Abstract
Plasmacytoid (p) dendritic cells (DC) are a specialized subset of DC whose primary role was initially defined by the production of type I interferons in response to viral infection. They are now known to also possess a repertoire of functions capable of determining T cell fate and activation. Under homeostatic conditions, non-lymphoid tissue-resident pDC play a critical role in the regulation of mucosal immunity, as well as the development of central and peripheral tolerance. Although these cells display a number of characteristics that differ from conventional DC, particularly altered costimulatory molecule expression and poor allostimulatory capacity when interacting with T cells, this phenotype favors the generation of alloantigen-specific regulatory CD4+ or CD8+ T cells critical to the development of graft tolerance. In this minireview we discuss pDC ontogeny, functional biology and the emerging data that demonstrate the importance of pDC in the induction of tolerance, as well as recent studies that define mechanisms underlying pDC-mediated tolerance to both solid organ and hematopoietic stem cell transplantats. We also highlight their use in clinical settings and the potential of pDC both as targets and cellular therapeutic agents to improve the outcome of organ transplantation.
Keywords: plasmacytoid dendritic cells, transplantation, immune regulation, tolerance
Introduction
Plasmacytoid (p) dendritic cells (DC), first identified in humans over 50 years ago, represent a previously enigmatic subset of DC (1) so-named for their plasma-cell like appearance when unstimulated. Their primary function was thought to be production of type I interferons (IFN) following viral infection (2). While pDC are phenotypically and functionally distinct in many respects from classical, or conventional DC (cDC), these subsets are connected by a defined role that links innate and adaptive immune responses. Recent progress in the identification of pDC-specific cell surface markers and growth-promoting factors has improved their purification, characterization and mobilization for comprehensive study. The limited immunostimulatory characteristics of pDC that distinguish them from cDC make the former an attractive therapeutic target for promoting tolerance in solid organ or hematopoietic stem cell transplantation.
pDC ontogeny
DC development exhibits considerable plasticity and complexity, and may be different for lymphoid and non-lymphoid tissue DC, or for DC generated during immune responses compared to the steady-state (3). Signaling pathways regulating DC subset ontogeny has been expertly reviewed by Belz and Nutt (4). DC can be detected in the murine thymus as early as embryonic day 17, coinciding with thymocyte development and the initiation of processes that selectively determine T cell fate. Both cDC and pDC appear concurrently and populate thymic tissue at equivalent rates (5). After birth, the proportion of pDC within the spleen decreases as cDC numbers increase, but remains constant within other secondary lymphoid tissues (lymph nodes).
Under homeostatic conditions, DC in peripheral organs have a half-life of only a few (5–7) days, although this may be as long as 25 days for pulmonary DC, and therefore undergo rapid and constant turnover from circulating and local precursors. Like cDC, pDC originate from CD34+ hematopoietic progenitors in the bone marrow (BM) and enter the peripheral circulation as precursor DC (pre-DC) before reaching their final tissue locations. The original paradigm of cDC having ‘myeloid’ and ‘lymphoid’ etiologies, and of pDC originating from the latter precursor group (6) based on a genetic profile that more closely resembles B and T cells, with D–J gene rearrangements and Rag gene expression (7), is now known to be incorrect. Both common lymphoid or myeloid progenitors can differentiate into pDC or cDC in vitro and in vivo (8). The zinc finger transcriptional regulator Ikaros is essential for DC development, and Ikaros gene knockout depletes all DC lineages (9), consistent with the role of this factor for normal hematopoietic progenitors. IFN regulatory factors (IRF) 4 and 8, and the transcriptional factors purine-rich nucleic acid binding protein.1 (PU.1) and growth factor independent 1 transcription repressor (Gfi1) also critically determine global DC development; Gfi1 deficient mice show defects in both DC subsets (10). Specific pDC lineage commitment is determined by the transcription factors E2-2 (which, in turn, regulates expression of IRF 7 and 8) (11) and Spi-B in humans (expressed exclusively in lymphoid cells) (12). However, no pDC precursor lacking the ability to transform into cDC has been identified, and BM pDC may alter their phenotype in the context of viral infection (13).
In addition to transcriptional programming for DC ontogeny, specific cytokines are required to promote the differentiation and expansion of DC subsets. Fms-like tyrosine kinase 3 ligand (Flt3L) is crucial for general DC diversification in both humans and mice and their amplification in vitro and in vivo, and it alone is critical for pDC propagation, although macrophage colony-stimulating factor (M-CSF) has also been implicated recently (14). Granulocyte-macrophage (GM)-CSF preferentially promotes cDC at the expense of pDC in a signal transducer and activator of transcription (STAT) 5-dependent manner (5), as STAT5-deficient hematopoietic progenitors can repopulate the pDC lineage. Flt3L has also been used successfully to mobilize circulating, lymphoid tissue-based, and interstitial hepatic and renal pre-DC subsets in non-human primates (NHP) and human volunteers, although a more substantial effect is seen in the cDC population (15).
pDC phenotype and receptor expression
The phenotypic characteristics of pDC are now well-defined and distinct from cDC. A current list of pDC surface markers and receptors and their functions is shown in Table 1. Within the lineage−CD4+MHC class II+CD11c− population, human pDC express high density CD123 (IL-3 receptor [R] α chain) and CD45RA, Ig-like transcript (ILT) 7 (16), C-type lectins blood DC Ag (BDCA)–2 and –4 (although the latter is not pDC-specific), leukocyte-associated Ig-like receptor-1 (LAIR1) (17) and CD2. Increased CD2 expression can distinguish pDC subsets capable of lysosomal and cytolytic activity (18). NHP pDC exhibit similar morphology, phenotype and function compared to their human counterparts (19). They are clearly identified within Lin−MHCII+CD123+CD11c−CD11b− populations, and express low-level CD40 and CD86. CD123 remains the only definitive NHP pDC marker, although limited to detection in old world primates.
Table 1. Plasmacytoid DC surface markers and receptors.
Markers/receptors that distinguish pDC from conventional DC are indicated in bolded type.
| Species | pDC Marker/Receptor | Function |
|---|---|---|
|
| ||
| Mouse | CD11cloCD11b−B220+Gr-1+ | |
| Siglec H* | Regulates type-I IFN production; endocytic receptor, no endogenous ligand known | |
| Ly6C | Transmits signals for T cell activation and cytotokine production | |
| CD8α | Increased expression following Flt3L treatment | |
| mPDCA-1(CD317/tetherin) | Up-regulated by inflammation; inhibits spread of virus | |
| CCR9 | Regulates transport of peripheral Ag to the thymus | |
| Ly49Q | Regulates type I IFN production | |
| LAG-3 | Binds MHC-II, down-regulates T cell stimulation | |
| CD200R | Ligation induces IDO expression | |
| PDC-TREM | Regulates type I IFN production | |
| TLR7,9 | Viral detection | |
|
| ||
| NHP (rhesus macaque) | Lin−MHC class II + | |
| CD123+ | IL-3 receptor | |
|
| ||
| Human | CD4+CD45RA+Lin−MHCII+CD11c− | |
| ILT7 | Inhibits type-I IFN production | |
| ILT3+ILT1− | Modulates type-I IFN production | |
| FcγRIIa (CD32) | Induces phagocytosis of opsonized microbes | |
| CD62L, CD36 | Ag internalization for T cell presentation | |
| CD123 | IL-3 receptor | |
| CD68 | Binds low density lipoprotein | |
| LAIR-1 | Regulates type I IFN production | |
| CD2hi | Cytolytic capacity | |
| BDCA2 (CD303) | Ag capture, inhibits type I IFN production | |
| BDCA4 (neuropilin-1) | Receptor for vascular endothelial growth factor | |
| TLR7, 9 | Viral detection | |
| L-selectin, PSGL | Ligands for E selectin | |
| CCR2+CCR4loCCR5hiCCR7+ | Chemokine receptors; CCR7↑ with maturation | |
| CXCR3hiCXCR4+CXCR2lo | Chemokine receptors | |
| C3aR+C5aR+ | Complement component receptors | |
| IL-18R | Binds IL-18 | |
Abbreviations: Flt3L: fms-like tyrosine kinase 3 ligand; NHP: non human primate; Siglec-H: sialic acid-binding Ig-like lectin; LAG-3: lymphocyte activation gene; PDC-TREM: triggering receptor expressed on myeloid cells; ILT: Ig-like transcript; IDO, indoleamine dioxygenase; LAIR-1: leukocyte-associated Ig-like receptor; PSGL, P-selectin glycoprotein ligand-1.
Naïve murine pDC express DC antigen (Ag) (PDCA)-1, also known as CD317/BM stromal cell Ag-2 (BST2)/tetherin (20). This molecule is not exclusive to pDC, but is upregulated in other cell types activated by exposure to type I IFN or IFN-γ. pDC also express several receptors that differentially regulate type-I IFN production in response to Toll-like receptor (TLR) ligation. Although they are incapable of independent signaling, they do so using adapters containing intracellular tyrosine-based activating or inhibitory motifs (ITAM or ITIM, respectively). Sialic acid-binding Ig-like lectin (Siglec)-H is a member of the Ig superfamily and signals via the transmembrane adaptor DNAX activation protein of 12kD (DAP12) or FcRγ adapters to mediate Ag endocytosis, although it is down-regulated in viral infections and impairs type-I IFN secretion (21). pDC-TREM (triggering receptor expressed on myeloid cells) and Ly49Q, the latter a C-type lectin binding MHC class I, both enhance IFNα production (22). Following their adoptive transfer, pDC typically lack TLR2-5 displayed by cDC, but contain TLR7 and -9 within endosomal compartments. Naïve pDC express nominal quantities of TLR4 (23), but these may be upregulated by exposure to LPS, and induce conversion of conventional CD4+CD25− T cells into forkhead box P3 (FoxP3+) regulatory T cells (Treg) with immunosuppressive capacity. TLR9 promotes responses to CpG-oligonucleotides (bacterial DNA); TLR7 (highly homologous to TLR9) may discriminate nucleic acid structures and single-stranded RNA, and responds to synthetic guanine analogues.
pDC location and trafficking
Under homeostatic conditions, pDC are found predominantly in T cell areas of peripheral lymphoid organs, but form clusters upon activation, compared to cDC that localize to marginal zones but distribute throughout T cell areas following activation. All DC subsets emigrate from the BM via the peripheral circulation to secondary lymphoid tissue (lymph nodes, spleen) facilitated by expression of L-selectin (non-inflamed states) or E-selectin (inflamed states) in high endothelial venules (24). Although chemokine receptor profiles are consistent between pDC and cDC (including CXCR3 & 4, CCR1, CCR2, CCR5 and CCR7), many are non-functional (CCR7 can mediate migration in vitro (25)) and are down-regulated in pDC in response to inflammatory stimuli. CCR9 has been identified recently as a marker of Ag-bearing pDC migrating to the thymus, as well as the small intestine (26).
Role of pDC in innate and adaptive immunity
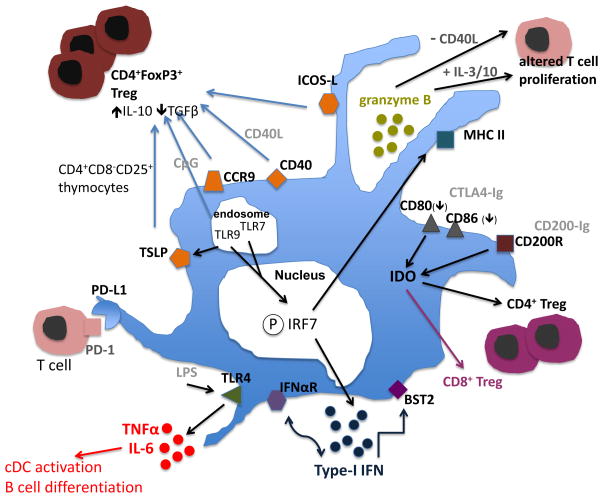
The primary function of pDC is their capacity to sense pathogens dependent upon TLR engagement, produce large amounts of type-I IFN (27), IL-6 and tumor necrosis factor (TNFα), and thus regulate long-term T cell survival and T helper 1 (Th1) cell skewing, cDC activation, and B cell differentiation. pDC display poor T cell (allo) stimulatory capacity since they lack the ample endocytic capacity shown by cDC (28), as well as mature cathepsins required for Ag processing. Moreover, they express only low levels of cell surface costimulatory molecules (28). However, they play a key role in linking and regulating anti-viral innate and adaptive immunity (29). Figure 1 depicts phenotypic and functional properties of pDC, indicating the molecules that regulate their activation and immune modulatory functions.
Figure 1. Phenotypic and functional characteristics of pDC.
pDC respond to viral infection via recognition of viral nucleic acids by endosomal TLR7 and 9, activated (phosphorylated; P) IRF7-mediated production of type-1 IFN, cytotoxic molecules (granzyme B) and expression of cell differentiation markers (CD40, CD86, CCR7), and to bacterial infection by secretion of pro-inflammatory cytokines (TNFα; IL-6) mediated through TLR4. BM stromal cell Ag-2 (BST2; tetherin = CD137) is modulated by IFN responses through the ITAM-bearing signaling receptor Ig-like transcript (ILT) 7, in a negative feedback fashion. TSLP receptor expression, produced in response to TLR ligation, inhibits IL-17 production by T cells and promotes CD4+ Treg development. CCR9 (homing receptor)+ pDC in lymphoid tissue are potent inducers of Treg function, and can suppress Ag-specific immune responses. Surface-expressed PD-L1 (= B7-H1; CD274) and ICOS-L are also implicated in regulation of alloimmune cell responses, Treg development and suppression of alloreactive T cells. pDC also act as tolerogenic cells by expressing the inducible immunoregulatory enzyme IDO. cDC=conventional DC
pDC and Central tolerance
There are increasing data showing that pDC play critical roles in the induction and maintenance of central tolerance. Circumstantial evidence suggests that this is through both the deletion of autoreactive T cells and the production of FoxP3+ Treg. A significant proportion of murine thymus-based DC arrive from the peripheral circulation laden with Ag and are capable of deleting Ag-specific T cells (30). pDC have also been identified in the cortex and medulla of human thymus. The murine thymus contains three major DC subsets, one of intra-thymic origin derived from thymic progenitors that develop into signal-regulatory protein [SIRP]α−CD11b−CD8αhi cDC and predominantly regulate T cell negative selection, and two extrathymic thymic-homing DC populations comprising pDC and a SIRPα+CD11b+CD8α− subset cDC (31). Indeed, the majority of pDC are immigrants from extrathymic tissues able to endocytose and transport peripheral Ag for subsequent presentation/promotion of central tolerance (32). Their entry into the thymus upregulates CD11c, MHC class II and CD8α expression. Thymic migration of pDC is dependent upon CCR9 and an absence of TLR signaling (33). The ability of thymic pDC to drive CD4+CD25+FoxP3+ Treg development has been documented (34). Additionally, there is evidence that human activation of pDC (via TLR7 or 9) induces expression of the thymic stromal lymphoprotein (TSLP) receptor and subsequent education in the presence of TSLP generates FoxP3+ Treg from CD4+CD8−CD25− thymocytes (35). A distinct feature of pDC-generated FoxP3+Treg is their greater production of IL-10 and TGFβ compared to those derived from interactions with cDC.
pDC and mucosal tolerance
Recent novel data have demonstrated the role of pDC in the induction and regulation of mucosal tolerance to innocuous ingested or inhaled Ag. Thus, murine hepatic pDC induce Ag-specific suppression of CD4+ and CD8+ T cell responses mediated by deletion or anergy. Moreover, tolerance is impaired by administration of either pDC-depleting Ab anti-Gr1 or 120G8 prior to Ag exposure (36). Human tonsillar pDC generate functional CD4+CD25+CD127− (IL-7Rα)− FoxP3+ Treg from naïve T cells, and co-localize in vivo in palatine and lingual tonsils with CD4+FoxP3+ Treg (37). Pulmonary pDC endocytose and present Ag to suppress effector T cell generation by cDC. Adoptive transfer of these pDC prevents the induction of murine allergic asthma and pDC depletion leads to cardinal features of Th2 cell-associated asthma (38). Altered pDC phenotype and function may also limit intestinal responses to commensal bacteria. Thus, Peyer’s patch pDC fail to produce type-I IFN, although the same alteration in response can be generated in splenic pDC exposed to TGFβ, prostaglandin (PG) E2 and IL-10, suggesting a role for local regulation of pDC function by the mucosal milieu (39). In addition, oral tolerance requires a two-step process, each phase mediated by pDC: initial allergen exposure depletes Ag-specific hepatic and mesenteric lymph node CD8+ T cells, followed by activation of lymphoid tissue-based Treg to suppress remaining effector T cells (40).
pDC and transplant tolerance
The advent of modern immunosuppressive agents (particularly calcineurin inhibitors) has remarkably diminished acute allograft loss, but now detrimentally influences long-term graft and patient survival. The burden of excess cardiovascular, infectious or malignant morbidity and mortality has led to strategies aimed at eliminating immunosuppression. Currently, no tolerizing immunosuppressive protocols are accepted into routine clinical practice, nor are there firmly established genetic signatures to predict operationally tolerant patients. However, harnessing the tolerogenic potential of immune cell therapy in transplantation (41, 42), including pDC-based therapy, may provide an opportunity to accomplish this goal.
(i) Pre-clinical models
There has been debate over whether pDC are capable of inducing an alloimmune response, as robust Ag presentation to T cells remains vital to their role as immunological mediators. It has been reported that adoptive transfer of MHC class II-competent pDC into MHC-deficient mice (resistant to graft-versus-host disease [GVHD]) allows priming of alloreactive CD4+ and CD8+ T cells to promote GVHD equivalent to that seen following reconstitution with cDC (43). Also, in a model of MHC-mismatched orthotopic murine lung transplantation, adoptive transfer of PDCA-1+ pDC led to T cell priming and induction of allograft rejection (44).
Despite these observations, considerable evidence has emerged in recent years that supports a role of pDC in regulating the induction and/or maintenance of tolerance to solid organ or hematopoietic stem cell (HSC) allografts (see Tables 2 & 3), although their tolerogenic function is less well-defined than that of cDC. Like cDC, pDC display a ‘dual’ functionality of immunogenicity versus tolerogenicity based on receptor ligation and maturation status. Generation of CD4+CD25+FoxP3+ Treg occurs following interaction with TLR9-ligated pDC (45), and regulatory CD8+ T cells are generated by CD40 ligand-activated pDC (46). Abe et al (47) and Bjorck et al (48) first reported prolongation of murine heterotopic cardiac allograft survival following infusion of Flt3L-mobilized BM-derived pre-pDC, or freshly-isolated splenic pDC respectively (in conjunction with CD40L mAb therapy). In a transplant tolerance model using donor-specific transfusion and CD40L mAb, Ochando et al (49) demonstrated an essential requirement for pDC in the induction of tolerance to cardiac allografts through Ag acquisition and presentation in draining lymph nodes, and the induction of alloAg-specific lymph node-based CD4+FoxP3+ Treg. Depletion of pDC using mAb inhibited Treg development and subsequent transplant tolerance. These findings have been corroborated in a model using CCR7-deficient mice that lack lymph node-based pDC and fail to develop cardiac allograft tolerance (50). Adoptive transfer of CCR7+ pDC (but not cDC) successfully restores tolerance. In rats, CD8+ Treg-mediated tolerance to heart allografts is also mediated by both allograft and splenic pDC (51).
Table 2.
Impact of adoptively-transferred pDC on organ allograft survival in rodents
| Model | pDC characteristics | pDC Source | Recipient Treatment | Outocme | Reference |
|---|---|---|---|---|---|
| Mouse HHT* | BM pre-pDC B220+CD11c+CD11b− (donor-derived) | Flt3L- expanded ex vivo | - | Prolonged graft survival; T cell hyporesponsiveness via B7-H1 | (47) |
| Mouse HHT | In vivo Flt3L-mobilized splenic pDC B220+CD11c+CD11b− (donor-derived) | Freshly- sorted pDC | Anti- CD40L mAb | Prolonged graft survival, MST 68d (50 % > 100d) | (48) |
| Mouse HHT | PDCA1+B220+CD11c+ Gr-1+ (CD19−) pDC | Visualized in situ | Anti- CD40L mAb | Ag-specific Treg in LN; pDC depletion abrogates tolerance | (49) |
| Rat HHT | B220+CD4+ | Flow-sorted from spleen | Adenoviral CD40Ig | Induction of CD8+ Treg, contact-dependent and independent (via IDO) | (51) |
| Mouse HHT | PDCA-1+ B220intCD11cint splenic and LN pDC | B16-Flt3L- secreting melanoma infusion | DST, anti- CD40L mAb | pDC from B6 mice facilitates graft survival in CCR7−/− mice | (50) |
| Mouse OLT | PDCA1+CD11cloB220+Gr1 | Flow-sorted pDC | mPDCA-1 mAb-treated | Adoptive transfer of pDC after depletion induces acute rejection, T cell priming | (44) |
Abbreviations: BM, bone marrow; DST, donor-specific transfusion; Flt3L, fms-like tyrosine kinase 3 ligand; HHT, heterotopic heart transplantation; IDO. indoleamine 2,3-dioxygenase; LN, lymph node; OLT, orthotopic lung transplantation
Table 3.
Impact of pDC on allogeneic HSC transplant outcome in rodents
| Model | pDC Characteristics | pDC Source | Outcome | Reference |
|---|---|---|---|---|
| Mouse allo- HSCT* | CCR9+B220+CD11cint pDC | B16-Flt3L- secreting melanoma infusion | Inhibition of acute GVHD; expansion of FoxP3+Treg, suppression Th17 cells, T cell hyporesponsiveness | (33) |
| Mouse alloHSCT (H2-Ab1−/−) | MHCII+ pDC | Flt3L-mobilized | pDC alone primed alloreactive T cells, caused GVHD | (43) |
| Mouse alloHSCT | FC (CD8α+TCR−) B220+CD11cloCD11b− | Freshly-sorted from Flt3L-treated mice | 95% survival with FC (51% survival with p-preDC); Ag- specific CD4+FoxP3+Treg induction | (52, 72) |
| Mouse alloHSCT | B220+Lin− CD11c+PDCA1+ | BM pDC, flow- sorted | pDC augment T cell GVL effect, attenuated GVHD effect; T cell IFNγ induces pDC IDO | (73) |
Abbreviations: FC, facilitating cell; Flt3L, fms-like tyrosine kinase 3 ligand; GVHD, graft-versus-host disease; GVL, graft-versus-leukemia; HSCT, hematopoietic stem cell transplant; IDO, indoleamine 2,3-dioxygenase
pDC constitute the major cell phenotype in the facilitating cell (FC) population shown to enable allogeneic HSC transplantation (52). These CD8+TCR− FC constitute a heterogeneous population that can be expanded in vitro and mobilized in vivo by Flt3L, and share specific functional traits with pDC (activation by CpG-ODN and subsequent production of IFNα and TNFα). Total FC infusion leads to long-term host survival following MHC-mismatched HSC transplantation, with donor-specific skin graft tolerance, which is abrogated by depletion of pre-pDC, but not recapitulated by infusion of pre-pDC alone (52). Depletion of pDC from BM grafts has been reported to accelerate acute GVHD (aGVHD) mortality; in addition, the influence of ongoing GVHD leads to an inability to durably reconstitute this cellular compartment and thus inhibit T cell proliferation (53).
In secondary lymphoid organs, immature pDC express CCR9 that is down-regulated in response to maturation-inducing pDC-restricted TLR ligands and induce CD4+CD25+ Treg with Ag-specific suppressive activity in vitro and in vivo (33). CCR9− DC appear to be precursors of cDC, rather than a separate pDC subset. Concurrent infusion of CCR9+ pDC with CD4+CD25− effector T cells and BM into lethally-irradiated mice rescues them from aGVHD.
(ii) Clinical transplant tolerance and relation between pDC and allograft outcome
Despite convincing evidence of pDC-mediated allograft tolerance in rodent models, few studies have been performed to ascertain the contribution of pDC in clinically–relevant transplant tolerance (see Table 4). Not surprisingly, assessment of pDC-based cell signatures and correlation with allograft outcome have been most common in recipients of liver transplants that experience the highest incidence of operational tolerance. Thus, operational tolerance in pediatric liver recipients is associated with higher peripheral blood pre-pDC numbers (compared to pre-cDC), as well as elevated B7-H1 (programed death ligand-1; CD274):CD86 ratios on these cells (54–56). Donor pDC subsets are consistently undetectable in recipients of orthotopic lung transplants (57), and although no correlative studies have has been performed, may account for higher rates of allograft rejection in these patients.
Table 4.
Association of pDC levels with outcome in clinical organ and HSC transplantation
| TRANSPLANT | pDC Characteristics | CLINICAL OUTCOME | REFERENCE |
|---|---|---|---|
| Liver | Precursor mDC and pDC | Tolerant or reduced IS* patients have higher precursor pDC:mDC ratio | (55) |
| AlloHSCT | Peripheral blood Lin−CD11c−ILT3− pDC 3 months after HSCT | Increased pDC count associated with absence of GVHD, improved survival, lower infections | (58) |
| Liver | pDC counts in tolerant and reduced IS patients | PD-L1:CD86 ratio on pre-pDC higher in tolerant patients and correlates with Treg | (66) |
| Liver, (pediatric) | CD11c+ mDC, CD123+ pDC | mDC:pDC ratio >1.78 associated with rejector status | (54) |
| AlloHSCT | Intra-graft pDC (CD123+) | Higher pDC associated with higher risk of relapse, lower overall survival | (59) |
Abbreviations: IS, immunosuppression; ILT3, Ig-like transcript 3
The role of pDC in clinical HSC transplantation and its relevance to distinct clinical outcomes (patient survival, event-free survival [EFS], incidence of acute or chronic (c) GVHD) is not clearly defined in the current literature (58, 59). The varying results may be influenced by differences in stem cell source, conditioning regimens, pDC identification by flow cytometry, and whether peripheral blood or BM pDC content are analyzed. Prior to more accurate determination of pDC, increased populations of BM-resident CD4brightCD3−Lin−HLA-DR+CD123+CD11c− cells (presumptive pDC) were associated with both lower EFS and cGVHD (60). Higher incidences of graft pDC have been associated with decreased EFS in more recent studies (59), although lower peripheral blood pDC are generally associated with augmented severity of acute and chronic GVHD (58).
In a recent landmark study, Leventhal et al (61) utilized HSC enriched for FC (composed predominantly of pre-pDC), in conjunction with non-myeloablative conditioning, to promote chimerism and tolerance in patients receiving an HLA-mismatched living-donor kidney allograft. The recently published phase II trial (62) reports on 15 transplant recipients, 9 of whom exhibited enduring chimerism and immune competence, with no GVHD despite HLA disparity.
Mechanisms of pDC-mediated tolerance
Limitations regarding successful in vitro propagation of pDC, as well as in vivo characterization and isolation of sufficient pDC numbers, have limited ability to extensively assess mechanisms underlying pDC-mediated allograft tolerance. Expansion of pDC ex vivo with Flt3L however, has enabled improved understanding (see Figure 1).
The well–recognized pDC characteristic of poor T cell allostimulatory capacity may be a function of persistent MHC class II ubiquitin E3 ligase membrane-associated RING-CH1 (MARCH1) expression despite maturation, a feature that is lost with cDC activation, allowing stable cell-surface exogenous Ag:MHC class II complexes for presentation (63) It has been posited that weak TCR stimulation and/or low-level Ag presentation by pDC promotes Treg induction, a key feature in multiple models of transplant tolerance (see Table 2). The transcription factor PU.1, critical to pan DC development (64) also modulates MHC class II expression and may be the ‘missing link’ in pDC provision of decreased T cell signaling.
Two additional but distinct patterns of cell surface marker expression on pDC underline their regulatory propensity. The overall lower level of co-stimulatory B7 family molecule expression and concomitant high level of co-inhibitory B7-H1, as well as elevated B7-H1: CD80/86 ratio, differs significantly from the intensity of costimulatory molecule expression by cDC and confers inhibition of T cell stimulation (47). B7-H1 up-regulation and induction of Treg by murine pDC appears to be governed by the IL-12 family member IL-27 and STAT3 (65). The clinical relevance of these findings has been demonstrated by Tokita et al (66), who showed that pre-pDC B7-H1: CD86 expression was highest in pediatric tolerant liver transplant patients and correlated with CD4+ Treg in peripheral blood. pDC location may also correlate with functional difference. For example, hepatic pDC produce less IL-12p70 (bioactive form), but equivalent IL-10 when compared to splenic pDC, in addition to biased production of Th2 cytokines and CD4+ T cell apoptosis (66).
The inducible co-stimulator (ICOS) pathway negatively regulates T cell activation via APC, and pDC increase their ICOS ligand (ICOS-L) expression following maturation via TLR or CD40 ligation to preferentially drive IL-10-producing Treg (67). This is in direct contrast to cDC, that require an immature phenotype to prevent priming of naïve T cells. The tryptophan-depleting enzyme indoleamine 2,3-dioxygenase (IDO) has been shown to regulate T cell cycle progression and thus potently inhibit T cell responses. Mellor et al (68) identified a mouse splenic DC subset (CD11c+B220+CD19: putative pDC) that expressed high-level IDO following cytotoxic T lymphocyte Ag (CTLA) 4-Ig administration to block B7-CD28 interaction in vivo and that subsequently blocked alloAg-induced T cell proliferation. There is more recent evidence of induction of phenotypically-stable, tolerogenic pDC following exposure to TGFβ, that function in a manner dependent on IDO, but independent of enzymatic activity (69).
pDC express a functional CD200 R that when engaged by an Ig-fusion protein concurrent with TLR ligation, induces functional IDO expression. This effect is mitigated by addition of the IDO inhibitor 1MT, or in pDC from IFNαβR−/− mice (70). A similar effect on IDO is seen with pDC-based glucocorticoid-induced TNFR-related ligand (GITRL) engagement by dexamethasone, resulting in protection from airway allergens (71). Additionally, in a rat model of allograft tolerance, regulation of alloreactive CD4+ T cells by pDC-induced CD8+ Treg occurs via both a contact- and IDO-dependent mechanism, and a contact-independent but IFNγ-dependent pathway (51).
Conclusions
The increasing body of knowledge regarding the ability of pDC to regulate immunological responses to self- and allo-Ag has enhanced understanding of immunity and tolerance in transplantation and autoimmune disease. It has also made these cells attractive targets and options for potential cell therapy, both in pre-clinical animal models (47, 48, 52) and humans (62). pDC clearly possess tolerogenic functions that may exceed the regulatory capacity of cDC, and may be a more appropriate cellular alternative for the induction of clinically-applicable tolerance.
Acknowledgments
The authors work is supported by National Institutes of Health grants R01 AI67541 and U01 AI51698 (AWT), R01 HL-108954 and 2R01HL089658 (JSI), P30 DK079307 O’Brien Kidney Center Award (JSI), an Australian National Health and Medical Research Council Overseas Biomedical Fellowship APP1016276 (NMR) and American Heart Association postdoctoral fellowship 13POST14520003 (NMR).
Abbreviations
- cDC
conventional dendritic cell
- Foxp3
forkhead box P3
- IFN
interferon
- pDC
plasmacytoid dendritic cell
- TLR
Toll-like receptor
- Treg
regulatory T cells
Footnotes
Disclosure
AWT is a co-inventor of a patent concerning use of dendritic cells to promote tolerance to organ allograft. JSI is chair of the scientific advisory boards of Vasculox, Inc. (St. Louis, MO) and Radiation Control Technologies, Inc. (Rockville, MD).
References
- 1.Grouard G, Rissoan M, Filgueira L, Durand I, Banchereau J, Liu Y. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinchieri G, Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7(1):19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 4.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2007;12(2):101–113. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26(6):741–750. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, O’Keeffe M, et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J Immunol. 2003;170(10):4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 7.Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, Busslinger M, et al. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105(11):4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8(11):1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 9.Allman D, Dalod M, Asselin-Paturel C, Delale T, Robbins SH, Trinchieri G, et al. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood. 2006;108(13):4025–4034. doi: 10.1182/blood-2006-03-007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathinam C, Geffers R, Yucel R, Buer J, Welte K, Moroy T, et al. The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity. 2005;22(6):717–728. doi: 10.1016/j.immuni.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135(1):37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schotte R, Nagasawa M, Weijer K, Spits H, Blom B. The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J Exp Med. 2004;200(11):1503–1509. doi: 10.1084/jem.20041231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5(12):1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fancke B, Suter M, Hochrein H, O’Keeffe M. M-CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood. 2008;111(1):150–159. doi: 10.1182/blood-2007-05-089292. [DOI] [PubMed] [Google Scholar]
- 15.Coates PT, Barratt-Boyes SM, Zhang L, Donnenberg VS, O’Connell PJ, Logar AJ, et al. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102(7):2513–2521. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 16.Rissoan MC, Duhen T, Bridon JM, Bendriss-Vermare N, Peronne C, de Saint Vis B, et al. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood. 2002;100(9):3295–3303. doi: 10.1182/blood-2002-02-0638. [DOI] [PubMed] [Google Scholar]
- 17.Bonaccorsi I, Cantoni C, Carrega P, Oliveri D, Lui G, Conte R, et al. The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNalpha production. PLoS One. 2010;5(11):e15080. doi: 10.1371/journal.pone.0015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui T, Connolly JE, Michnevitz M, Chaussabel D, Yu CI, Glaser C, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182(11):6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jesudason S, Collins MG, Rogers NM, Kireta S, Coates PT. Non-human primate dendritic cells. J Leukoc Biol. 2012;91(2):217–228. doi: 10.1189/jlb.0711355. [DOI] [PubMed] [Google Scholar]
- 20.Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010;18(9):388–396. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107(9):3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 22.Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, Taniguchi M. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci U S A. 2008;105(8):2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183(11):6922–6932. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16(7):915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 25.Krug A, Uppaluri R, Facchetti F, Dorner BG, Sheehan KC, Schreiber RD, et al. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J Immunol. 2002;169(11):6079–6083. doi: 10.4049/jimmunol.169.11.6079. [DOI] [PubMed] [Google Scholar]
- 26.Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104(15):6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 28.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185(6):1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, et al. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101(9):3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 30.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7(10):1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206(3):607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36(3):438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9(11):1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115(26):5366–5375. doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 35.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184(6):2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29(3):464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palomares O, Ruckert B, Jartti T, Kucuksezer UC, Puhakka T, Gomez E, et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J Allergy Clin Immunol. 2012;129(2):510–520. 520 e511–519. doi: 10.1016/j.jaci.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 38.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200(1):89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Contractor N, Louten J, Kim L, Biron CA, Kelsall BL. Cutting edge: Peyer’s patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J Immunol. 2007;179(5):2690–2694. doi: 10.4049/jimmunol.179.5.2690. [DOI] [PubMed] [Google Scholar]
- 40.Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137(3):1019–1028. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 41.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 42.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12(6):417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 43.Koyama M, Hashimoto D, Aoyama K, Matsuoka K, Karube K, Niiro H, et al. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113(9):2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 44.Benson HL, Suzuki H, Lott J, Fisher AJ, Walline C, Heidler KM, et al. Donor lung derived myeloid and plasmacytoid dendritic cells differentially regulate T cell proliferation and cytokine production. Respir Res. 2012;13:25. doi: 10.1186/1465-9921-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173(7):4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 46.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195(6):695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5(8):1808–1819. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 48.Bjorck P, Coates PT, Wang Z, Duncan FJ, Thomson AW. Promotion of long-term heart allograft survival by combination of mobilized donor plasmacytoid dendritic cells and anti-CD154 monoclonal antibody. J Heart Lung Transplant. 2005;24(8):1118–1120. doi: 10.1016/j.healun.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Mishra P, Yu S, Beckmann J, Wendland M, Kocks J, et al. Tolerance induction towards cardiac allografts under costimulation blockade is impaired in CCR7-deficient animals but can be restored by adoptive transfer of syngeneic plasmacytoid dendritic cells. Eur J Immunol. 2011;41(3):611–623. doi: 10.1002/eji.201040877. [DOI] [PubMed] [Google Scholar]
- 51.Li XL, Menoret S, Bezie S, Caron L, Chabannes D, Hill M, et al. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol. 2010;185(2):823–833. doi: 10.4049/jimmunol.1000120. [DOI] [PubMed] [Google Scholar]
- 52.Fugier-Vivier IJ, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, et al. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201(3):373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banovic T, Markey KA, Kuns RD, Olver SD, Raffelt NC, Don AL, et al. Graft-versus-host disease prevents the maturation of plasmacytoid dendritic cells. J Immunol. 2009;182(2):912–920. doi: 10.4049/jimmunol.182.2.912. [DOI] [PubMed] [Google Scholar]
- 54.Gupta A, Kumar CA, Ningappa M, Sun Q, Higgs BW, Snyder S, et al. Elevated myeloid: plasmacytoid dendritic cell ratio associates with late, but not early, liver rejection in children induced with rabbit anti-human thymocyte globulin. Transplantation. 2009;88(4):589–594. doi: 10.1097/TP.0b013e3181b11f12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazariegos GV, Zahorchak AF, Reyes J, Ostrowski L, Flynn B, Zeevi A, et al. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant. 2003;3(6):689–696. doi: 10.1034/j.1600-6143.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 56.Tokita D, Sumpter TL, Raimondi G, Zahorchak AF, Wang Z, Nakao A, et al. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J Hepatol. 2008;49(6):1008–1018. doi: 10.1016/j.jhep.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paantjens AW, van de Graaf EA, Heerkens HD, Kwakkel-van Erp JM, Hoefnagel T, van Kessel DA, et al. Chimerism of dendritic cell subsets in peripheral blood after lung transplantation. J Heart Lung Transplant. 2011;30(6):691–697. doi: 10.1016/j.healun.2011.01.706. [DOI] [PubMed] [Google Scholar]
- 58.Mohty M, Blaise D, Faucher C, Bardou VJ, Gastaut JA, Viens P, et al. Impact of plasmacytoid dendritic cells on outcome after reduced-intensity conditioning allogeneic stem cell transplantation. Leukemia. 2005;19(1):1–6. doi: 10.1038/sj.leu.2403558. [DOI] [PubMed] [Google Scholar]
- 59.Rajasekar R, Lakshmi KM, George B, Viswabandya A, Thirugnanam R, Abraham A, et al. Dendritic cell count in the graft predicts relapse in patients with hematologic malignancies undergoing an HLA-matched related allogeneic peripheral blood stem cell transplant. Biol Blood Marrow Transplant. 2010;16(6):854–860. doi: 10.1016/j.bbmt.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Waller EK, Rosenthal H, Jones TW, Peel J, Lonial S, Langston A, et al. Larger numbers of CD4(bright) dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood. 2001;97(10):2948–2956. doi: 10.1182/blood.v97.10.2948. [DOI] [PubMed] [Google Scholar]
- 61.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4(124):124ra128. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott MJ, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95(1):169–176. doi: 10.1097/TP.0b013e3182782fc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9(11):1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 64.Carotta S, Dakic A, D’Amico A, Pang SH, Greig KT, Nutt SL, et al. The transcription factor PU. 1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32(5):628–641. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188(11):5227–5237. doi: 10.4049/jimmunol.1103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tokita D, Mazariegos GV, Zahorchak AF, Chien N, Abe M, Raimondi G, et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85(3):369–377. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 67.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204(1):105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171(4):1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 69.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 70.Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, et al. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173(6):3748–3754. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- 71.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13(5):579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 72.Huang Y, Bozulic LD, Miller T, Xu H, Hussain LR, Ildstad ST. CD8α+ plasmacytoid precursor DCs induce antigen-specific regulatory T cells that enhance HSC engraftment in vivo. Blood. 2011;117(8):2494–2505. doi: 10.1182/blood-2010-06-291187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu Y, Giver CR, Sharma A, Li JM, Darlak KA, Owens LM, et al. IFN-gamma and indoleamine 2,3-dioxygenase signaling between donor dendritic cells and T cells regulates graft versus host and graft versus leukemia activity. Blood. 2012;119(4):1075–1085. doi: 10.1182/blood-2010-12-322891. [DOI] [PMC free article] [PubMed] [Google Scholar]