Abstract
Isolated pancreatic metastasis from malignant melanoma (IPMMM) is rare because most melanoma patients already have a widespread disease at diagnosis. No adjuvant systemic treatment is known to be efficient in this setting. Experience with pancreatic resection for IPMMM is limited and controversial. We report here the case of an IPMMM patient successfully treated by pancreaticoduodenectomy with a prolonged survival of 6 years.
Keywords: Chemotherapy, isolated metastasis, melanoma, pancreas, pancreatic surgery
INTRODUCTION
Pancreatic metastatic tumors are uncommon and account for less than 2% of all pancreatic carcinomas.[1] Renal-cell cancer, colorectal cancer, melanoma and sarcoma are the most common sites of primary malignancy.[1] Around one-third of patients with malignant melanoma develop metastases.[2] Metastatic melanoma has a poor prognosis; the median survival for patients with stage IV melanoma ranges from 8 to 18 months after diagnosis, depending on the sub-stage. Isolated Pancreatic Metastasis (IPM) is a rare event that represents about 1% of metastatic melanomas.[3] In patients who do not have a widespread disease (10-25%),[4] resection, which proved to be effective for the management of some types of cancer, such as colorectal and renal cancers,[5] is the gold standard; however, despite curative-intent treatment, survival remains poor (the 5-years survival rate is less than 10% and the median survival of 9 months). We report here the case of a patient who developed isolated pancreatic metastasis from malignant melanoma (IPMMM). Treatment by pancreaticoduodenectomy (PD) provided a prolonged survival of 6 years.
CASE REPORT
In 1989, a 29-year-old woman was diagnosed with a dorsal malignant melanoma treated by a wide R0 resection. After 4 years of follow-up (1993), she developed a local recurrence treated by resection; the analysis of sentinel lymph nodes was negative. Sixteen years after initial diagnosis (2005), she presented an epigastric pain and a 5 kg weight loss. An echoendoscopy revealed a 50 mm tumor located in the head of the pancreas, without vascular invasion and the biopsy confirmed an IPMMM. Exhaustive radiologic staging did not reveal any other metastasis [Figure 1a and b]. Primary chemotherapy was not delivered because of the long delay between resection of the primary tumor and recurrence and the only modest antitumor activity that could be expected with the conventional cytotoxic regimen (i.e., dacarbazine single-agent) used in metastatic melanoma. PD was performed and the histological analysis revealed a 60 mm tumor with clear resection margins. Immunohistochemistry detected the expression of protein S100, HMB45 but not of vimentin and melanA, features compatible with a metastasis of primary melanoma. Due to post-operative hemorrhage (day 7) arising from splenic vessels, the patient was re-operated and a totalization of pancreatectomy was performed. A new intervention was done at day 15 for gastro-jejunal anastomosis leak. Nineteen months after resection (2007), the patient presented a para-aortic lymph node recurrence that was treated by peri-aortic lymph node clearance [Figure 2]. After both initial PD and subsequent resection of the lymph node relapse, adjuvant treatment was not proposed because of (a) poor post-operative courses, (b) potential toxicities in a patient with complete pancreatic exocrine and endocrine insufficiency and (c) lack of available evidence demonstrating any impact of adjuvant systemic treatment in this setting. Since 2007, the patient has been under regular clinical and radiological surveillance without evidence of disease recurrence.
Figure 1.
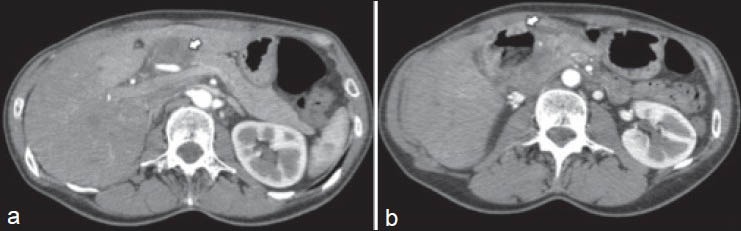
Pre-operative abdominal computed tomography showing a mass in the head of the pancreas (white arrow) (a) with central necrosis (white arrow) (b)
Figure 2.
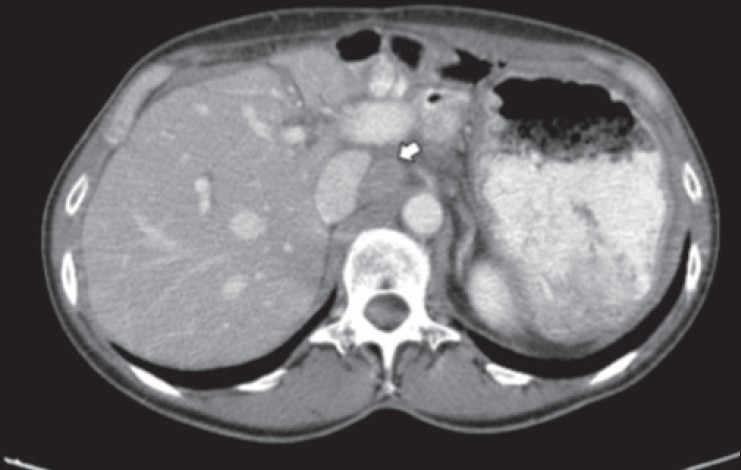
Abdominal computed tomography showing para-aortic lymph node recurrence (white arrow)
DISCUSSION
Metastasis to the pancreas occurs from a variety of primary cancers. Because IPM is asymptomatic in more than 50% of cases[6] it is usually detected during follow-up for a primary lesion.
IPM is rare because most patients usually have a widespread disease at diagnosis. Approximately 1% of pancreatic resections are conducted for IPM.[7] Management of IPM is controversial, and the benefit of resection regarding overall and disease-free survival has not been clearly established. The type and extent of surgical resection for IPM remain controversial. Because pancreas-sparing pancreatectomies are not optimal oncologic resections – with limited margins and no formal lymphadenectomy - it is unclear if they can be applied to IPM. Standard pancreatic resections, i.e., PD and distal pancreatectomy are associated, even in experienced hands, with significant post-operative morbidity and mortality and disappointing long-term functional results. The main advantage is easier lymphadenectomy. However, the usefulness of removing lymph nodes in this setting is also controversial.[8,9] Some authors advocate standard pancreatic resections instead of atypical resection because of the risk of early local recurrence and high morbidity.[10,11] Reported mortality and morbidity rates of pancreatectomies for metastasis are 5% and 48%, respectively.[12] Thus, resection for IPM has to be decided after balancing peri-operative risks and expected survival benefit.
Survival after pancreatectomy for IPM is strongly related to the primary cancer type.[6] Resections in patients with metastasis from renal-cell carcinoma[7,12] or colorectal cancer have a better prognosis than IPMMM patients. The reported 5-years survival in 321 patients undergoing resection of pancreatic renal cell cancer metastases was 72% with a 5-years disease-free survival of 57% compared to a 5-years overall survival rate of 14% for non-operated patients.[13] It has been suggested that completion of resection directly impacts on survival of IPMMM patients after pancreatectomy.[14] Furthermore, survival of patients undergoing complete surgical resection for IPMMM seems higher than that of patient receiving non-surgical treatments.[4,7,12,14] The median survival and the 5-years disease-free survival of patients with complete resection of IPMMM is 24 months and 50%, respectively, while their 5-years survival rate is 0% when resection is incomplete.[14,15] Even though previous data are derived from retrospective and potentially biased non-controlled studies, they are consistent with the marginal activity of chemotherapy alone in this setting.[16] Thus, dacarbazine, the only cytotoxic agent approved by the Food and Drug Administration in metastatic melanoma, provides a response rate of 7-12% and a median overall survival of 5.6-7.8 months after the initiation of treatment.[17] Higher response rates can be achieved with some combination of cytotoxics, but these combinations have failed to improve overall survival.
Experience with pancreatic resection for IPMMM is limited and controversial. It is important to emphasize that surgery has to be performed only when a complete resection is possible. Thus, pre-operative exhaustive staging is needed to confirm both the absence of local invasion of major vasculature and the absence of distant metastasis. Positron emission tomography scanning seems to have a higher sensitivity and specificity than conventional imaging for detecting metastasis from malignant melanoma.[18] The need of histological diagnosis before surgery is seldom necessary. Percutaneous fine-needle biopsy is needed especially in unresecable patients to use chemotherapy. However, staging of the disease should not preclude a meticulous examination of the medical history of the patient: A long disease-free interval after treatment of primary malignancy is strongly related to improved outcome.[1,7,12]
Major advances in systemic treatment of metastatic melanoma were recently reported. Thus, ipilimumab, a monoclonal antibody that blocks cytotoxic T-lymphocyte – associated antigen 4 on lymphocytes, was shown to improve overall survival in advanced disease, either alone in pre-treated patients or in combination with dacarbazine in first-line setting.[19] In addition, vemurafenib, a v-raf murine sarcoma viral oncogene homolog B (BRAF) kinase inhibitor, significantly increased rates of survival over dacarbazine in patients with previously untreated BRAF V600E-mutated metastatic melanoma (40-60% of melanomas).[20] Whether or not these innovative approaches, either alone or in combination with aggressive surgical procedures, have the potential to improve outcome in IPMMM remains to be determined.
The rather successful management of this new case of IPM melanoma favors the choice of a surgical act.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Z’graggen K, Fernández-del Castillo C, Rattner DW, Sigala H, Warshaw AL. Metastases to the pancreas and their surgical extirpation. Arch Surg. 1998;133:413–7. doi: 10.1001/archsurg.133.4.413. [DOI] [PubMed] [Google Scholar]
- 2.Allen PJ, Coit DG. The surgical management of metastatic melanoma. Ann Surg Oncol. 2002;9:762–70. doi: 10.1007/BF02574498. [DOI] [PubMed] [Google Scholar]
- 3.Nikfarjam M, Evans P, Christophi C. Pancreatic resection for metastatic melanoma. HPB (Oxford) 2003;5:174–9. doi: 10.1080/13651820310015284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer T, Merkel S, Goehl J, Hohenberger W. Surgical therapy for distant metastases of malignant melanoma. Cancer. 2000;89:1983–91. doi: 10.1002/1097-0142(20001101)89:9<1983::aid-cncr15>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Lee WS, Yun HR, Yun SH, Chun HK, Lee WY, Kim SJ, et al. Treatment outcomes of hepatic and pulmonary metastases from colorectal carcinoma. J Gastroenterol Hepatol. 2008;23:e367–72. doi: 10.1111/j.1440-1746.2007.05178.x. [DOI] [PubMed] [Google Scholar]
- 6.Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM, et al. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol. 2008;15:3199–206. doi: 10.1245/s10434-008-0140-7. [DOI] [PubMed] [Google Scholar]
- 7.Harrison LE, Merchant N, Cohen AM, Brennan MF. Pancreaticoduodenectomy for nonperiampullary primary tumors. Am J Surg. 1997;174:393–5. doi: 10.1016/s0002-9610(97)00121-9. [DOI] [PubMed] [Google Scholar]
- 8.Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: Which patients benefit from surgical resection? Ann Surg Oncol. 2008;15:1161–8. doi: 10.1245/s10434-007-9782-0. [DOI] [PubMed] [Google Scholar]
- 9.Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: An indication for pancreatic surgery. Ann Surg Oncol. 2006;13:75–85. doi: 10.1245/ASO.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 10.Bassi C, Butturini G, Falconi M, Sargenti M, Mantovani W, Pederzoli P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br J Surg. 2003;90:555–9. doi: 10.1002/bjs.4072. [DOI] [PubMed] [Google Scholar]
- 11.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10:287–93. doi: 10.1016/S1470-2045(09)70065-8. [DOI] [PubMed] [Google Scholar]
- 12.Hiotis SP, Klimstra DS, Conlon KC, Brennan MF. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002;9:675–9. doi: 10.1007/BF02574484. [DOI] [PubMed] [Google Scholar]
- 13.Tanis PJ, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg. 2009;96:579–92. doi: 10.1002/bjs.6606. [DOI] [PubMed] [Google Scholar]
- 14.Wood TF, DiFronzo LA, Rose DM, Haigh PI, Stern SL, Wanek L, et al. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol. 2001;8:658–62. doi: 10.1007/s10434-001-0658-4. [DOI] [PubMed] [Google Scholar]
- 15.Vagefi PA, Stangenberg L, Krings G, Forcione DG, Wargo JA. Ocular melanoma metastatic to the pancreas after a 28-year disease-free interval. Surgery. 2010;148:151–4. doi: 10.1016/j.surg.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Crosby T, Fish R, Coles B, Mason MD. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2000;2:CD001215. doi: 10.1002/14651858.CD001215. [DOI] [PubMed] [Google Scholar]
- 17.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–66. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 18.Rinne D, Baum RP, Hör G, Kaufmann R. Primary staging and follow-up of high risk melanoma patients with whole-body 18F-fluorodeoxyglucose positron emission tomography: Results of a prospective study of 100 patients. Cancer. 1998;82:1664–71. doi: 10.1002/(sici)1097-0142(19980501)82:9<1664::aid-cncr11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]


