Abstract
Cilia and flagella (interchangeable terms) are evolutionarily conserved organelles found on many different types of eukaryotic cells where they fulfill important functions in motility, sensory reception and signaling. The process of Intraflagellar Transport (IFT) is of central importance for both the assembly and maintenance of cilia, as it delivers building blocks from their site of synthesis in the cell body to the ciliary assembly site at the tip of the cilium. A key player in this process is the multi-subunit IFT-complex, which acts as an adapter between the motor proteins required for movement and the ciliary cargo proteins. Since the discovery of IFT more than 15 years ago, considerable effort has gone into the purification and characterization of the IFT complex proteins. Even though this has led to very interesting findings and has greatly improved our knowledge of the IFT process, we still know very little about the overall architecture of the IFT complex and the specific functions of the various subunits. In this review we will give an update on the knowledge of the structure and function of individual IFT proteins, and the way these proteins interact to form the complex that facilitates IFT.
Keywords: Cilium, Intraflagellar Transport, IFT complex, Protein domain, Protein–protein interaction
1. Introduction
Cilia and flagella can be found on nearly all eukaryotic cells with the exception of fungi and higher plants. They consist of a highly organized microtubule-based axoneme, consisting of 9 outer microtubule doublets and in motile cilia also 2 single central microtubules (Ishikawa and Marshall, 2011). The ciliary membrane surrounds the axoneme and is continuous with the plasma membrane of the cell body, but has a unique composition of lipids and membrane proteins essential for sensory functions (Emmer et al., 2010; Rohatgi and Snell, 2010). The axoneme is assembled onto the ‘basal body’, a modified centriole consisting of 9 microtubule triplets, two of which are extended during the formation of the axoneme (Ishikawa and Marshall, 2011). At its distal end, the basal body is attached to the plasma membrane by proteinaceous fibers, the so-called ‘transition fibers’, which are believed to form a selective ‘ciliary pore’ in order to retain ciliary proteins in their proper compartment (Nachury et al., 2010; Rosenbaum and Witman, 2002). The region of the axoneme immediately distal to the basal body, where the microtubule doublets extend from the basal body triplets, is called the ‘transition zone’ and is characterized by extensive contacts between the microtubules and the overlying ciliary membrane (Craige et al., 2010; Ishikawa and Marshall, 2011).
Proteomic analyses of isolated flagella from the green alga Chlamydomonas reinhardtii (Cr) have revealed that this organelle contains around 600 proteins (Pazour et al., 2005). The lack of protein synthesis machinery in cilia/flagella leads to the logistical problem of targeting proteins to the ciliary base and specifically importing them into the ciliary compartment. Furthermore, axonemal assembly occurs at the tip of the microtubules (Johnson and Rosenbaum, 1992), leading to the requirement of transport of axonemal subunits along the entire length of the axoneme (Hao et al., 2011). A key player in all these steps is Intraflagellar Transport (IFT), the bi-directional movement of large protein complexes along the axoneme. Defective IFT in Chlamydomonas leads to defects in flagellar assembly (fla mutants), resulting in cells with either absent, shortened, or malformed flagella (Adams et al., 1982). IFT defects in the nematode Caenorhabditis elegans result in abnormal chemosensation (che mutants), osmotic avoidance (osm mutants), dye-filling of ciliated sensory neurons (dyf mutants) and dauer-formation (daf mutants), a fact that has been extensively used to identify important factors in ciliogenesis (Inglis et al., 2007). Mammals contain various forms of both motile and immotile cilia, necessary for diverse functions such as mucus clearance in the respiratory tract, movement of the egg in the fallopian tubes, light-sensing in the retina and many more. In addition to these specialized cilia found on various cell types, most mammalian cells contain a primary cilium with important functions in signaling. As a consequence of all these important functions of both motile and immotile cilia, defective ciliary assembly is the cause of a number of severe genetic syndromes, commonly referred to as ‘ciliopathies’, with phenotypes including respiratory defects, obesity, blindness, kidney defects, skeletal abnormalities, mental retardation and many more (reviewed in Fliegauf et al. (2007)).
2. Discovery of IFT and purification of the IFT complex
IFT was discovered in the early 1990s in the laboratory of Joel Rosenbaum by Differential Interference Contrast (DIC) microscopy of paralyzed Chlamydomonas flagella (Kozminski et al., 1993). Granule-like particles were observed to move at a speed of ~2 μm/s from the base to the tip (anterograde transport) and at a speed of ~3.5 μm/s from the tip to the base (retrograde transport). Movement of these particles was later shown to be dependent on the FLA10 protein, a subunit of heterotrimeric kinesin II (Cole et al., 1993; Walther et al., 1994) because the particles disappeared after shifting a temperature-sensitive fla10 mutant to the restrictive temperature (Kozminski et al., 1995). In the same study, comparison of light microscopic and electron microscopic images showed that the particles seen by DIC microscopy corresponded to ‘raft-like’ electron dense material located between the outer doublet microtubules of the axoneme and the overlying flagellar membrane (Kozminski et al., 1995). Further work on the identities of the molecular motors powering IFT in Chlamydomonas clearly showed that heterotrimeric kinesin II, containing two motor subunits (KIF3A and KIF3B) and one accessory subunit (KAP) (Cole, 1999), is the anterograde motor (Cole et al., 1998; Kozminski et al., 1995; Matsuura et al., 2002; Walther et al., 1994), and that cytoplasmic dynein 2, previously known as cytoplasmic dynein 1b (Pfister et al., 2005), is required for retrograde transport (Hou et al., 2004; Pazour et al., 1999; Perrone et al., 2003; Porter et al., 1999). The situation is more complex in animals where homodimeric kinesin II (OSM-3 in C. elegans and Kif17 in vertebrates) represents a second anterograde motor, which functions redundantly with heterotrimeric kinesin II in building the ‘proximal’ segment of the cilium (containing microtubule doublets), and is specifically required for the formation of the ‘distal’ segment containing only single microtubules (Insinna et al., 2009; Insinna et al., 2008; Signor et al., 1999; Snow et al., 2004).
The discovery that the IFT ‘trains’ disappeared from fla10 mutant flagella after shifting the cells to the restrictive temperature (Kozminski et al., 1995) paved the way for the biochemical purification of these particles. Piperno and Mead were the first to report the existence of a ‘17S’ complex containing at least 13 polypeptides in the matrix of Chlamydomonas flagella, which was absent in the fla10 mutants at the restrictive temperature (Piperno and Mead, 1997). Soon after this discovery, Cole et al. also presented the purification of this complex and analyzed its content by two-dimensional gel electrophoresis (Cole et al., 1998). This study identified a 15-subunit complex that readily dissociated into two smaller sub-complexes (IFT-A and IFT-B) at increased ionic strength (>50 mM NaCl). Functional analysis of individual IFT proteins revealed that the IFT-A and IFT-B complexes not only represent distinct biochemical entities, but also function in different aspects of the IFT process. Mutations in IFT-B proteins lead to absent or dramatically shortened cilia (Brazelton et al., 2001; Hou et al., 2007; Pazour et al., 2000), similar to kinesin II mutants (Matsuura et al., 2002; Walther et al., 1994), whereas IFT-A defects result in malformed cilia with prominent bulges containing accumulations of IFT proteins (Iomini et al., 2009; Piperno et al., 1998), reminiscent of mutations in cytoplasmic dynein 2 (Pazour et al., 1999; Pazour et al., 1998; Porter et al., 1999; Schafer et al., 2003). These findings led to the notion that the IFT-B and IFT-A complexes are specifically involved in anterograde and retrograde transport, respectively. The proteins of the purified IFT complex were named after their apparent molecular weight in SDS-PAGE as p144, p140, p139 and p122 (for complex A), and p172, p88, p81, p80, p74, p72, p57/55, p52, p46, p27 and p20 (for complex B). Purification of the IFT complex under even higher ionic strength (300 mM NaCl) stripped the IFT-B complex of weakly associated subunits (IFT172, IFT80, IFT57 and IFT20), leaving behind a salt-stable IFT-B core complex containing IFT88/81/74/72/52/46/27 (Lucker et al., 2005). It should be noted that IFT74 and IFT72 are encoded by a single gene and that IFT72 is likely to represent a proteolysed fragment of IFT74 (Taschner et al., 2011). Similarly, IFT57 was observed to migrate as two close bands and consequently named p57/55 (Cole et al., 1998), which could be a result of proteolysis, but might also represent a post-translational modification of this factor in Chlamydomonas. Although these initial studies probably identified most of the IFT complex proteins, additional subunits were discovered later in various organisms. These include the IFT-B proteins IFT70/Dyf-1 (Fan et al., 2010; Ou et al., 2005a), IFT25 (Follit et al., 2009; Lechtreck et al., 2009b; Wang et al., 2009), IFT54/Elipsa (Follit et al., 2009; Kunitomo and Iino, 2008; Li et al., 2008; Omori et al., 2008) and IFT22/IFTA-2/RabL5 (Schafer et al., 2006), as well as the IFT-A proteins IFT121 (Blacque et al., 2006) and IFT43 (Piperno et al., 1998). Most importantly, some of the Chlamydomonas proteins identified as members of the IFT complex were shown to be the orthologs of proteins which are essential for the formation of sensory cilia in C. elegans, hinting at a conserved role for the IFT complex in ciliary assembly and maintenance across species. Because of the high degree of conservation of IFT complex proteins across ciliated organisms, it is the accepted view that almost all cilia rely on IFT for assembly and maintenance (Jékely and Arendt, 2006).
In addition to the IFT complex, another large protein complex, the BBSome, has been proposed to be involved specifically in the trafficking of membrane proteins to the cilium (Jin et al., 2010; Nachury et al., 2007). In C. elegans the IFT-A and -B sub-complexes have been shown to be held together by this multi-subunit BBSome (Ou et al., 2005a) and mutations in BBSome proteins can lead to the pathogenesis of Bardet–Biedl syndrome (BBS), a prominent ciliopathy (reviewed in Beales (2005)). Mutations in BBS proteins in C. elegans lead to defects in IFT (Blacque et al., 2004) and to a dissociation of IFT-complexes A and B, whereas in Chlamydomonas BBS mutants have normal IFT but lack the ability for phototaxis (Lechtreck et al., 2009a).
In this contribution, we will review the current knowledge regarding the function and structure of individual IFT subunits and give an update on how these proteins come together to form a complex that allows IFT-facilitated ciliogenesis.
3. IFT proteins contain multiple protein–protein interaction domains
The IFT complex contains at least 20 IFT proteins that have to interact with each other but also with motor and cargo proteins. This necessitates the presence of a significant number of regions capable of forming protein–protein interactions. Indeed, bioinformatic analysis of IFT protein sequences clearly identifies a number of well-known protein–protein interaction motifs, such as tetratricopeptide repeats (TPRs) (Blatch and Lässle, 1999), WD-40 repeats (Smith et al., 1999) and coiled-coils (Burkhard et al., 2001). While several IFT proteins contain many of those well-characterized sequences, others do not display significant similarity to known domains or motifs (Cole, 2003; Jékely and Arendt, 2006). Strikingly, only very few molecular structures of IFT proteins have been determined and currently no high-resolution structural studies have been carried out for larger IFT complexes. The most detailed view of the IFT particles to date is an electron tomographic study that visualized the IFT-trains but is of insufficient resolution to resolve individual IFT proteins (Pigino et al., 2009). In the next sections we will first summarize our current knowledge about the individual IFT proteins, including an analysis of recognizable structural elements and a description of the experimentally characterized interactions between individual polypeptides. Using recently available crystal and NMR structures, highly sensitive domain recognition programs (Soding, 2005), secondary structure prediction programs (Jones, 1999) and prediction programs for coiled-coil and TPR motifs (Andrade et al., 2000; Lupas et al., 1991), an updated schematic representation of domains/motifs within individual IFT proteins as well as an up-to-date interaction map is presented in Fig. 1.
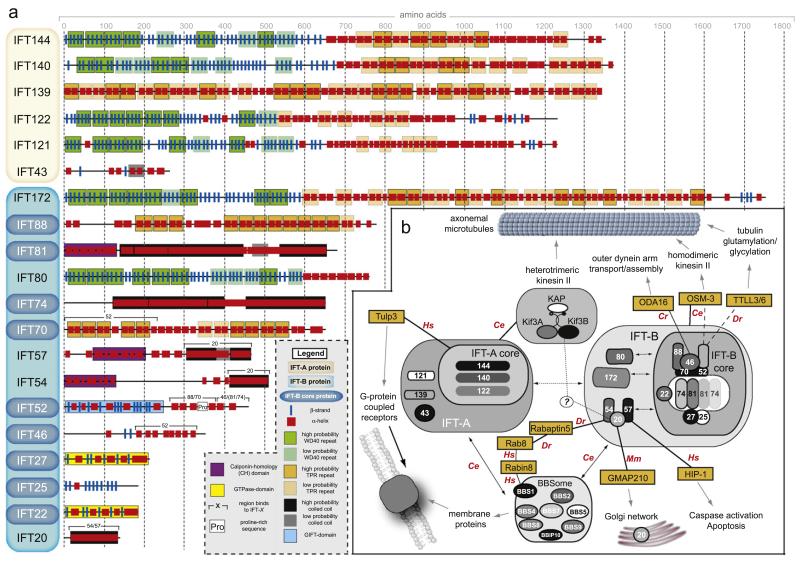
Fig. 1.
(a) Schematic representations of the primary structures of the 20 C. reinhardtii IFT proteins identified to date. Predicted secondary structure elements and domains/motifs identified using bioinformatics are indicated according to the legend. For IFT25, IFT27 and the N-terminal domain of IFT54, the available crystal and NMR structures where used to identify domains and derive the secondary structure. For subunits with no 3D structure determined, the secondary structure was predicted using the PSIPRED algorithm (Jones, 1999). Tetratricopeptide repeats (TPR) were predicted using the algorithms TPRpred (http://toolkit.tuebingen.mpg.de/tprpred) and REP (http://www.embl.de/andrade/papers/rep/search.html). The program REP was also used to predict WD40 repeats. Coiled coils were identified using COILS (Lupas et al., 1991; http://toolkit.tuebingen.mpg.de/pcoils) and Calponin-homology (CH) domains were identified using the HHPred algorithm (Soding et al., 2005; http://toolkit.tuebingen.mpg.de/hhpred) for IFT81 and IFT57. The CH domain of IFT54 was identified based on the determined NMR structure of this domain (PDB code 2EQO). (b) Simplified interaction map of IFT proteins and associated factors (see text for details). Direct interactions are indicated with solid lines and potential interactions with dashed lines. Abbreviation for the organisms in which the interactions have been reported are as follows: Cr, Chlamydomonas reinhardtii; Ce, Caenorhabditis elegans; Hs, Homo sapiens, Dr, Danio rerio; Mm, Mus musculus.
4. The IFT-A proteins
The six subunits of IFT-A identified to date are known to purify as a stable complex (Piperno et al., 1998), but little is known about direct protein–protein interactions between individual subunits, apart from a reported interaction between IFT121 and IFT43 (Cole and Snell, 2009). A reason for this lack of information might be that these factors are quite large, making studies using recombinant proteins a difficult task. A recent study used co-immunoprecipitation of IFT-A subunits to identify a core of IFT144/WDR19, IFT140 and IFT122 that remained associated after knockdown of the other two IFT-A components IFT139/THM1 and IFT121/WDR35 (Mukhopadhyay et al., 2010). However, the architecture of the IFT-A complex and the molecular nature of the interaction with IFT-B, the BBSome, IFT cargos and motors remain largely unknown and will undoubtedly be the focus of future studies.
With the exception of IFT43, the members of the IFT-A complex are large proteins with molecular masses above 120 kDa. IFT144, IFT140, IFT122 and IFT121 protein sequences are predicted to have a remarkably similar domain organization with the N-terminal regions containing WD-repeats that are known to fold into β-propellers, while the C-terminal part is predicted to contain α-helical TPR repeats (Fig. 1). This overall structural organization is reminiscent of nucleoporins and vesicle coat proteins, and it has been hypothesized that these proteins evolved from a protocoatomer in a pre-karyotic cell (Devos et al., 2004; Jékely and Arendt, 2006). Interestingly, the BBSome complex also contains multiple subunits with a similar predicted domain organization and was recently shown to form a membrane coat likely to be important for the trafficking of membrane proteins to the cilium (Jin et al., 2010). Two IFT-A proteins do not have predicted β-propellers, namely IFT139 that appears to mainly consist of TPRs, and IFT43 that does not have any motifs predicted with high probability (Fig. 1). It is noteworthy that none of the IFT-A proteins have any predicted domains with enzymatic activity. Since both β-propellers and TPRs are often found to be involved in protein–protein interactions, the IFT-A subcomplex is likely a structural platform that mediates the binding to cargo and motor proteins as well as to the IFT-B subcomplex and possibly also the BBSome. In the following sections we will review the current state of knowledge about the architecture and function of individual IFT-A proteins.
IFT144 has 56 predicted β-strands at the N-terminal half, consistent with two 7-bladed β-propellers (each blade having 4 β-strands) although only the first β-propeller is predicted with high confidence. In agreement with such a domain organization, searches against the data base of known protein structures (Protein Data Bank, PDB) using the CrIFT144 sequence reveal similarity (E=1.7 × 10−2) to the C. elegans AIP1 protein that indeed adopts the structure of two consecutive β-propellers (Mohri et al., 2004; Voegtli et al., 2003). CrIFT144 is encoded by the FLA15 gene in which a temperature-sensitive mutation results in decreased velocities of retrograde IFT particles, decreased concentration of IFT-A proteins in flagella, and to the formation of a characteristic flagellar ‘bulge’ containing IFT-B proteins at the permissive temperature, while at the restrictive temperature the flagella are resorbed (Iomini et al., 2009; Piperno et al., 1998). WDR19 and DYF-2 are the IFT144 orthologs in humans and C. elegans, respectively (Efimenko et al., 2006; Lin et al., 2003). Consistent with a function of an IFT-A protein in retrograde transport, IFT-B components accumulate in cilia of a dyf-2 mutant. Surprisingly, however, in a bbs-8 mutant background, in which the IFT-A and IFT-B complexes move at different speeds (Ou et al., 2005a), GFP-tagged DYF-2 moved at a speed characteristic of an IFT-B associated protein although the mechanistic implications of this observation are currently not completely clear (Efimenko et al., 2006). Mutations in human IFT144 were recently found to be the cause of the ciliopathies Sensenbrenner syndrome and Jeune syndrome with skeletal anomalies and renal insufficiency phenotypes (Bredrup et al., 2011).
IFT140, like IFT144, contains one clearly predicted β-propeller at the very N-terminus followed by another potential β-propeller. IFT140 has not been studied extensively and not much literature is available for this protein. The C. elegans ortholog, CHE-11, was shown to move together with IFT-B and IFT-A proteins in ciliated sensory neurons (Qin et al., 2001). In Drosophila, mutations in the rempA locus, which encodes the fly ortholog, result in defects in mechanosensory cilia and lead to accumulations of IFT-B proteins, consistent with a defect in retrograde IFT (Lee et al., 2008).
IFT139 is an atypical IFT-A protein in the sense that it does not contain β-propellers but is predicted to contain TPRs along the entire sequence (Fig. 1). The C-terminal half of the protein (residues 700-Cterm of Cr139) displays significant sequence similarity (E=10−6 −10−3) to proteins in the PDB known to adopt a TPR structure. In Chlamydomonas, IFT139 is encoded by the FLA17 gene, and a temperature-sensitive mutation leads to similar phenotypes as those already described for IFT144/FLA15 (Iomini et al., 2009; Piperno et al., 1998). Mutations of the mammalian ortholog TTC21B/THM1 cause accumulation of IFT-B proteins in primary cilia together with an over-activation of the Sonic Hedgehog (Shh) pathway, indicative of defects in retrograde IFT (Goetz and Anderson, 2010; Tran et al., 2008).
IFT122 and IFT121 (sometimes referred to as IFT122A and IFT122B, respectively) both have β-propellers predicted at the N-termini and low-confidence TPRs predicted at the C-terminus (Fig. 1). In Tetrahymena, IFT122 is not required for ciliogenesis, but a knock-out strain accumulates IFT-B proteins at ciliary tips (Tsao and Gorovsky, 2008b). The IFT122 orthologs in animals are DAF-10 in C. elegans (Bell et al., 2006; Qin et al., 2001) and WDR10 in humans (Gross et al., 2001). A null allele for IFT122 in mice leads to clear defects in retrograde IFT, including accumulation of complex B proteins at the tips of primary cilia and defective Shh signaling (Cortellino et al., 2009; Qin et al., 2011). Mutation of IFTA-1, the C. elegans ortholog of IFT121, leads to chemosensory and dye-filling defects, abnormal cilia structure, and to accumulations of IFT-B proteins in cilia, clear indicators of a bona-fide IFT-A complex protein (Blacque et al., 2006). Patients with mutations in WDR35, the human ortholog of IFT121, suffer from various ciliopathies including Sensenbrenner syndrome (Gilissen et al., 2010) and short-rib polydactyly syndromes (Mill et al., 2011).
IFT43, the smallest IFT-A protein, does not contain any clearly identifiable domains, apart from a short central coiled-coil region only predicted with low probability. IFT43 was not identified in the original purification of the IFT-A complex (Cole et al., 1998), which might be explained by a different purification procedure used in a subsequent study that identified IFT43 (Piperno et al., 1998). Recently, a mutation in IFT43, similarly to mutations in IFT121/WDR35, was identified as the cause of Sensenbrenner syndrome (Arts et al., 2011; Gilissen et al., 2010). Patients suffering from this genetic disease display skeletal and renal defects, phenotypes commonly observed in ciliogenic disorders. Furthermore, cells from affected individuals show a specific defect in retrograde IFT, as expected for dysfunctional IFT-A components (Arts et al., 2011).
5. The IFT-B ‘core’ proteins
The IFT-B complex is biochemically divided into a salt-stable core of nine subunits that associates with a number of peripheral subunits. This salt-stable IFT-B core complex was first isolated from Chlamydomonas flagella (Lucker et al., 2005), but subsequent studies also identified a similar complex in the mouse (Follit et al., 2009). Initially, it was shown to contain the six subunits IFT88/81/74/52/46/27 (Lucker et al., 2005) with IFT70 and IFT25 identified later (Fan et al., 2010; Wang et al., 2009). Although IFT22 was not originally described as a core member, it is referred to as such in a subsequent publication (Lucker et al., 2010). Our own work with recombinant complexes show that IFT22, IFT25 and IFT70 all remain associated with the IFT-B core at higher salt concentrations (Bhogaraju et al., 2011 and Taschner M, unpublished data) and consequently the salt-stable core complex is currently believed to have nine members.
IFT88 is predicted to be a TPR containing protein, and the comparison of the human IFT88 sequence to the PDB shows significant similarity to members of N-acetylglucosamine transferases (OGT) (E=4 × 10−7) that in turn show structural similarity to importin α (Jinek et al., 2004). Both OGT and importin α contain numerous TPRs that form an elongated superhelix where the concave surface is lined by conserved asparagines necessary for the recognition of binding partners. Most of these asparagines are conserved in the IFT88 sequence indicating that a similar mechanism for target recognition may apply. However, if such an interaction surface exists in IFT88, it is currently not known if the target is another protein of the IFT complex or possibly a ciliary recognition motif of cargo proteins.
IFT88 was shown to be absolutely required for flagellar assembly in Chlamydomonas and for ciliogenesis in vertebrates (Pazour et al., 2002a; Pazour et al., 2000). Interestingly, the mammalian ortholog of IFT88 (encoded by the Tg737 gene) is mutated in the Oak Ridge Polycystic Kidney (orpk) mouse, a model for autosomal recessive polycystic kidney disease (ARPKD) (Moyer et al., 1994; Pazour et al., 2000). This link between an IFT protein and polycystic kidney disease caused a significant increase in IFT research. Polycystin-1, polycystin-2 and cystin, the proteins affected in autosomal dominant polycystic kidney disease (ADPKD), were subsequently shown to localize to cilia (Pazour et al., 2002b; Yoder et al., 2002a), further highlighting the link between PKD and cilia. The orpk mouse model is now an important tool to study the requirement for IFT in various organs and processes, and it was shown that IFT88 (and therefore IFT) is required not only for assembly of renal cilia (Pazour et al., 2000; Yoder et al., 2002b), but also for left-right axis determination (Murcia et al., 2000), skeletal patterning (Haycraft et al., 2007; Zhang et al., 2003) and more (reviewed in Lehman et al. (2008)). The mutation in the orpk mouse is hypomorphic, while complete loss-of-function of Tg737 (in the flexo mutant) leads to early embryonic lethality (Huangfu et al., 2003). The C. elegans ortholog of IFT88 is OSM-5 and has been shown to be required for formation of cilia on the dendritic tips of sensory neurons (Haycraft et al., 2001).
IFT70, like IFT88, is predicted to contain a number of TPRs along most of the sequence. It was first identified in C. elegans as DYF-1 and shown to link the homodimeric OSM-3 motor to the IFT-B complex (Ou et al., 2005a; Starich et al., 1995), although it remains to be seen if IFT70/DYF1 interacts directly with OSM-3. Consequently, dyf-1 mutant worms have a specific defect in the assembly of the distal segment of cilia. The zebrafish fleer gene encodes the ortholog of IFT70 in this organism, and fleer mutations lead to defects in ciliary structure, including deformation of the axonemal B-tubules and severely reduced tubulin glutamylation and glycylation (Pathak et al., 2011; Pathak et al., 2007). These modifications correspond to the addition of peptide polymers consisting of multiple glutamates or glycines to the sidechain carboxyl group of a glutamate residue in tubulin, and are carried out by the tubulin tyrosine ligase-like enzymes TTLL3 (for glycylation) and TTLL6 (for glutamylation). Interestingly, the combined knockdown of TTLL3 and TTLL6 causes ciliary defects similar to those observed for fleer (Pathak et al., 2011). It is thus tempting to speculate that IFT70/Fleer might specifically recognize these enzymes as IFT-cargos, although this notion remains to be proven. Defective tubulin glutamylation was also shown to occur in the C. elegans dyf-1 mutant (Pathak et al., 2007). Even though these results suggest a conserved role for IFT70/DYF-1 in this enzymatic tubulin modification, the situation is different in Tetrahymena, where DYF-1 is also required for the proper formation of the axoneme, but axonemal tubulin subunits are hyper-rather than hypo-glutamylated in the mutant strain, and tubulin glycylation is not significantly affected (Dave et al., 2009). Chlamydomonas IFT70 was recently cloned based on sequence similarity to C. elegans DYF-1 (Fan et al., 2010). IFT70/CrDYF-1 depletion causes axonemal defects and destabilization of the IFT-B complex, but the effect on post-translational tubulin modification by glutamylation and/or glycylation has not been examined (Fan et al., 2010).
IFT74 and IFT81 display a similar structural organization with long predicted coiled-coil regions (Fig. 1). The two proteins appear to be distant homologs as they share about 15-20% sequence identity over approx. 650 residues. IFT81 was shown to associate with itself and with IFT74 via the coiled-coil regions in Y2H studies and, based on these results, an IFT74-81-81-74 heterotetramer was suggested to exist (Lucker et al., 2005). A subsequent study in C. elegans confirmed a direct IFT81/IFT74 interaction using Y2H, and also showed that the localization patterns of these two proteins overlap significantly with other IFT-B proteins and that mutations in their genes lead to chemosensory defects (Kobayashi et al., 2007). Additionally, our recombinant expression and purification has verified the existence of a stable sub-complex containing IFT81/74/27/25 (Taschner et al., 2011). There is thus sufficient evidence that IFT74 and IFT81 interact directly, but it remains to be verified if IFT74 and IFT81 form a heterodimer or a heterotetramer in the context of the IFT-B complex. We found in our reconstitution and purification experiments that an IFT88/81/74/70/52/46/27/25 octamer is formed in vitro, in which only one copy of each protein is present, and so the formation of an (IFT81)2/(IFT74)2 heterotetramer is not a prerequisite for the assembly of such a complex (Taschner M, unpublished).
Additional protein domains/motifs in IFT81/74, apart from the long coiled-coil regions, were not reported previously. Secondary structure predictions for the N-terminus of IFT74 does not show any significant α-helices or β-sheets, but the first 140 amino acids of IFT81 are clearly predicted as mainly α-helical and could constitute a small N-terminal domain. Using the HHpred algorithm (Soding et al., 2005), we found that this region shows significant similarity to a calponin-homology (CH) domain (E=2.2 × 10−2) that is known to bind microtubules in case of end-binding protein 1(EB1) (Hayashi and Ikura, 2003) and the NDC80 protein that is involved in kinetochore-microtubule attachment during cell division (Ciferri et al., 2008). However, further studies are needed to determine the function of this domain during IFT.
IFT52 is the IFT-B core subunit mutated in the bld1 strain of Chlamydomonas (Brazelton et al., 2001), and is the ortholog of OSM-6 previously characterized in C. elegans (Collet et al., 1998) and NGD5 in mammals (Wick et al., 1995). Mutation of IFT52 in green algae leads to ‘bald’ cells, which (like ift88 mutants) are completely unable to assemble flagella, highlighting a central role of this protein in IFT. These results may be explained by the observation that IFT52 is crucial to the IFT-B core complex assembly and stability as it interacts directly with the IFT46, IFT70 and IFT88 proteins as well as with the IFT81/74/27/25 heterotetramer (Taschner et al., 2011). High-resolution localization studies by immunoelectron microscopy showed that IFT52 localizes to the periphery of the transitional fibers, suggesting that these structures form a docking site for IFT particles (Deane et al., 2001). Regarding specific domains/motifs in IFT52, it should be noted that the protein is predicted to contain an N-terminal ‘GIFT’-domain (GldG/IFT) that is suggested to bind oligosaccharides (Beatson and Ponting, 2004), but as we could not detect significant binding to any glycan ligand in a screen using recombinantly produced CrIFT52 protein (Taschner et al., 2011) the importance of this region for the IFT process remains to be addressed. Additionally, IFT52 contains a proline-rich stretch (residues 330-360 in CrIFT52) that is predicted as an SH3 domain interacting region. This proline-rich sequence is part of a region of IFT52 (residues 281-381) that interacts directly with both IFT70 and IFT88 (Taschner et al., 2011). Further experiments in our lab have revealed that the proline-rich motif of IFT52 appears to solubilize and directly bind to IFT70 (M. Taschner and E. Lorentzen, unpublished). Curiously, IFT70 is not predicted to contain any SH3 domain and the molecular nature of the IFT52/70 interaction is currently unknown.
IFT46 sequence analysis does not reveal any recognizable domains or motifs. The gene encoding IFT46 was first cloned from C. elegans as DYF-6 (Bell et al., 2006) and later from Chlamydomonas (Hou et al., 2007), and in both cases the cilia/flagella of the mutants are dramatically shortened, but the defect is not as severe as in strains lacking IFT88 or IFT52. Western blots for IFT-B components in Chlamydomonas ift46 mutants revealed that the levels of several IFT-B proteins are dramatically reduced, indicating that IFT46 is required for the stability of the IFT-B subcomplex. These observations agree well with experiments showing that IFT46 interacts directly with IFT52, IFT70 and IFT88 within the IFT-B core (Fan et al., 2010; Lucker et al., 2010; Taschner et al., 2011). Furthermore, the mutant flagella displayed a lack of axonemal outer dynein arms, pointing towards a specific role of IFT46 in the cargo-recognition of these complexes. Interestingly, a suppressor mutant was identified, which was able to assemble full-length flagella, which still lacked outer dynein arms and were immotile (Hou et al., 2007). The authors suggested that the C-terminus of IFT46 was expressed in this suppressor strain, restoring the stability of the IFT-B complex. It follows from this that IFT46 could have two functions, one general role in maintaining IFT-B complex stability and a specific role in the recognition of outer dynein arms as an IFT-cargo. Indeed it has been reported that the protein ODA16 was found to bind directly to IFT46 and appears to function as a specific adapter between the IFT complex and outer dynein arms (Ahmed et al., 2008).
IFT27 and IFT25 were found to interact with each other in green algae (Wang et al., 2009), mouse (Follit et al., 2009) and human (Rual et al., 2005). IFT27 shows clear homology to small Rab GTPases typically involved in the regulation of membrane trafficking and has thus been suggested to have a regulatory role in IFT (Qin et al., 2007). In green algae, a complete knockdown with siRNA of IFT27 is lethal whereas a partial knockdown leads to significantly shorter cilia (Qin et al., 2007). It is however not completely clear if the lethality of the IFT27 knockdown can be attributed solely to IFT27 or if other important GTPases might also be affected in the siRNA experiment (Qin et al., 2007). IFT25 (also referred to as C1orf41, Hsp16.2 or FAP232) was not identified in the initial biochemical characterization of the IFT-B complex (Cole et al., 1998; Lucker et al., 2005), but was found in later studies both in Chlamydomonas (Lechtreck et al., 2009b; Wang et al., 2009) and in the mouse (Follit et al., 2009). IFT25 is a calcium-binding protein and adopts a jelly-roll fold (Ramelot et al., 2009), and appears to have a function in solubilizing and/or stabilizing IFT27 (Bhogaraju et al., 2011). Interestingly, in green algae, a significant amount of IFT27/25 was found in a stable subcomplex that only associates with the remaining IFT-B complex upon entrance into the cilium, suggesting a regulatory function of IFT initiation by the IFT27/25 complex (Wang et al., 2009). We recently determined the crystal structure of the CrIFT27/25 complex and carried out a biochemical investigation of nucleotide binding and GTPase activity in vitro (Bhogaraju et al., 2011). In this study, we were able to show that IFT27 is indeed a GTPase as it displays very low but significant intrinsic GTPase activity, and that IFT27 binds GDP and GTP weakly (Kd in the micromolar range). These results suggest that IFT27 may not need a factor for the exchange of GDP for GTP but is likely to rely on a GTPase activation protein (GAP) for robust GTP turnover, although such a potential GAP has not yet been identified (Bhogaraju et al., 2011). In summary, IFT27 may serve a crucial role in IFT complex assembly and in IFT initiation, even though the molecular mechanism of such a regulation remains to be unraveled.
IFT22 is another core subunit that was not immediately identified in the pioneering characterizations of the Chlamydomonas IFT-B complex (Cole et al., 1998; Lucker et al., 2005), but was discovered subsequently in C. elegans (Schafer et al., 2006), C. reinhardtii (Wang et al., 2009) and Trypanosoma brucei (Adhiambo et al., 2009). Like IFT27, IFT22 displays significant sequence similarity to Rab-like GTPases (and is known as RabL5 in mammals), but its GTPase activity has not yet been analyzed. Curiously, both IFT22 and IFT27 lack the prenylation motif containing multiple cysteines at the C-termini that typically allow Rab GTPases to associate with membranes (Qin et al., 2007; Schafer et al., 2006). The IFT22 ortholog in C. elegans is the IFTA-2 protein, that was found to undergo IFT and lead to an increased life-span of worms when mutated (Schafer et al., 2006). Mutation of IFTA-2 in worms, however, was not found to cause any abnormalities in cilia (Schafer et al., 2006). In the trypanosome flagellum, IFT22 was shown to undergo IFT and its knockdown by RNAi surprisingly results in a retrograde IFT inactivation phenotype with short flagella filled with IFT material (Adhiambo et al., 2009). The same authors make the interesting observation that IFT22 does not have the G4 GTPase-motif conferring specificity for guanine over adenine conserved (also noted by Schafer et al., 2006). Because other predicted small GTPases with G4 mutated has turned out to be specific for ATP rather than GTP (Espinosa et al., 2009), it is currently not clear if IFT22 is a GTPase or an ATPase. Further characterization of IFT22 is thus likely to yield interesting insights into its molecular function.
6. The peripheral IFT-B proteins
IFT172, IFT80, IFT57, IFT54 and IFT20 are not counted as IFT-B core complex members as they dissociate at NaCl concentrations above 300 mM (Lucker et al., 2005). These peripheral IFT-B subunits, however, serve important functions in IFT regulation and carrier vesicle targeting from the Golgi network to the cilium as described in the following sections.
IFT172 is the largest IFT protein and has a structural organization similar to that of the large IFT-A proteins, with predicted N-terminal WD-40 repeats and C-terminal α-helical TPRs. A total of 52 β-strands are predicted, which is more compatible with two rather than one β-propeller at the N-terminus, a notion that is further corroborated by the similarity to the double β-propeller protein AIP1 (E=3.8 × 10−2). IFT172 is encoded by the FLA11 gene in Chlamydomonas and is required for the localization of the microtubule plus-end binding protein EB1 to the tips of cilia (Pedersen et al., 2003; Pedersen et al., 2005). Strikingly, the phenotype of a temperature sensitive fla11 mutant is similar to IFT-A mutants, with accumulation of IFT complexes at flagellar tips, indicating that IFT172 has a role in tip turnaround (Iomini et al., 2001). Similar results for IFT172 have been obtained in Tetrahymena (Tsao and Gorovsky, 2008a). In zebrafish, IFT172 is absolutely required for photoreceptor assembly (Sukumaran and Perkins, 2009), and the mutation of IFT172 (wimple) in the mouse is embryonic lethal, as observed also for IFT88, due to a strong defect in Shh signaling during embryogenesis (Huangfu et al., 2003).
IFT80 is the only other IFT-B protein, apart from IFT172, where WD-40 repeats are predicted with a high probability, but no TPRs are found at the C-terminus, which is predicted to contain only a short stretch of α-helical structure (Fig. 1). Not much work has been carried out on Chlamydomonas IFT80, but mutation of the C. elegans ortholog, CHE-2, leads to defects in ciliogenesis (Fujiwara et al., 1999). Similarly, knockdown of this factor in Tetrahymena leads to absent or significantly shortened cilia, and knockdown in the zebrafish causes cystic kidneys and photoreceptor degeneration (Beales et al., 2007; Hudak et al., 2010). IFT80 is also relevant for human disease, because mutations have been identified as the underlying cause for Jeune asphyxiating thoracic dystrophy, which often leads to death in early childhood because of respiratory insufficiency (Beales et al., 2007). Interesting results were recently obtained for IFT80 in the mouse. Hypomorphic levels of IFT80 lead to embryonic lethality associated with severe defects in hedgehog signaling (Rix et al., 2011), reminiscent of mutation in other IFT-B components such as IFT172 and IFT88 (Huangfu et al., 2003). Interestingly, other characteristic ciliary phenotypes (e.g. left-right asymmetry defects and retinal degeneration) were not observed, and cilia structure seemed unaffected. The authors therefore concluded that the low background levels of IFT80 are sufficient for ciliary assembly and maintenance, but that high levels of the protein are required for efficient hedgehog signaling (Rix et al., 2011). It is tempting to speculate that IFT80 might be required for the specific movement of hedgehog signaling components, such as the membrane protein Smoothened (Corbit et al., 2005) or the Gli transcription factors (Haycraft et al., 2005), which is a prerequisite for hedgehog signal transduction (Wong and Reiter, 2008).
IFT57 is known as CHE-13 in C. elegans, where it displays ciliary localization (Haycraft et al., 2003), and che-13 mutants show defects in ciliogenesis comparable to other IFT-B factors (Perkins et al., 1986). Knock-out of the mouse IFT57 ortholog, Hippi, leads to defects in nodal cilia, left-right patterning defects, sonic hedgehog signaling defects, and embryonic lethality (Houde et al., 2006). Interestingly, Hippi has been found to bind to Huntingtin-interacting protein 1 (HIP-1) in a complex that recruits and activates caspase-8, which induces apoptosis and might be relevant for the pathogenesis of Huntington’s disease (Gervais et al., 2002). Further studies have also implicated Hippi in the regulation of gene expression (reviewed in Bhattacharyya et al. (2008)). Regarding its structural organization, IFT57 has clearly predicted coiled-coils at the C-terminus, that is required for the interaction with IFT20 (Baker et al., 2003). In addition to the coiled-coil region, we found a potential CH-domain in the N-terminal half of IFT57 using the HHpred algorithm (Soding et al., 2005), but the functional significance of this remains to be determined.
IFT54 is known as DYF-11 in C. elegans (Bacaj et al., 2008; Kunitomo and Iino, 2008; Li et al., 2008) and is required for sensory cilia formation. The closest mammalian relative was shown to be TRAF3IP1/MIP-T3, a protein, which contains a microtubule- and β-tubulin binding domain at its N-terminus (Kunitomo and Iino, 2008; Ling and Goeddel, 2000) and a coiled coil region at the C-terminus that was shown to interact with IFT20 (Follit et al., 2009; Omori et al., 2008). The solution structure of the N-terminal domain of human TRAF3IP1 is available (pdb entry 2EQO, Dang and Yokoyama, unpublished) and displays a CH domain most similar to domains found in actin binding proteins, but its significance and functionality in IFT has not been addressed. In mice, the knock-out of the gene encoding IFT54/TRAF3IP1 is lethal at the embryonic stage, demonstrating an important function of this protein in development (Berbari et al., 2011). Elipsa, the zebrafish version of IFT54, localizes to cilia, and elipsa mutation causes phenotypes indicative of ciliary defects, including a curly body axis and kidney cysts (Omori et al., 2008). In the same study, the authors identified a direct interaction between Elipsa and Rabaptin-5, which in turn binds to the small GTPase Rab8. Rab8 plays an important role in the transport of vesicles to the base of the cilium and their subsequent fusion with the plasma membrane (Moritz et al., 2001; Nachury et al., 2007). It is thus speculated that an important function of Elipsa might be to bridge the IFT complex with membrane-associated complexes (Omori et al., 2008).
IFT20, the smallest IFT protein, differs from all the other IFT complex proteins with respect to its subcellular localization. It is not only found in the peri-basal body region and inside the cilium, but also at the Golgi complex in mammalian cells (Follit et al., 2006). Localization to this compartment is achieved by interaction with the Golgi protein GMAP210 (Follit et al., 2008), and several lines of evidence suggest that IFT20 functions at the Golgi in the sorting and/or transport of proteins destined for the ciliary membrane. Moderate knockdown of IFT20 decreases the ciliary concentration of the membrane protein polycystin-2, an effect also observed in GMAP210-deficient cells (Follit et al., 2008; Follit et al., 2006). Furthermore, IFT20 has been shown to bind to the C-terminal tail of photoreceptor proteins rhodopsin and opsin in pull-down experiments, and IFT20 deletion in cone photoreceptor cells causes accumulation of these proteins at the Golgi-complex, indicating a defect in their targeting to the photoreceptor outer segment (Keady et al., 2011).
Several studies reported direct protein–protein interactions involving IFT20 and the other two peripheral IFT-B subunits IFT57 and IFT54. The Besharse lab was the first to show a direct interaction between mammalian IFT20 and IFT57 using the yeast-two-hybrid system, and mapped the IFT-20 interacting region to the IFT57 C-terminus, which contains the predicted coiled-coil region (Baker et al., 2003). IFT54/Elipsa was also shown to associate with IFT20 by yeast-two-hybrid and pull-down experiments (Omori et al., 2008), indicating that these three peripheral IFT-B proteins might form a trimeric complex. Baker and colleagues not only reported a direct interaction between IFT57 and IFT20, but also with IFT20 and KIF3B, one of the motor subunits of heterotrimeric kinesin II (Baker et al., 2003). Subsequent studies have, however, not been able to confirm such an interaction (Follit et al., 2006). This reported interaction between IFT20 and KIF3B could be unspecific binding to a part of the coiled-coil region of KIF3B that in the context of a native heterotrimeric kinesin complex interacts with KIF3A and is thus not exposed. Further studies are needed to elucidate the relevance of this putative interaction.
7. Functions of IFT proteins outside of ciliogenesis
Apart from their critical role in the formation and maintenance of cilia, IFT proteins are also found in non-ciliated cells and have been implicated in cellular processes such as mitosis and formation of the immune synapse (Baldari and Rosenbaum, 2010; Griffiths et al., 2010; Sedmak and Wolfrum, 2010). IFT88, a core component of the IFT-B complex, localizes to centrosomes in actively proliferating cells (Robert et al., 2007). The knockdown of this gene results in increased cell proliferation, and overexpression leads to prevention of S phase entry and activation of apoptosis. Interestingly, this IFT88 mutant phenotype is linked to its physical interaction with Che-1, a regulator of the E2F transcription factor. In another related study, Doxsey and co-workers showed that IFT88 mutants have very specific defects in the cell division machinery. In HeLa cells, IFT88 knockdown leads to misorientation of mitotic spindle poles and shortening or absence of astral microtubules. Akin to IFT in cilia, IFT88 exists in complex with other IFT proteins (IFT52 and IFT20) and is involved in the transport of microtubule clusters from the periphery to the poles during mitosis in a motor driven process involving dynein (Delaval et al., 2011).
Surprisingly, another important role of IFT proteins outside cilia is in the assembly of the immune synapse (Finetti et al., 2009). IFT20, apart from its role in the sorting of ciliary proteins at the Golgi network, is found to be involved in the polarized recycling of T-cell receptors (TCRs). IFT20 knockdown cells (Jurkat T-lymphoma cells) failed to form clusters of TCR/CD3 at the immune synapse and thus were defective in activation by antigen presenting cells (APCs). Apart from IFT20, other IFT proteins that localize to the immune synapse include IFT57 and IFT88. Although distinct from IFT in cilia, the processes mentioned above share some very interesting mechanistic aspects with IFT. These include, but may not be limited to, microtubule-dependent motor-driven transport, vesicular trafficking and centrosome orientation. It will be very interesting to test if the other proteins of the IFT complex are also involved in these processes, and to see if the IFT complex plays a role in other mechanistically similar cellular processes.
8. Conclusions
In this review article we summarized the current knowledge about the function and architecture of IFT proteins and complexes. Although 20 subunits of the IFT complex have so far been described, it seems likely that additional subunits or accessory factors remain to be identified. Some of these potential IFT proteins that were not discussed in detail in this review include CrFAP22/CeDyf-3/HsCLUAP1/Qilin (Murayama et al., 2005; Ou et al., 2005b; Sun et al., 2004) and CrDyf13/CeDyf-13/HsTTC26 (Blacque et al., 2005; Inglis et al., 2009), which have been clearly shown to have an influence on IFT and have recently been described as ‘IFT accessory factors’ (Ishikawa and Marshall, 2011).
Although the overall picture of IFT has been put in place, most of the molecular details underlying this process still remain to be unraveled. Very little is known about the molecular functions of the individual IFT proteins. Which IFT subunits have structural roles in holding the IFT particle together, which mediate contacts to cargo and motor proteins and which serve regulatory roles in initiation and remodeling to switch from anterograde to retrograde transport at the ciliary tip? Another unresolved question is the nature of putative ciliary ZIP codes analogous to nuclear localization signals. With about 600 proteins in the cilium it is clear that ciliary targeting sequences are likely to exist. However, the nature of such sequences remains largely enigmatic. With a lot of important discoveries still awaiting, we can be sure that the field of IFT research will stay exciting for many years to come.
References
- Adams GM, Huang B, Luck DJ. Temperature-sensitive, assembly-defective flagella mutants of Chlamydomonas reinhardtii. Genetics. 1982;100:579–586. doi: 10.1093/genetics/100.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhiambo C, Blisnick T, Toutirais G, Delannoy E, Bastin P. A novel function for the atypical small G protein Rab-like 5 in the assembly of the trypanosome flagellum. Journal of Cell Science. 2009;122:834–841. doi: 10.1242/jcs.040444. [DOI] [PubMed] [Google Scholar]
- Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. The Journal of Cell Biology. 2008;183:313–322. doi: 10.1083/jcb.200802025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MA, Ponting CP, Gibson TJ, Bork P. Homology-based method for identification of protein repeats using statistical significance estimates. Journal of Molecular Biology. 2000;298:521–537. doi: 10.1006/jmbi.2000.3684. [DOI] [PubMed] [Google Scholar]
- Arts HH, Bongers EMHF, Mans DA, van Beersum SEC, Oud MM, Bolat E, Spruijt L, Cornelissen EAM, Schuurs-Hoeijmakers JHM, de Leeuw N, Cormier-Daire V, Brunner HG, Knoers NVAM, Roepman R. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. Journal of Medical Genetics. 2011;48:390–395. doi: 10.1136/jmg.2011.088864. [DOI] [PubMed] [Google Scholar]
- Bacaj T, Lu Y, Shaham S. The conserved proteins CHE-12 and DYF-11 are required for sensory cilium function in Caenorhabditis elegans. Genetics. 2008;178:989–1002. doi: 10.1534/genetics.107.082453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC. IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. The Journal of Biological Chemistry. 2003;278:34211–34218. doi: 10.1074/jbc.M300156200. [DOI] [PubMed] [Google Scholar]
- Baldari CT, Rosenbaum J. Intraflagellar transport: it’s not just for cilia anymore. Current Opinion in Cell Biology. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales PL. Lifting the lid on Pandora’s box: the Bardet-Biedl syndrome. Current Opinion in Genetics and Development. 2005;15:315–323. doi: 10.1016/j.gde.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, Johnson C, Irving M, Elcioglu N, Winey M, Tada M, Scambler PJ. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nature Genetics. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- Beatson S, Ponting CP. GIFT domains: linking eukaryotic intraflagellar transport and glycosylation to bacterial gliding. Trends in Biochemical Sciences. 2004;29:396–399. doi: 10.1016/j.tibs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Bell LR, Stone S, Yochem J, Shaw JE, Herman RK. The molecular identities of the Caenorhabditis elegans intraflagellar transport genes dyf-6, daf-10 and osm-1. Genetics. 2006;173:1275–1286. doi: 10.1534/genetics.106.056721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Kin NW, Sharma N, Michaud EJ, Kesterson RA, Yoder BK. Mutations in Traf3ip1 reveal defects in ciliogenesis, embryonic development, and altered cell size regulation. Developmental Biology. 2011 doi: 10.1016/j.ydbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya NP, Banerjee M, Majumder P. Huntington’s disease: roles of huntingtin-interacting protein 1 (HIP-1) and its molecular partner HIPPI in the regulation of apoptosis and transcription. The FEBS Journal. 2008;275:4271–4279. doi: 10.1111/j.1742-4658.2008.06563.x. [DOI] [PubMed] [Google Scholar]
- Bhogaraju S, Taschner M, Morawetz M, Basquin C, Lorentzen E. Crystal structure of the intraflagellar transport complex 25/27. The EMBO Journal. 2011;30:1907–1918. doi: 10.1038/emboj.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque OE, Li C, Inglis PN, Esmail MA, Ou G, Mah AK, Baillie DL, Scholey JM, Leroux MR. The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Molecular Biology of the Cell. 2006;17:5053–5062. doi: 10.1091/mbc.E06-06-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque OE, Perens EA, Boroevich KA, Inglis PN, Li C, Warner A, Khattra J, Holt RA, Ou G, Mah AK, McKay SJ, Huang P, Swoboda P, Jones SJM, Marra MA, Baillie DL, Moerman DG, Shaham S, Leroux MR. Functional genomics of the cilium, a sensory organelle. Current Biology: CB. 2005;15:935–941. doi: 10.1016/j.cub.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RHA, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes & Development. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatch GL, Lässle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Brazelton WJ, Amundsen CD, Silflow CD, Lefebvre PA. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Current Biology: CB. 2001;11:1591–1594. doi: 10.1016/s0960-9822(01)00485-7. [DOI] [PubMed] [Google Scholar]
- Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbo M, Filhol E, Bole-Feysot C, Nitschke P, Gilissen C, Haugen OH, Sanders JS, Stolte-Dijkstra I, Mans DA, Steenbergen EJ, Hamel BC, Matignon M, Pfundt R, Jeanpierre C, Boman H, Rodahl E, Veltman JA, Knappskog PM, Knoers NV, Roepman R, Arts HH. Ciliopathies with Skeletal Anomalies and Renal Insufficiency due to Mutations in the IFT-A Gene WDR19. The American Journal of Human Genetics. 2011 doi: 10.1016/j.ajhg.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard P, Stetefeld J, Strelkov SV. Coiled coils: a highly versatile protein folding motif. Trends in Cell Biology. 2001;11:82–88. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, Salek M, Rappsilber J, Moores CA, Salmon ED, Musacchio A. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG. Kinesin-II, the heteromeric kinesin. Cellular and Molecular Life Sciences: CMLS. 1999;56:217–226. doi: 10.1007/s000180050423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic (Copenhagen, Denmark) 2003;4:435–442. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- Cole DG, Chinn SW, Wedaman KP, Hall K, Vuong T, Scholey JM. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 1993;366:268–270. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. The Journal of Cell Biology. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG, Snell WJ. SnapShot: intraflagellar transport. Cell. 2009;137(784-784):e781. doi: 10.1016/j.cell.2009.04.053. [DOI] [PubMed] [Google Scholar]
- Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Cortellino S, Wang C, Wang B, Bassi MR, Caretti E, Champeval D, Calmont A, Jarnik M, Burch J, Zaret KS, Larue L, Bellacosa A. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Developmental Biology. 2009;325:225–237. doi: 10.1016/j.ydbio.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige B, Tsao C-C, Diener DR, Hou Y, Lechtreck K-F, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. The Journal of Cell Biology. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave D, Wloga D, Sharma N, Gaertig J. DYF-1 Is required for assembly of the axoneme in Tetrahymena thermophila. Eukaryotic Cell. 2009;8:1397–1406. doi: 10.1128/EC.00378-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Current Biology: CB. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nature Cell Biology. 2011;13:461–468. doi: 10.1038/ncb2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biology. 2004;2:e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimenko E, Blacque OE, Ou G, Haycraft CJ, Yoder BK, Scholey JM, Leroux MR, Swoboda P. Caenorhabditis elegans DYF-2, an orthologue of human WDR19, is a component of the intraflagellar transport machinery in sensory cilia. Molecular Biology of the Cell. 2006;17:4801–4811. doi: 10.1091/mbc.E06-04-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. Journal of Cell Science. 2010;123:529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa EJ, Calero M, Sridevi K, Pfeffer SR. RhoBTB3: a Rho GTPase-family ATPase required for endosome to golgi transport. Cell. 2009;137:938–948. doi: 10.1016/j.cell.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z-C, Behal RH, Geimer S, Wang Z, Williamson SM, Zhang H, Cole DG, Qin H. Chlamydomonas IFT70/CrDYF-1 is a core component of IFT particle complex B and is required for flagellar assembly. Molecular Biology of the Cell. 2010;21:2696–2706. doi: 10.1091/mbc.E10-03-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature Cell Biology. 2009;11:1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nature Reviews. Molecular Cell Biology. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ. The golgin GMAP210/TRIP11 anchors IFT20 to the golgi complex. PLoS Genetics. 2008;4:e1000315. doi: 10.1371/journal.pgen.1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the golgi complex and is required for cilia assembly. Molecular Biology of the Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Xu F, Keady BT, Pazour GJ. Characterization of mouse IFT complex B. Cell Motility and the Cytoskeleton. 2009;66:457–468. doi: 10.1002/cm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Ishihara T, Katsura I. A novel WD40 protein, CHE-2, acts cell-autonomously in the formation of C. elegans sensory cilia. Development (Cambridge, England) 1999;126:4839–4848. doi: 10.1242/dev.126.21.4839. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Singaraja R, Xanthoudakis S, Gutekunst C-A, Leavitt BR, Metzler M, Hackam AS, Tam J, Vaillancourt JP, Houtzager V, Rasper DM, Roy S, Hayden MR, Nicholson DW. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nature Cell Biology. 2002;4:95–105. doi: 10.1038/ncb735. [DOI] [PubMed] [Google Scholar]
- Gilissen C, Arts HH, Hoischen A, Spruijt L, Mans DA, Arts P, van Lier B, Steehouwer M, van Reeuwijk J, Kant SG, Roepman R, Knoers NVAM, Veltman JA, Brunner HG. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. American Journal of Human Genetics. 2010;87:418–423. doi: 10.1016/j.ajhg.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature Reviews Genetics. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: a focal point for endocytosis and exocytosis. Journal of Cell Biology. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, De Baere E, Lo A, Chang W, Messiaen L. Cloning and characterization of human WDR10, a novel gene located at 3q21 encoding a WD-repeat protein that is highly expressed in pituitary and testis. DNA and Cell Biology. 2001;20:41–52. doi: 10.1089/10445490150504684. [DOI] [PubMed] [Google Scholar]
- Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nature Cell Biology. 2011 doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Ikura M. Crystal structure of the amino-terminal microtubule-binding domain of end-binding protein 1 (EB1) The Journal of Biological Chemistry. 2003;278:36430–36434. doi: 10.1074/jbc.M305773200. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genetics. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Schafer JC, Zhang Q, Taulman PD, Yoder BK. Identification of CHE-13, a novel intraflagellar transport protein required for cilia formation. Experimental Cell Research. 2003;284:251–263. doi: 10.1016/s0014-4827(02)00089-7. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Swoboda P, Taulman PD, Thomas JH, Yoder BK. The C. elegans homolog of the murine cystic kidney disease gene Tg737 functions in a ciliogenic pathway and is disrupted in osm-5 mutant worms. Development (Cambridge, England) 2001;128:1493–1505. doi: 10.1242/dev.128.9.1493. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development (Cambridge, England) 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Hou Y, Pazour GJ, Witman GB. A dynein light intermediate chain, D1bLIC, is required for retrograde intraflagellar transport. Molecular Biology of the Cell. 2004;15:4382–4394. doi: 10.1091/mbc.E04-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, Witman GB. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. The Journal of Cell Biology. 2007;176:653–665. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde C, Dickinson RJ, Houtzager VM, Cullum R, Montpetit R, Metzler M, Simpson EM, Roy S, Hayden MR, Hoodless PA, Nicholson DW. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Developmental Biology. 2006;300:523–533. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Hudak LM, Lunt S, Chang C-H, Winkler E, Flammer H, Lindsey M, Perkins BD. The intraflagellar transport protein ift80 is essential for photo-receptor survival in a zebrafish model of jeune asphyxiating thoracic dystrophy. Investigative Ophthalmology & Visual Science. 2010;51:3792–3799. doi: 10.1167/iovs.09-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis PN, Blacque OE, Leroux MR. Functional genomics of intraflagellar transport-associated proteins in C. elegans. Methods in Cell Biology. 2009;93:267–304. doi: 10.1016/S0091-679X(08)93014-4. [DOI] [PubMed] [Google Scholar]
- Inglis PN, Ou G, Leroux MR, Scholey JM. The sensory cilia of Caenorhabditis elegans. WormBook: The Online Review of C. elegans Biology. 2007:1–22. doi: 10.1895/wormbook.1.126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Humby M, Sedmak T, Wolfrum U, Besharse JC. Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 2009;238:2211–2222. doi: 10.1002/dvdy.21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Developmental Biology. 2008;316:160–170. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. The Journal of Cell Biology. 2001;153:13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini C, Li L, Esparza JM, Dutcher SK. Retrograde intraflagellar transport mutants identify complex A proteins with multiple genetic interactions in Chlamydomonas reinhardtii. Genetics. 2009;183:885–896. doi: 10.1534/genetics.109.101915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nature Reviews. Molecular Cell Biology. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Jékely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 2006;28:191–198. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nature Structural and Molecular Biology. 2004;11:1001–1007. doi: 10.1038/nsmb833. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. The Journal of Cell Biology. 1992;119:1605–1611. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. Journal of Molecular Biology. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- Keady BT, Le YZ, Pazour GJ. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Molecular Biology of the Cell. 2011;22:921–930. doi: 10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Gengyo-Ando K, Ishihara T, Katsura I, Mitani S. IFT-81 and IFT-74 are required for intraflagellar transport in C. elegans. Genes to Cells. 2007;12:593–602. doi: 10.1111/j.1365-2443.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. The Journal of Cell Biology. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitomo H, Iino Y. Caenorhabditis elegans DYF-11, an orthologue of mammalian Traf3ip1/MIP-T3, is required for sensory cilia formation. Genes to Cells. 2008;13:13–25. doi: 10.1111/j.1365-2443.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- Lechtreck K-F, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. The Journal of Cell Biology. 2009a;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck K-F, Luro S, Awata J, Witman GB. HA-tagging of putative flagellar proteins in Chlamydomonas reinhardtii identifies a novel protein of intraflagellar transport complex B. Cell Motility and the Cytoskeleton. 2009b;66:469–482. doi: 10.1002/cm.20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Sivan-Loukianova E, Eberl DF, Kernan MJ. An IFT-A protein is required to delimit functionally distinct zones in mechanosensory cilia. Current Biology: CB. 2008;18:1899–1906. doi: 10.1016/j.cub.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge polycystic kidney mouse: modeling ciliopathies of mice and men. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 2008;237:1960–1971. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Inglis PN, Leitch CC, Efimenko E, Zaghloul NA, Mok CA, Davis EE, Bialas NJ, Healey MP, Héon E, Zhen M, Swoboda P, Katsanis N, Leroux MR. An essential role for DYF-11/MIP-T3 in assembling functional intraflagellar transport complexes. PLoS Genetics. 2008;4:e1000044. doi: 10.1371/journal.pgen.1000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, White JT, Utleg AG, Wang S, Ferguson C, True LD, Vessella R, Hood L, Nelson PS. Isolation and characterization of human and mouse WDR19,a novel WD-repeat protein exhibiting androgen-regulated expression in prostate epithelium. Genomics. 2003;82:331–342. doi: 10.1016/s0888-7543(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Ling L, Goeddel DV. MIP-T3, a novel protein linking tumor necrosis factor receptor-associated factor 3 to the microtubule network. The Journal of Biological Chemistry. 2000;275:23852–23860. doi: 10.1074/jbc.M001095200. [DOI] [PubMed] [Google Scholar]
- Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. The Journal of Biological Chemistry. 2005;280:27688–27696. doi: 10.1074/jbc.M505062200. [DOI] [PubMed] [Google Scholar]
- Lucker BF, Miller MS, Dziedzic SA, Blackmarr PT, Cole DG. Direct interactions of intraflagellar transport complex B proteins IFT88, IFT52, and IFT46. The Journal of Biological Chemistry. 2010;285:21508–21518. doi: 10.1074/jbc.M110.106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Lefebvre PA, Kamiya R, Hirono M. Kinesin-II is not essential for mitosis and cell growth in Chlamydomonas. Cell Motility and The Cytoskeleton. 2002;52:195–201. doi: 10.1002/cm.10051. [DOI] [PubMed] [Google Scholar]
- Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MAM, Keighren M, Bahlo M, Bromhead CJ, Budd P, Aftimos S, Delatycki MB, Savarirayan R, Jackson IJ, Amor DJ. Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. American Journal of Human Genetics. 2011;88:508–515. doi: 10.1016/j.ajhg.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri K, Vorobiev S, Fedorov AA, Almo SC, Ono S. Identification of functional residues on Caenorhabditis elegans actin-interacting protein 1 (UNC-78) for disassembly of actin depolymerizing factor/cofilin-bound actin filaments. The Journal of Biology Chemistry. 2004;279:31697–31707. doi: 10.1074/jbc.M403351200. [DOI] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Hurd LL, Peränen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Molecular Biology of the Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science (New York, NY) 1994;264:1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes & Development. 2010;24:2180–2193. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T, Toh Y, Ohshima Y, Koga M. The dyf-3 gene encodes a novel protein required for sensory cilium formation in Caenorhabditis elegans. Journal of Molecular Biology. 2005;346:677–687. doi: 10.1016/j.jmb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge polycystic kidney (orpk) disease gene is required for left-right axis determination. Development (Cambridge, England) 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annual Review of Cell and Developmental Biology. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nature Cell Biology. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005a;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- Ou G, Qin H, Rosenbaum JL, Scholey JM. The PKD protein qilin undergoes intraflagellar transport. Current Biology: CB. 2005b;15:R410–411. doi: 10.1016/j.cub.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Pathak N, Austin CA, Drummond IA. Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. The Journal of Biological Chemistry. 2011;286:11685–11695. doi: 10.1074/jbc.M110.209817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Molecular Biology of the Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. The Journal of Cell Biology. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. The Journal of Cell Biology. 2002a;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. The Journal of Cell Biology. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. The Journal of cell Biology. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Current Biology: CB. 2002b;12:R378–380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) The Journal of Cell Biology. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Geimer S, Sloboda RD, Rosenbaum JL. The Microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Current Biology: CB. 2003;13:1969–1974. doi: 10.1016/j.cub.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Miller MS, Geimer S, Leitch JM, Rosenbaum JL, Cole DG. Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Current Biology: CB. 2005;15:262–266. doi: 10.1016/j.cub.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Developmental Biology. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Perrone CA, Tritschler D, Taulman P, Bower R, Yoder BK, Porter ME. A novel dynein light intermediate chain colocalizes with the retrograde motor for intraflagellar transport at sites of axoneme assembly in Chlamydomonas and mammalian cells. Molecular Biology of the Cell. 2003;14:2041–2056. doi: 10.1091/mbc.E02-10-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister KK, Fisher EMC, Gibbons IR, Hays TS, Holzbaur ELF, McIntosh JR, Porter ME, Schroer TA, Vaughan KT, Witman GB, King SM, Vallee RB. Cytoplasmic dynein nomenclature. The Journal of Cell Biology. 2005;171:411–413. doi: 10.1083/jcb.200508078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. The Journal of Cell Biology. 2009;187:135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Siuda E, Henderson S, Segil M, Vaananen H, Sassaroli M. Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. The Journal of Cell Biology. 1998;143:1591–1601. doi: 10.1083/jcb.143.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Molecular Biology of the Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Rosenbaum JL, Barr MM. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Current Biology: CB. 2001;11:457–461. doi: 10.1016/s0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Current Biology: CB. 2007;17:193–202. doi: 10.1016/j.cub.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1456–1461. doi: 10.1073/pnas.1011410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramelot TA, Raman S, Kuzin AP, Xiao R, Ma LC, Acton TB, Hunt JF, Montelione GT, Baker D, Kennedy MA. Improving NMR protein structure quality by Rosetta refinement: a molecular replacement study. Proteins. 2009;75:147–167. doi: 10.1002/prot.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rix S, Calmont A, Scambler PJ, Beales PL. An Ift80 mouse model of short rib polydactyly syndromes shows defects in hedgehog signalling without loss or malformation of cilia. Human Molecular Genetics. 2011;20:1306–1314. doi: 10.1093/hmg/ddr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Margall-Ducos G, Guidotti J-E, Brégerie O, Celati C, Bréchot C, Desdouets C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. Journal of Cell Science. 2007;120:628–637. doi: 10.1242/jcs.03366. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Snell WJ. The ciliary membrane. Current Opinion in Cell Biology. 2010;22:541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nature Reviews. Molecular Cell Biology. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- Schafer JC, Haycraft CJ, Thomas JH, Yoder BK, Swoboda P. XBX-1 encodes a dynein light intermediate chain required for retrograde intraflagellar transport and cilia assembly in Caenorhabditis elegans. Molecular Biology of the Cell. 2003;14:2057–2070. doi: 10.1091/mbc.E02-10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JC, Winkelbauer ME, Williams CL, Haycraft CJ, Desmond RA, Yoder BK. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. Journal of Cell Science. 2006;119:4088–4100. doi: 10.1242/jcs.03187. [DOI] [PubMed] [Google Scholar]
- Sedmak T, Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. The Journal of Cell Biology. 2010;189:171–186. doi: 10.1083/jcb.200911095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signor D, Wedaman KP, Rose LS, Scholey JM. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Molecular Biology of the Cell. 1999;10:345–360. doi: 10.1091/mbc.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends in Biochemical Sciences. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MRS, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nature Cell Biology. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics. 2005;21:951–960. doi: 10.1093/bioinformatics/bti125. [DOI] [PubMed] [Google Scholar]
- Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Research. 2005;33:W244–248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Herman RK, Kari CK, Yeh WH, Schackwitz WS, Schuyler MW, Collet J, Thomas JH, Riddle DL. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics. 1995;139:171–188. doi: 10.1093/genetics/139.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Perkins BD. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vision Research. 2009;49:479–489. doi: 10.1016/j.visres.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development (Cambridge, England) 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Vetter M, Morawetz M, Lorentzen E. Biochemical mapping of interactions within the intraflagellar transport (IFT) B core complex: IFT52 binds directly to four other IFT-B subunits. The Journal of Biological Chemistry. 2011;286(30):26344–52. doi: 10.1074/jbc.M111.254920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nature Genetics. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C-C, Gorovsky MA. Different effects of tetrahymena IFT172 domains on anterograde and retrograde intraflagellar transport. Molecular Biology of the Cell. 2008a;19:1450–1461. doi: 10.1091/mbc.E07-05-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C-C, Gorovsky MA. Tetrahymena IFT122A is not essential for cilia assembly but plays a role in returning IFT proteins from the ciliary tip to the cell body. Journal of Cell Science. 2008b;121:428–436. doi: 10.1242/jcs.015826. [DOI] [PubMed] [Google Scholar]
- Voegtli WC, Madrona AY, Wilson DK. The structure of Aip1p, a WD repeat protein that regulates cofilin-mediated actin depolymerization. Journal of Biological Chemistry. 2003;278:34373–34379. doi: 10.1074/jbc.M302773200. [DOI] [PubMed] [Google Scholar]
- Walther Z, Vashishtha M, Hall JL. The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. The Journal of Cell Biology. 1994;126:175–188. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fan Z-C, Williamson SM, Qin H. Intraflagellar transport (IFT) protein IFT25 is a phosphoprotein component of IFT complex B and physically interacts with IFT27 in Chlamydomonas. PloS One. 2009;4:e5384. doi: 10.1371/journal.pone.0005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick MJ, Ann DK, Loh HH. Molecular cloning of a novel protein regulated by opioid treatment of NG108-15 cells. Brain research. Molecular Brain Research. 1995;32:171–175. doi: 10.1016/0169-328x(95)00090-f. [DOI] [PubMed] [Google Scholar]
- Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Current Topics in Developmental Biology. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. Journal of the American Society of Nephrology: JASN. 2002a;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. American Journal of Physiology. Renal Physiology. 2002b;282:F541–552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, Yoder BK. Loss of the Tg737 protein results in skeletal patterning defects. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 2003;227:78–90. doi: 10.1002/dvdy.10289. [DOI] [PubMed] [Google Scholar]