Abstract
Objective
Ability to work and independent living capacity are of particular concern for patients with Parkinson’s disease (PD). We utilized a series of PD patients able to work or live independently at baseline, and evaluated potential risk factors for the two separate outcomes of loss of ability to work and loss of ability to live independently.
Methods
The series was comprised of 495 PD patients followed prospectively. Ability to work and ability to live independently were based on clinical interview and examination. Cox regression models adjusted for age and disease duration were used to evaluate associations of baseline characteristics with loss of ability to work and loss of ability to live independently.
Results
Higher UPDRS dyskinesia score, UPDRS instability score, UPDRS total score, Hoehn and Yahr stage, and presence of intellectual impairment at baseline were all associated with increased risk of future loss of ability to work and loss of ability to live independently (P≤0.0033). Five years after initial visit, for patients ≤70 years of age with a disease duration ≤4 years at initial visit, 88% were still able to work and 90% to live independently. These estimates worsened as age and disease duration at initial visit increased; for patients >70 years of age with a disease duration >4 years, estimates at 5 years were 43% and 57%, respectively.
Conclusions
The information provided in this study can offer useful information for PD patients in preparing for future loss of ability to perform activities of daily living.
Keywords: Parkinson disease, work ability, independence
Introduction
Parkinson’s disease (PD) is a chronic and progressive disorder associated with functional decline. Ability to work and independent living capacity are two important activities of daily living (ADL) of particular concern for patients with PD wondering how long their capability may last. Inability to continue working causes financial concerns and place a major individual and societal burden. Moreover, motor disabilities and increasing dependence on others in daily life has an important influence on patients’ and caregivers’ quality of life.
Previous research has shown that the rate of premature unemployment due to PD ranges from 27% to 70%. Older age, later disease onset, longer disease duration, greater severity of symptoms and lack of support from coworkers are the main factors responsible for giving up work [1-6]. Moreover, a recent report and systematic review presented axial impairment and difficulties with ambulation as important predictors of disability in patients with PD [7-9].
Although these are important findings, methodological limitations of previous studies (retrospective or cross-sectional nature, limited range of predictive factors, narrowed age at onset and severity of disease, univariate analysis, lack of adjustment for multiple testing) influence the understanding of predictive factors associated with the ability to work and live independently. Moreover, since ability to work and independent living are influenced by an individual’s cultural background, it is more important to assess whether the individual could perform a task if needed (present study) than to determine whether the individual is currently involved in doing it (most previous studies).
The current study uses a prospective, clinical cohort of patients with PD able to work or live independently at baseline visit, with a broad range of demographic, historical and clinical characteristics. In order to provide clinicians, patients, and families with information regarding the likely length of time patients will be able to function in everyday life, we examined associations of patient characteristics and disease information with the two separate outcomes of loss of ability to work and loss of ability to live independently, and estimated the future likelihood of these two outcomes after baseline visit.
Design and Methods
Patients and procedures
The original cohort consisted of 825 consecutive patients diagnosed with PD by one movement disorders neurologist (RJU) at the Mayo Clinic, Jacksonville, Florida, from 1994 to 2002, who were still able to work or live independently at baseline visit. Patients were excluded if they had no follow-up visit, had follow-up visits occurring less than 90 days after the baseline visit, or if information was not collected regarding age or disease duration at baseline visit. Of the 825 patients, 495 met inclusion criteria. After baseline visit, patients were generally seen on a yearly basis. Follow-up visits included in the present study extend to the year 2008. Of the 495 patients, 303 were able to work at baseline and as such were included in analysis involving loss of ability to work, while 491 were able to live independently at baseline and were included in analysis involving loss of ability to live independently.
In order to assess potential bias, we compared characteristics of the 330 patients who were excluded. These patients differed significantly (P≤0.05) from the 495 patients included in the study in regard to disease duration (Median: 58 vs. 45 months), personal history of depression (22% vs. 31%), current depression (19% vs. 12%), Hoehn and Yahr stage >2 (39% vs. 27%), any impairment on ADL score (39% vs. 17%), Unified Parkinson’s Disease Rating Scale (UPDRS) instability score (Median: 3 vs. 2), and UPDRS dyskinesia score >0 (24% vs. 16%). There were no other significant differences in demographic or disease information between the two groups.
The diagnosis of PD was made by virtue of patients having at least two of the following features: bradykinesia, resting tremor, rigidity, and postural instability, without any other explanation for parkinsonism or atypical features [10, 11].
Demographic, historical, and clinical information was collected as previously described in detail [12, 13]. We used the modified Hoehn and Yahr scale and the modified UPDRS motor score with additional items for arm swing and dyskinesia, as reported previously [13]. All data were entered prospectively into an electronic database in accordance with the Mayo Clinic IRB-approved protocol. Written informed consent was obtained from all patients participating in the study at baseline visit.
Two separate primary endpoints were captured as part of a routine semi-structured, physician-based (RJU) interview. One endpoint, loss of ability to work, was defined as inability to work in a job with light physical/social requirements (e.g. work in a retail department store). An important point to highlight is that because loss of ability to work can be considered as a measure of ability to function socially in everyday life, it was appropriate to consider patients of all ages in this assessment, not just in those younger than usual retirement age. The second endpoint, loss of ability to live independently was measured uniformly by observation and asking patients, spouses and caregivers about abilities to dress, eat, use toilet, take care of own hygiene, walk, travel and perform household activities independently.
Statistical analysis
The Kaplan-Meier method was used to estimate the proportion of patients still able to work, and the proportion of patients still able to live independently after baseline visit, censoring at the date of last follow-up. Due to the fact that both age and disease duration are known to be highly related to each of the two endpoints, we stratified our sample into four groups based on the combination of approximate median age at baseline (≤70 years, >70 years) and approximate median symptomatic disease duration (≤4 years, >4 years), and calculated Kaplan-Meier estimates for each of these four patient groups. Relative risks (RRs) and 95% confidence intervals (CIs) resulting from Cox proportional hazards regression models adjusted for age and disease duration were used to evaluate associations of baseline patient characteristics with the two separate endpoints of loss of ability to work and loss of ability to live independently. For easier interpretation of results, Hoehn and Yahr stage was dichotomized as ≤2 vs. >2, and UPDRS dyskinesia score was dichotomized as 0 vs. >0 in all analyses.
In evaluation of associations of patient baseline characteristics with the endpoints of loss of ability to work and loss of ability to live independently, we adjusted for multiple testing using the Bonferroni method. With 15 different statistical tests performed in association analysis for each endpoint, p-values ≤ 0.0033 were considered statistically significant. Because we did not aim to evaluate whether age and disease duration are associated with loss of ability to work and loss of ability to live independently as these associations are already known, we did not include these two association tests in our Bonferroni adjustment for multiple testing. All statistical analyses were performed using S-Plus (version 8.0.1; Insightful Corporation, Seattle, Washington).
Results
Patient demographic and disease information
In the overall cohort of 495 patients, individuals were predominantly males (68%), with a median age of 66 years at PD symptomatic onset and of 71 years at the time of their first (baseline) visit. Median length of follow-up from baseline visit was 4.0 years (min=92 days, max=12.7 years). These and other demographic and clinical characteristics are provided in Table 1.
Table 1.
Patient demographic and disease information at baseline visit
| Variable | Summary (N=495) |
|---|---|
| Age at first visit (years) | 71 (38, 93) |
| Gender (Male) | 338 (68%) |
| Age at PD onset | 66 (16, 90) |
| Disease duration (months) | 45 (2, 621) |
| Personal history of depression | 154 (31%) |
| Current depression | 60 (12%) |
| Family history of movement disorders | 136 (27%) |
| Family history of PD | 76 (15%) |
| Intellectual impairment | 29 (6%) |
| Hoehn and Yahr stage (>2) | 131 (27%) |
| ADL score (any impairment) | 83 (17%) |
| UPDRS – tremor | 2 (0, 18) |
| UPDRS – rigidity | 7 (0, 16) |
| UPDRS – bradykinesia | 14 (0, 39) |
| UPDRS – instability | 2 (0, 11) |
| UPDRS – dyskinesia (>0) | 76 (16%) |
| UPDRS – total | 25 (0, 64) |
• The sample median (minimum, maximum) is given for numerical variables. Information was unavailable regarding intellectual impairment (N=16), Hoehn and Yahr stage (N=16), UPDRS – tremor (N=16), UPDRS – rigidity (N=16), UPDRS – bradykinesia (N=16), UPDRS – instability (N=16), UPDRS – dyskinesia (N=16), UPDRS – total (N=16), and UPDRS – dominance score (N=16). PD, Parkinson’s disease; UPDRS, Unified Parkinson’s Disease Rating Scale.
Loss of ability to work and to live independently
Kaplan-Meier estimated proportions of patients still able to work and still able to live independently after baseline visit are shown in Table 2, and Figures 1 and 2. These Kaplan-Meier estimates are provided separately according to median age at baseline (≤70 years, >70 years) and median symptomatic disease duration (≤4 years, >4 years). At 5 years following baseline visit, the proportions of patients still able to work were as follows: 88% (age≤70, disease duration ≤4), 66% (age≤70, disease duration >4), 58% (age>70, disease duration≤4), and 43% (age>70, disease duration>4). At 5 years following baseline visit, the proportion of patients still able to live independently were 90% (age ≤70, disease duration ≤4), 68% (age ≤70, disease duration >4), 80% (age >70, disease duration ≤4), and 57% (age >70, disease duration >4).
Table 2.
Kaplan-Meier estimated proportion of patients still able to work and still able to live independently after baseline visit, stratified by age at baseline and disease duration
| Proportion of patients still able to work or live independently (95% CI) | ||||
|---|---|---|---|---|
|
| ||||
| Time after baseline visit | Age ≤70, Disease duration ≤4 years | Age ≤70, Disease duration >4 years | Age >70, Disease duration ≤4 years | Age >70, Disease duration >4 years |
| Ability to work | N=108 | N=60 | N=85 | N=50 |
| 1 year | 99% (97% - 100%) | 95% (89% - 100%) | 95% (91% - 100%) | 89% (80% - 99%) |
| 2 years | 97% (94% - 100%) | 88% (79% - 97%) | 90% (83% - 97%) | 79% (68% - 92%) |
| 3 years | 94% (89% - 99%) | 82% (73% - 93%) | 77% (68% - 87%) | 61% (48% - 78%) |
| 4 years | 91% (86% - 97%) | 72% (61% - 85%) | 70% (60% - 81%) | 56% (42% - 74%) |
| 5 years | 88% (81% - 95%) | 66% (55% - 80%) | 58% (47% - 71%) | 43% (30% - 63%) |
| 6 years | 82% (74% - 91%) | 58% (46% - 73%) | 52% (41% - 66%) | 27% (16% - 48%) |
| 8 years | 64% (52% - 77%) | 38% (26% - 55%) | 35% (24% - 50%) | 14% (6% - 33%) |
| 10 years | 44% (28% - 59%) | 26% (15% - 44%) | 8% (3% - 23%) | 3% (1% - 23%) |
| Ability to live independently | N=129 | N=111 | N=129 | N=122 |
| 1 year | 100% (97% - 100%) | 97% (94% - 100%) | 98% (95% - 100%) | 92% (87% - 97%) |
| 2 years | 98% (96% - 100%) | 91% (85% - 97%) | 95% (91% - 99%) | 85% (79% - 93%) |
| 3 years | 95% (91% - 99%) | 88% (82% - 95%) | 90% (85% - 96%) | 78% (70% - 87%) |
| 4 years | 92% (87% - 98%) | 78% (69% - 87%) | 88% (81% - 95%) | 67% (57% - 78%) |
| 5 years | 90% (84% - 96%) | 68% (58% - 79%) | 80% (72% - 90%) | 57% (47% - 70%) |
| 6 years | 87% (80% - 94%) | 65% (55% - 76%) | 76% (67% - 87%) | 41% (30% - 56%) |
| 8 years | 83% (75% - 92%) | 49% (38% - 64%) | 58% (45% - 74%) | 27% (17% - 43%) |
| 10 years | 64% (50% - 83%) | 31% (20% - 49%) | 23% (12% - 46%) | 5% (1% - 20%) |
• The patient cohort consisted of 495 patients either able to work at baseline or able to live independently at baseline. Kaplan-Meier estimates for loss of ability to work were evaluated for the 303 patients who were able to work at baseline. Kaplan-Meier estimates for loss of ability to live independently at baseline were evaluated for the 491 patients who were able to live independently at baseline. Patients were stratified into four different age and disease duration groups based on the median value of each measure, in order to provide meaningful Kaplan-Meier estimates for specific patient groups.
Figure 1.
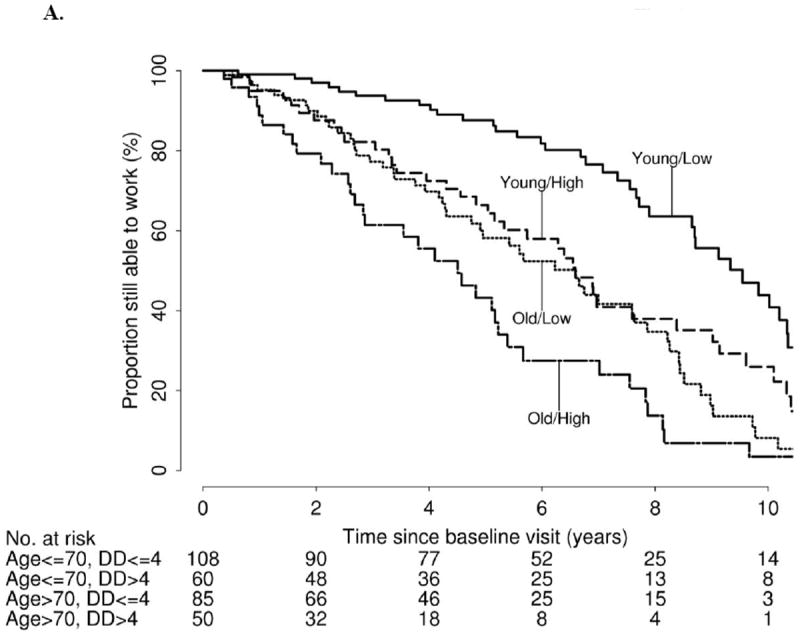
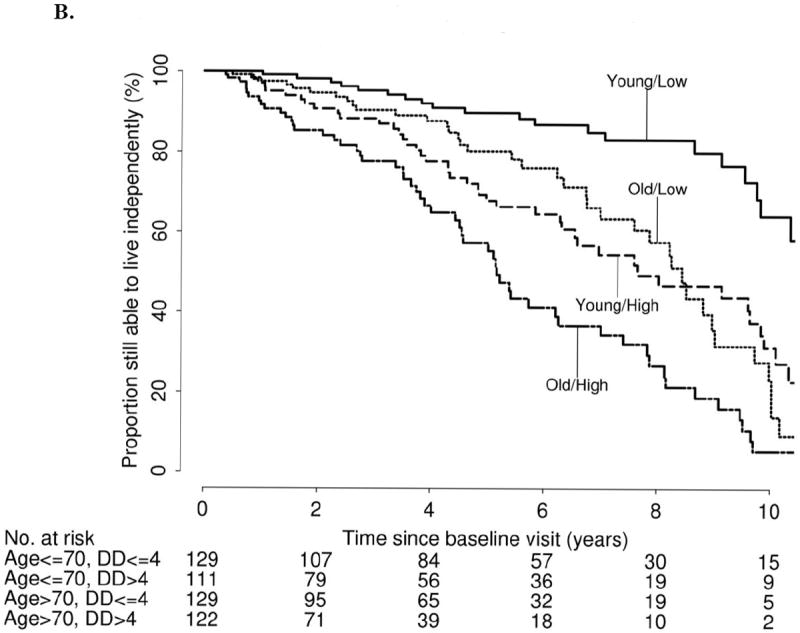
A. Kaplan-Meier estimated proportion of patients still able to work after baseline visit, stratified by age at baseline visit and disease duration (DD)
B. Kaplan-Meier estimated proportion of patients still able to live independently after baseline visit, stratified by age at baseline visit and disease duration (DD)
Predictors of loss of ability to work and loss of ability to live independently
Associations of baseline demographic and disease information with the separate endpoints of loss of ability to work and loss of ability to live independently are displayed in Table 3.
Table 3.
Associations of baseline demographic and disease information with loss of ability to work and loss of ability to live independently
| Association with loss of ability to work (N=303) | Association with loss of ability to live independently (N=491) | |||
|---|---|---|---|---|
|
| ||||
| Variable | Estimated RR (95% CI) | P-value | Estimated RR (95% CI) | P-value |
| Gender (Male) | 1.08 (0.78 – 1.50) | 0.64 | 1.09 (0.79 – 1.51) | 0.60 |
| Personal history of depression | 0.92 (0.66 – 1.29) | 0.64 | 1.21 (0.88 – 1.68) | 0.25 |
| Current depression | 0.83 (0.51 – 1.35) | 0.45 | 1.10 (0.68 – 1.78) | 0.70 |
| Family history of movement disorders | 1.06 (0.74 – 1.50) | 0.77 | 0.87 (0.61 – 1.24) | 0.44 |
| Family history of PD | 0.84 (0.53 – 1.31) | 0.44 | 0.87 (0.56 – 1.34) | 0.52 |
| Intellectual impairment | 3.58 (1.53 – 8.41) | 0.0033 | 3.55 (2.14 – 5.98) | 1×10-5 |
| Hoehn and Yahr stage (>2) | 2.05 (1.34 – 3.13) | 0.0009 | 1.99 (1.42 – 2.80) | 7×10-5 |
| ADL score (any impairment) | 2.30 (1.20 – 4.41) | 0.012 | 2.13 (1.47 – 4.11) | 8×10-5 |
| UPDRS – tremor (5 point increase) | 1.06 (0.76 – 1.48) | 0.72 | 0.89 (0.66 – 1.18) | 0.41 |
| UPDRS – rigidity (5 point increase) | 1.37 (1.07 – 1.74) | 0.012 | 1.26 (1.02 – 1.55) | 0.033 |
| UPDRS – bradykinesia (10 point increase) | 1.40 (1.09 – 1.81) | 0.0094 | 1.38 (1.13 – 1.70) | 0.0019 |
| UPDRS – instability (3 point increase) | 2.04 (1.54 – 2.69) | 6×10-7 | 1.73 (1.43 – 2.09) | 2×10-8 |
| UPDRS – dyskinesia (>0) | 1.87 (1.19 – 2.93) | 0.0067 | 2.39 (1.61 – 3.57) | 2×10-5 |
| UPDRS – total (20 point increase) | 1.60 (1.19 – 2.15) | 0.0017 | 1.44 (1.14 – 1.82) | 0.0019 |
• Estimated relative risks and p-values result from Cox proportional hazards models adjusted for age and disease duration. Relative risks correspond to presence of the given characteristic or the increase given in parenthesis. P-values ≤ 0.0033 were considered statistically significant after a Bonferroni adjustment for the 15 statistical tests of association that were performed for each endpoint. The patient cohort consisted of 495 patients either able to work at baseline or able to live independently at baseline. Associations with loss of ability to work were evaluated for the 303 patients who were able to work at baseline. Associations with loss of ability to live independently at baseline were evaluated for the 491 patients who were able to live independently at baseline. PD, Parkinson’s disease; UPDRS, Unified Parkinson’s Disease Rating Scale.
A significantly increased risk of future loss of ability to work was observed for patients with intellectual impairment (RR: 3.58, P=0.0033), Hoehn and Yahr stage > 2 (RR: 2.05, P=0.0009), higher UPDRS instability score (RR: 2.04 [3 point increase], P=6×10-7), and higher UPDRS total score (RR: 1.60 [20 point increase], P=0.0017). No significant association with loss of ability to work was observed for the other items evaluated after adjustment for multiple testing.
We identified a significantly increased risk of future loss of ability to live independently in patients with intellectual impairment (RR: 3.55, P=1×10-5), Hoehn and Yahr stage >2 (RR: 1.99, P=7×10-5), higher UPDRS bradykinesia score (RR: 1.38 [10 point increase], P=0.0019), higher UPDRS instability score (RR: 1.73 [3 point increase], P=2×10-8), higher UPDRS dyskinesia score >0 (RR: 2.39, P=2×10-5), higher UPDRS total score (RR: 1.44 [20 point increase], P=0.0019), and any impairment on ADL score (RR: 2.13, P=8×10-5). No significant association with loss of ability to work was noted for the remaining items evaluated after adjustment for multiple testing. Of note, as expected, increased age was strongly associated with risk of loss of ability to work (RR: 2.62, P=4×10-9) and loss of ability to live independently (RR: 2.32, P=2×10-7), as well increased disease duration was strongly associated with risk of loss of ability to work (RR: 1.81, P=0.0002) and loss of ability to live independently (RR: 2.49, P=2×10-8).
Discussion
The present study examines the associations of patient characteristics and disease information with future loss of ability to work and loss of ability to live independently, in individuals diagnosed with PD and able to work or live independently at baseline. In addition to older age at baseline and longer disease duration [1, 5, 7], our study has identified a number of predictors associated with an increased risk for loss of ability to work and to live independently, providing patients and caregivers with valuable information.
Our results indicate that more advanced disease with intellectual impairment, higher Hoehn and Yahr stage, increased UPDRS instability score, and increased UPDRS total score are related to an increased risk of future loss of ability to work. Most strikingly, patients with intellectual impairment at baseline were at an approximate 3.5-fold risk of future loss of ability to work compared to others, while UPDRS instability score was strongly associated with increased risk of future loss of ability to work, with a 2-fold increased risk for each 3 point increase. Severity of PD symptoms was also presented by others as an important factor involved in early cessation of work in individuals with PD [1, 6], together with lack of support in the workplace, and available “opportunities” for early retirement [6].
Loss of ability to live independently after baseline visit was significantly associated with all UPDRS scores except tremor, rigidity, and dominance score, and was also significantly associated with intellectual impairment, higher Hoehn and Yahr stage, and any impairment on ADL score. Consistent with findings regarding loss of ability to work, loss of ability to live independently was also most pronounced in patients with intellectual impairment (RR~3.5) and an increased UPDRS instability score (RR~2). Interestingly, rigidity and tremor at baseline visit did not noticeably influence either future loss of ability to work or future loss of ability to live independently.
One previous study found that most patients report loss of independence on ≥2 ADL/IADL domains when their UPDRS score reaches >60 at best functioning, approximately 7 years after PD diagnosis [9]. Another study identified axial impairment as strongly associated with disability assessed with three different ADL scales [8]. However, this cross-sectional study was conducted exclusively in patients with mild to moderate PD. Comparably, the systematic review by Post et al. found strong evidence for axial impairment being a predictor for progression of disability [7], and a study by Shulman et al [9] found that needing help with walking preceded problems with housework, dressing, transferring in and out of bed, and traveling in the community. These data are in line with our results showing that Hoehn and Yahr stage >2 and higher instability score are strongly associated with an increased risk for loss of ability to live independently.
In our cohort from the US, the estimated proportion of patients still able to work 5 years after baseline visit ranged from 88% (age ≤70, disease duration ≤4 years) to 43% (age >70, disease duration >4 years). After 10 years, these figures reached 44% and 3%, respectively. These results are considerably higher than those of previous surveys in the UK and Finland, which could reflect cultural and social differences [1, 6]. The patients in this study may also have benefited from care provided by a movement disorders specialist. However, despite methodological differences with our study, the survival analysis by Schrag et al. revealed that 54% of patients with disease onset <65 years still worked after 5 years from disease onset, and that >80 % of patients with PD with disease onset <65 years lost their employment within 10 years of disease onset [5]. Additionally, previous studies on quality of life in PD reported that about 25% of patients prematurely retired or were unemployed because of PD [2, 4]. In the study by Banks et al. it was reported that the mean age of premature retirement due to PD was 50.1 years in the UK [6].
Our study has several limitations. The length of time to loss of ability to work and loss of ability to live independently was likely overestimated. That is, time of clinical assessment was used as the event date, although the event may have occurred prior to the follow-up visit. Also, we excluded 330 patients due to a lack of follow-up or insufficient data, which raises the possibility that our results may be biased. Excluded patients may have had slightly more advanced disease than the 495 patients included in this study based on our comparisons of the two groups, which could result in our Kaplan-Meier estimates being biased too high. However, this would not affect the results of the association analysis presented in Table 2. Finally, we acknowledge that this is a clinic-based cohort. However, our previous reports would suggest that both demographic and clinical features are similar to population-based series [13].
Despite the above limitations, the present study includes prospective data collected from a large cohort of patients with an extended follow-up period and at least one follow-up visit, as opposed to the retrospective and cross-sectional nature of other studies from literature. Additionally, we analyzed a wide range of demographic, historical and clinical features as potential predictors for future loss of ability to work or live independently. We identified a number of associations that were significant after correcting for the number of statistical tests performed, and also provide concrete data that can be used to inform patients about their likelihood of future loss of ability to work and loss of ability to live independently based on their current age and disease duration.
PD has a significant economic impact on patients, families and society [14, 19]. The most recent study on the cost of PD in the US estimated the total annual cost of the disease to be $23 billion in 2002. As calculated per diagnosed individual per year, the total direct cost of PD was established at $23,101 and the indirect cost at $25,326 [20, 21]. The loss of income due to the premature discontinuing of work is one of the largest primary components of indirect costs associated with PD and moreover, the loss of personal productivity accounts for almost 50% of the total financial burden [20].
Movement disorders specialists acknowledge that making an effort to prevent disability and premature termination of employment is needed [22-24]. We also believe that the data presented here may prevent unnecessary nihilism concerning expectations for work and independently living, as many patients were able to maintain both of these abilities. Examining the associations of patient characteristics and disease information with ability to work, estimating time to loss of ability to work as well as identifying its predictors may help reducing both the risk and the impact of future unemployment. Similarly, time before overt clinical disability and dependent living may provide a window for therapeutic interventions preventing or delaying the occurrence of disability.
Acknowledgments
We are grateful to all patients for their collaborations. R.J.U. is partially supported by the NIH/NINDS P50-NS072187 and NIH/NINDS NS057567, the research funding from Advanced Neuromodulation Systems, Inc., and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch (MCF #90052031/PAU #90052). Z.K.W. is partially supported by the NIH/NINDS 1RC2NS070276, NS057567, P50NS072187, Mayo Clinic Florida (MCF) Research Committee CR programs (MCF #90052030), and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch (MCF #90052031/PAU #90052). B.J-M. was supported by the Robert and Clarice Smith Fellowship Program and partially by the Pacific Alzheimer Research Foundation (PARF) C06-01 grant. C.W. was supported by the Swiss Parkinson Foundation. B.J.-M. and C.W. worked on this project during their clinical research fellowship at the Mayo Clinic Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martikainen KK, Luukkaala TH, Marttila RJ. Parkinson’s disease and working capacity. Mov Disord. 2006;21(12):2187–91. doi: 10.1002/mds.21171. [DOI] [PubMed] [Google Scholar]
- 2.Chrischilles EA, Rubenstein LM, Voelker MD, Wallace RB, Rodnitzky RL. The health burdens of Parkinson’s disease. Mov Disord. 1998;13(3):406–13. doi: 10.1002/mds.870130306. [DOI] [PubMed] [Google Scholar]
- 3.Schrag A, Ben-Shlomo Y, Brown R, Marsden CD, Quinn N. Young-onset Parkinson’s disease revisited--clinical features, natural history, and mortality. Mov Disord. 1998;13(6):885–94. doi: 10.1002/mds.870130605. [DOI] [PubMed] [Google Scholar]
- 4.Clarke CE, Zobkiw RM, Gullaksen E. Quality of life and care in Parkinson’s disease. Br J Clin Pract. 1995;49(6):288–93. [PubMed] [Google Scholar]
- 5.Schrag A, Banks P. Time of loss of employment in Parkinson’s disease. Mov Disord. 2006;21(11):1839–43. doi: 10.1002/mds.21030. [DOI] [PubMed] [Google Scholar]
- 6.Banks P, Lawrence M. The Disability Discrimination Act, a necessary, but not sufficient safeguard for people with progressive conditions in the workplace? The experiences of younger people with Parkinson’s disease. Disabil Rehabil. 2006;28(1):13–24. doi: 10.1080/09638280500165120. [DOI] [PubMed] [Google Scholar]
- 7.Post B, Merkus MP, de Haan RJ, Speelman JD. Prognostic factors for the progression of Parkinson’s disease: a systematic review. Mov Disord. 2007;22(13):1839–51. doi: 10.1002/mds.21537. quiz 988. [DOI] [PubMed] [Google Scholar]
- 8.Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70(23):2241–7. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- 9.Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23(6):790–6. doi: 10.1002/mds.21879. [DOI] [PubMed] [Google Scholar]
- 10.de Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MM, Maraganore DM. A population perspective on diagnostic criteria for Parkinson’s disease. Neurology. 1997;48(5):1277–81. doi: 10.1212/wnl.48.5.1277. [DOI] [PubMed] [Google Scholar]
- 11.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson’s disease. Adv Neurol. 1990;53:245–9. [PubMed] [Google Scholar]
- 12.Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson’s disease phenotype. J Neurol. 2005;252(10):1201–5. doi: 10.1007/s00415-005-0835-7. [DOI] [PubMed] [Google Scholar]
- 13.Uitti RJ, Baba Y, Wszolek ZK, Putzke DJ. Defining the Parkinson’s disease phenotype: initial symptoms and baseline characteristics in a clinical cohort. Parkinsonism Relat Disord. 2005;11(3):139–45. doi: 10.1016/j.parkreldis.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein LM, DeLeo A, Chrischilles EA. Economic and health-related quality of life considerations of new therapies in Parkinson’s disease. Pharmacoeconomics. 2001;19(7):729–52. doi: 10.2165/00019053-200119070-00003. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Oroz MC, Gorospe A, Guridi J, Ramos E, Linazasoro G, Rodriguez-Palmero M, et al. Bilateral deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. Neurology. 2000;55(12 Suppl 6):S45–51. [PubMed] [Google Scholar]
- 16.Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology. 1999;53(1):85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- 17.Krause M, Fogel W, Heck A, Hacke W, Bonsanto M, Trenkwalder C, et al. Deep brain stimulation for the treatment of Parkinson’s disease: subthalamic nucleus versus globus pallidus internus. J Neurol Neurosurg Psychiatry. 2001;70(4):464–70. doi: 10.1136/jnnp.70.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wider C, Pollo C, Bloch J, Burkhard PR, Vingerhoets FJ. Long-term outcome of 50 consecutive Parkinson’s disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord. 2008;14(2):114–9. doi: 10.1016/j.parkreldis.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Dowding CH, Shenton CL, Salek SS. A review of the health-related quality of life and economic impact of Parkinson’s disease. Drugs Aging. 2006;23(9):693–721. doi: 10.2165/00002512-200623090-00001. [DOI] [PubMed] [Google Scholar]
- 20.Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S, Lenhart G. Burden of illness in Parkinson’s disease. Mov Disord. 2005;20(11):1449–54. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- 21.Whetten-Goldstein K, Sloan F, Kulas E, Cutson T, Schenkman M. The burden of Parkinson’s disease on society, family, and the individual. J Am Geriatr Soc. 1997;45(7):844–9. doi: 10.1111/j.1532-5415.1997.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 22.Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease (2001): treatment guidelines. Neurology. 2001;56(11 Suppl 5):S1–S88. doi: 10.1212/wnl.56.suppl_5.s1. [DOI] [PubMed] [Google Scholar]
- 23.Calne SM, Lidstone SC, Kumar A. Psychosocial issues in young-onset Parkinson’s disease: current research and challenges. Parkinsonism Relat Disord. 2008;14(2):143–50. doi: 10.1016/j.parkreldis.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Poewe W, Mahlknecht P. The clinical progression of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 4):S28–32. doi: 10.1016/S1353-8020(09)70831-4. [DOI] [PubMed] [Google Scholar]


