could also serve as an historical context from which to appraise the outcomes yielded by restructuring efforts formulated by the CTWG and IOM reports.
Limitations of this study include an under-representation of nonmedical experimental modalities. Trials evaluating surgery or radiation therapy are too infrequent within this sample to draw valid conclusions. A second limitation stems from data source because not all important factors impacting accrual sufficiency are attainable through CTCG documents. Certain reasons previously cited as affecting accrual success, such as clinical relevance of the scientific question and control arm deviation from standard therapy [6,22], could not be identified in this study. Furthermore, reasons for redesigns or for adding CTCGs after trial activation are not always clearly documented. Reasons for poor accrual can be complex, highly variable, and specific to individual trials [14]. The clinical relevance and timeliness of a trial influences its likelihood of successful accrual [23], yet are challenging to measure. Our ongoing research includes additional information sources, such as a survey, to augment and refine factors considered for accrual sufficiency. The methods used to determine accrual benchmark feasibility were based on the assumption that percent actual/predicted average monthly accrual rate attains some uniformity during the trial course. If percent actual/predicted average monthly accrual rate was highly variable, then we would conclude that this rate is not a suitable parameter for defining such benchmarks. Although such erratic variability in percent actual/predicted average monthly accrual rate seems unlikely, an alternative approach involves evaluating all trials by the proportion of total patient accrual achieved by a specific time. An additional limitation stems from trial sample construction. The expanded trial set may introduce bias since it does not represent a consecutive series of trials. However, the only aspects of these trials known prior to their inclusion were disease site, trial type, and accrual sufficiency status. These selection criteria assured trial diversity to meet a study goal, namely, assessing feasibility of identifying trial factors associated with accrual sufficiency across a broad selection of trials from all participating CTCGs.
In summary, our findings confirm a high closure rate of phase III trials due to insufficient accrual, demonstrate that actual and predicted accrual vary greatly, and indicate that certain trial features appear associated with a greater likelihood of attaining sufficient accrual. Further research is needed to better inform accrual prediction practices and trial prioritization processes. Our ongoing research goals include identifying factors at the time of trial design or prioritization predictive of sufficient accrual and of scientific success; the earliest time point at which the accrual record reliably predicts an inability to attain sufficient accrual; and the subset of trials experiencing slow accrual that merit redesign. Reducing trial closures due to insufficient accrual is critical in realizing the promises of clinical cancer care.
Acknowledgments
This research was supported by the Commonwealth Foundation for Cancer Research support to the University of Virginia Cancer Center.
References
- 1.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–33. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 2.Cassileth BR. Clinical trials: time for action. J Clin Oncol. 2003;21:765–66. doi: 10.1200/JCO.2003.12.070. [DOI] [PubMed] [Google Scholar]
- 3.Prescott RJ, Counsell CE, Gillespie WJ, et al. Factors that limit the quality, number and progress of randomized controlled trials. Health Technol Assess. 1999;3:1–143. [PubMed] [Google Scholar]
- 4.Grunfeld E, Zitzelsberger L, Coristine M, Aspelund F. Barriers and facilitators to enrollment in cancer clinical trials: qualitative study of the perspectives of clinical research associates. Cancer. 2002;95:1577–83. doi: 10.1002/cncr.10862. [DOI] [PubMed] [Google Scholar]
- 5.Ross S, Grant A, Counsell C, et al. Barriers to participation in randomized controlled trials: a systematic review. J Clin Epidemiol. 1999;52:1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 6.Ellis PM. Attitudes towards and participation in randomized clinical trials in oncology: a review of the literature. Ann Oncol. 2000;11:939–45. doi: 10.1023/a:1008342222205. [DOI] [PubMed] [Google Scholar]
- 7.Mapstone J, Elbourne D, Roberts IG. Strategies to improve recruitment to research studies. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.MR000013.pub3. Art. No.:MR000013. [DOI] [PubMed] [Google Scholar]
- 8.Mills EJ, Seely D, Rachlis R, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–48. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B. On clinical trial participation. J Clin Oncol. 1991;9:1927–30. doi: 10.1200/JCO.1991.9.11.1927. [DOI] [PubMed] [Google Scholar]
- 10.Comis RL, Miller JD, Aldige CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–35. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 11.Trauth JM, Musa D, Siminoff L, Jewell IK, et al. Public attitudes regarding willingness to participate in medical research studies. J Health Soc Policy. 2000;12:23–43. doi: 10.1300/J045v12n02_02. [DOI] [PubMed] [Google Scholar]
- 12.IOM (Institute of Medicine) A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. The National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- 13.McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomized controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDaid C, Hodges Z, Fayter D, et al. Increasing participation of cancer patients in randomized controlled trials: a systematic review. Trials. 2006;7:16. doi: 10.1186/1745-6215-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Horwitz RI. Applying results of randomized trials to clinical practice: impact of losses before randomization. Brit Med J. 1984;289:1281–84. doi: 10.1136/bmj.289.6454.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vale C, Stewart L, Tierney J. Trends in UK cancer trials: results from the UK Coordinating Committee for Cancer Research National Register of Cancer Trials. Br J Cancer. 2005;92:811–14. doi: 10.1038/sj.bjc.6602425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical Trials Working Group of the National Cancer Advisory Board. Restructuring the National Cancer Clinical Trials Enterprise. [accessed June 2005]; Available at: http://integrated-trials.nci.nih.gov/ict/CTWG_report_June2005.pdf.
- 18.Taylor KM, Feldstein ML, Skeel RT, et al. Fundamental dilemmas of the randomized clinical trial process: results of a survey of the 1737 Eastern Cooperative Oncology Group investigators. J Clin Oncol. 1994;12:1796–1805. doi: 10.1200/JCO.1994.12.9.1796. [DOI] [PubMed] [Google Scholar]
- 19.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–45. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 20.Mann H, Djulbegovic B. Choosing a control intervention for a randomized clinical trial. BMC Med Res Methodol. 2003;3:7–12. doi: 10.1186/1471-2288-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dilts DM, Sandler AB, Baker M, et al. Processes to activate phase III clinical trials in a cooperative oncology group: the case of Cancer and Leukemia Group B. J Clin Oncol. 2006;24:4553–57. doi: 10.1200/JCO.2006.06.7819. [DOI] [PubMed] [Google Scholar]
- 22.Fayter D, McDaid C, Eastwood A. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. J Clin Epidemiol. 2007;60:990–1001. doi: 10.1016/j.jclinepi.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Campbell MK, Snowdon C, Francis D, et al. Recruitment to randomized trials: strategies for trial enrolment and participation studies. The STEPS study. Health Technol Assess. 2007;11:1–72. doi: 10.3310/hta11480. [DOI] [PubMed] [Google Scholar]