ABSTRACT
Many pathogens produce the β-(1−6)-linked poly-N-acetylglucosamine (PNAG) surface polysaccharide that is being developed as a broadly protective antimicrobial vaccine. However, it is unknown whether systemically injected PNAG vaccines or antibodies would provide protective immunity against pathogens confined to the gastrointestinal tract such as Shiga toxin (Stx)-producing Escherichia coli (STEC), an important group of gastrointestinal (GI) pathogens for which effective immunotherapeutics are lacking. To ascertain whether systemic IgG antibody to PNAG impacts this infectious situation, a vaccine consisting of a synthetic nonamer of nonacetylated PNAG, 9GlcNH2, conjugated to the Shiga toxin 1b subunit (9GlcNH2-Stx1b) was produced. Rabbit antibodies raised to the conjugate vaccine were tested for bacterial killing and toxin neutralization in vitro and protection against infection in infant mice. Cell surface PNAG was detected on all 9 STEC isolates tested, representing 6 STEC serogroups, including E. coli O157:H7. Antibody to the 9GlcNH2-Stx1b conjugate neutralized Stx1 potently and Stx2 modestly. For O157:H7 and O104:H4 STEC strains, antibodies elicited by the 9GlcNH2-Stx1b conjugate possessed opsonic killing and bactericidal activity. Following intraperitoneal injection, antibodies to both PNAG and Stx were needed for infant mouse protection against O157 STEC. These antibodies also mediated protection against the Stx2-producing O104:H4 strain that was the cause of a recent outbreak in Germany, although sufficient doses of antibody to PNAG alone were protective against this strain in infant mice. Our observations suggest that vaccination against both PNAG and Stx, using a construct such as the 9GlcNH2-Stx1b conjugate vaccine, would be protective against a broad range of STEC serogroups.
IMPORTANCE
The presence of poly-N-acetylglucosamine (PNAG) on many pathogens presents an opportunity to target this one structure with a multispecies vaccine. Whether antibodies to PNAG can protect against pathogens confined to the gastrointestinal tract is not known. As Shiga toxin (Stx)-producing Escherichia coli (STEC) bacteria are serious causes of infection whose virulence is dependent on elaboration of Stx, we prepared a vaccine containing a synthetic nonamer of PNAG (9GlcNH2) conjugated to Shiga toxin 1b subunit (9GlcNH2-Stx1b) to evaluate bacterial killing, toxin neutralization, and protective efficacy in infant mice. All nine (100%) clinical strains of STEC from different serogroups expressed PNAG. Vaccine-induced antibody mediated in vitro killing of STEC and neutralization of both Stx1 and Stx2. Passive administration of antibody to the conjugate showed protection requiring immunity to both PNAG and Stx for O157 strains, although for an O104 strain, antibody to PNAG alone was protective. Immunity to PNAG may contribute to protection against STEC infections.
INTRODUCTION
Outbreaks and sporadic cases of intestinal infections caused by Shiga toxin (Stx)-producing Escherichia coli (STEC) have become increasingly common (1, 2). While E. coli O157:H7 remains the most common STEC serogroup (1), additional STEC serogroups are being reported more frequently as causes of infection (2). In 2011, a large outbreak of diarrhea and hemolytic-uremic syndrome (HUS) caused by a novel Stx-producing strain of serogroup O104:H4 occurred in Germany (3), and a recent study of virulence factors of this strain in an infant rabbit model of intestinal colonization and diarrhea indicated that Stx and chromosomally encoded autotransporters, but not the aggregative adherence plasmid pAA, were required for disease induction (4). STEC bacteria are estimated to cause over 265,000 cases of infection annually in the United States, with more than 3,600 hospitalizations and 30 deaths (5). The gastrointestinal illnesses caused by STEC range from nonbloody diarrhea to hemorrhagic colitis, and approximately 5% to 10% of patients with STEC infections develop HUS, a life-threatening complication, with a case fatality rate of 3% to 5% (6). In the 2011 epidemic in Germany, HUS developed in >20% of individuals infected with the O104:H4 strain (3, 7).
Shiga toxins are the principal cause of the diarrhea and HUS associated with STEC infections (8). They are categorized into two antigenically distinct groups, Stx1 and Stx2, which are potent cytotoxins composed of a single toxic A subunit and five B subunits (8). Stx2 is more frequently associated with severe disease and is more potent in toxicity and lethality models in mice (9). Treatment of STEC infection is supportive, and antibiotic use is controversial (10), as it has been reported to promote production and release of the phage-encoded Stxs (11, 12) and may increase the risk of HUS (13). However, not all studies have detected an impact of antibiotic treatment on the development of HUS (14). In light of this controversy, vaccines and/or immunotherapeutics that target Shiga toxins are considered a valuable approach for prevention and treatment of these common infections.
To date, several Shiga toxin-based vaccine strategies using either nontoxic subunits, recombinant proteins, or inactivated holotoxins have been undertaken (15–17), and several protein-based vaccines have been shown to reduce fecal shedding and carriage in cows (14). In addition to Stx, other vaccine antigens that are under development include the lipopolysaccharide (LPS) O-antigens (e.g., O157) (2, 18) and surface proteins (15); however, the existence of a variety of STEC O-antigens may limit the utility of the former approach.
In contrast to O-antigens, poly-N-acetylglucosamine (PNAG), a polymer of β-(1−6)-linked N-acetylglucosamine units, is a widely conserved carbohydrate that has been detected on the surface of a broad range of pathogens following its discovery in Staphylococcus epidermidis and Staphylococcus aureus (19, 20). The highly acetylated glycoform of PNAG naturally expressed on microbial surfaces elicits nonkilling, nonprotective antibody (21, 22) due to the antibody’s inability to deposit opsonically active complement onto the microbial surface (23). Removal of the majority of acetates from the native PNAG molecule (21, 22) or use of a synthetic oligosaccharide consisting of β-(1-6)-linked glucosamines (i.e., no N-linked acetates) conjugated to carrier proteins (24, 25) generates opsonic or bactericidal antibody protective against microbes producing the native, highly acetylated PNAG (24), including a variety of E. coli strains isolated from the urinary tract (26). However, the evaluation of PNAG-based vaccine candidates against STEC or any other pathogen confined to the gastrointestinal (GI) tract has not been conducted thus far. To assess protective potential in the GI tract, a bivalent vaccine was synthesized by conjugating a synthetic β-(1−6)-oligoglucosamine consisting of 9 glucosamine units (nonaglucosamine [9GlcNH2]) to the carrier protein Shiga toxin 1b subunit (Stx1b). Stx1b was chosen as opposed to Stx2b, because the latter was reported to be poorly stable (27) and thus not readily amenable to conjugation. We found that for O157 STEC strains, antibody induced by the conjugate vaccine could neutralize both Stx1 and Stx2 and mediate PNAG-specific opsonic/bactericidal killing. Antibodies to both of these antigens were required for in vivo protection against O157 STEC strains, but antibody to PNAG alone could protect mice against O104 STEC infection. Since immunity to PNAG can contribute to resistance to STEC infections in a mouse infection model, this conserved carbohydrate may be a key component for development of a broad-spectrum vaccine for STEC.
RESULTS
Detection of surface-associated PNAG in STEC.
We used a fluorescently labeled human monoclonal antibody (MAb) (F598) that specifically binds PNAG (25) and confocal microscopy to assay for surface-associated polysaccharide. All four O157:H7 strains exhibited robust staining with F598 and only background staining with an isotype-matched control human MAb (Fig. 1A). MAb F598 did not bind to ΔpgaC or ΔpgaABCD derivatives of these 4 strains, which are not capable of PNAG synthesis (Fig. 1A). MAb F598 binding to cell surfaces of non-O157 STEC strains was observed, and binding was lost after digestion with the PNAG-degrading enzyme dispersin B (28) and PNAG-destroying sodium periodate (Fig. 1B). Thus, all 9 STEC strains produce surface-associated PNAG.
FIG 1 .
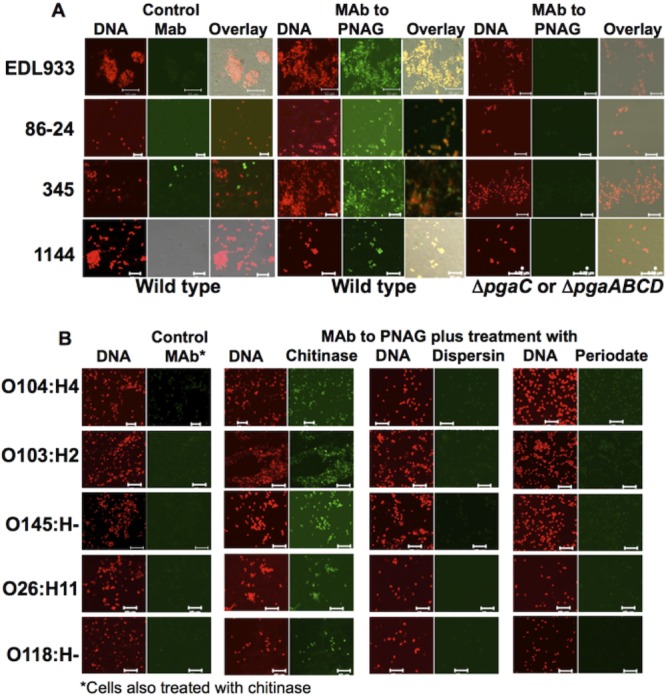
Expression of poly-N-acetylglucosamine (PNAG) by STEC strains. (A) PNAG production by four O157 STEC strains, EDL933, 86-24, 345, and 1144 and their respective pgaC (EDL933) or pgaABCD (other 3 strains) deletion mutants. (B) PNAG production by non-O157 STEC strains. In panel B, demonstration of specificity for PNAG was shown by maintenance of MAb F598 binding after treatment with the control enzyme chitinase but loss of binding after treatment with the PNAG-specific hydrolytic enzyme dispersin B or reaction with sodium periodate, which hydrolyzes PNAG by breaking the bonds between vicinal carbons 3 and 4 that contain hydroxyl groups in N-acetylglucosamine. The red channel shows DNA, while the green channel shows control MAb or MAb to PNAG and columns labeled Overlay show fluorescence in both channels. Green fluorescence that does not overlap red fluorescence (i.e., strain 345) indicates likely nonspecific binding of a MAb to non-cell-associated material. Bars, 10 µm.
Characterization of the antisera elicited by the 9GlcNH2-Stx1b conjugate vaccine.
The 9GlcNH2-Stx1b conjugate elicited high-titered IgG antibodies that bound to purified native PNAG at levels slightly less than to a reference control raised to 9GlcNH2-TT (9GlcNH2 conjugated to tetanus toxoid) (see Fig. S1A in the supplemental material) (24, 25) and to purified Stx1 holotoxin (Fig. S1B). There was also clearly detectable (albeit at a lower titer) binding of the antibody raised to the Stx1b carrier to Stx2 (Fig. S1C).
Antisera raised to the 9GlcNH2-Stx1b conjugate mediated opsonic killing of PNAG-producing STEC strains in the presence of the HL-60 phagocytic cells comparable to that of the control immune serum to 9GlcNH2-TT (Fig. 2A and B). In phagocyte-independent bactericidal killing assays, antibodies to both the 9GlcNH2-TT and 9GlcNH2-Stx1b exhibited bactericidal killing of four out of five STEC strains tested (Fig. 2C and D).
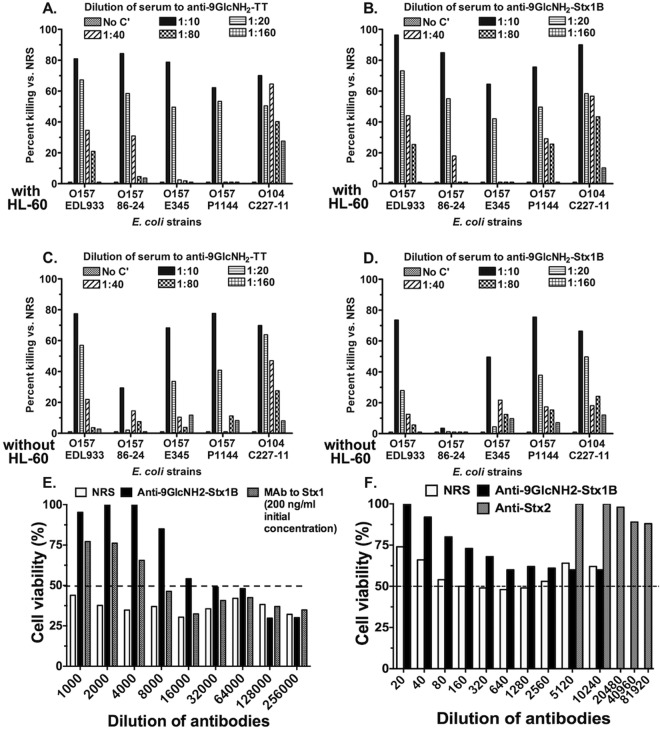
FIG 2 .
In vitro opsonophagocytic killing (OPK), bactericidal activity, and Shiga toxin neutralizing activity of the antibodies induced by PNAG-based conjugate vaccines. (A and B) Opsonophagocytic killing (with HL-60 cells) of the STEC strains indicated on the x axis by antibody in sera raised to 9GlcNH2-TT (A) or 9GlcNH2-Stx1b (B). (C and D) Bactericidal killing (without HL-60 cells) of the STEC strains indicated on the x axis by antibody in sera raised to 9GlcNH2-TT (C) or 9GlcNH2-Stx1b (D). There was no killing in the absence of complement in any assay (small bar to the left of each 1:10 serum dilution bar). Bars represent means of three replicate samples within an assay. (E and F) Normal rabbit serum (NRS), antibody to the 9GlcNH2-Stx1b conjugate vaccine, MAb to Stx1, or polyclonal antibody to Stx2 was diluted as indicated on the x axis and mixed with an amount of either Stx1 or Stx2 predetermined to give ~50% killing (dashed lines) of the Vero cells in 96-well culture plates. The control for Stx1 neutralization was a MAb initially used at 200 ng/ml. The bars represent the calculated mean cell viabilities from six duplicate wells, and the graphs depict a typical experiment representative of 3 repeat assays.
Toxin neutralization assays using a concentration of purified native Stx1 (40 pg/ml) that killed ~50% of Vero cells in the absence of added serum along with twofold serial dilutions of the antiserum raised to 9GlcNH2-Stx1b, control normal rabbit serum (NRS), or the positive-control MAb to Stx1b revealed a high level of toxin neutralizing activity, with an endpoint neutralization titer of ~16,000 (Fig. 2E). The control MAb to Stx1b had detectable neutralizing activity at a concentration of ~25 ng/ml (Fig. 2E). Antibody to Stx2 had no neutralizing activity against Stx1 at the concentrations tested. Antibody to 9GlcNH2-Stx1b also contained detectable neutralizing activity against Stx2, with an endpoint dilution of 1:320 (Fig. 2F).
Requirements for Stx and PNAG in a lethal, infant mouse-based STEC infection model.
We found we could consistently achieve a moribund/lethal infection 50 to 120 h postinoculation following oral gavage of 5-day-old CD1 mice with ≥5 × 108 CFU/mouse of various STEC. Following oral gavage, we sacrificed animals 0.25, 3, 30, and 50 h postgavage and measured the STEC CFU in the stomachs, small intestines, large intestines, and systemic tissues, including the livers, kidneys, and spleens (see Fig. S2 in the supplemental material). Within 15 min of infection, STEC bacteria were detected in the stomach, small intestine, and large intestine, where they remained until at least 50 h. STEC CFU in the stomach and small intestine declined during this period, but high levels persisted within the large intestine (Fig. S2). No major pathological changes were observed in the GI tracts or kidneys by hematoxylin and eosin staining of tissue sections after 72 to 96 h of infection. STEC was not recovered from any extraintestinal tissues at this point (Fig. S2).
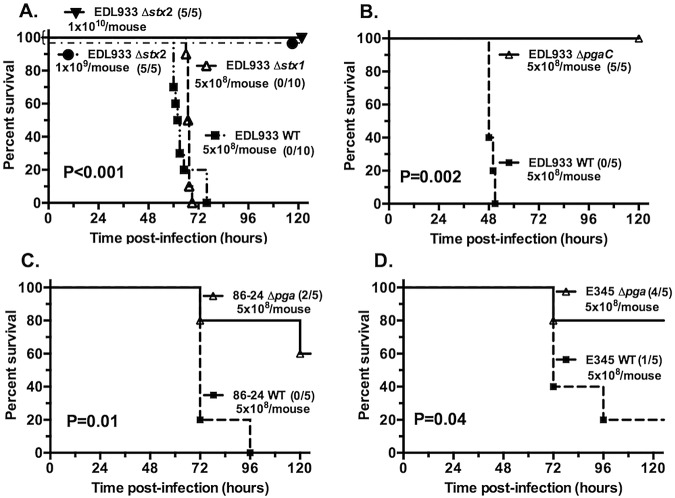
We deleted the complete stx1 and stx2 genes in E. coli O157:H7 strain EDL933 to test whether either of the Shiga toxins is required for lethality. All isogenic mutant strains had in vitro growth curves identical to that of the wild-type (WT) parent, and PCR analysis, along with detection of toxin expression in vitro, confirmed that all mutants retained or lost the intended genes and corresponding protein production. Deletion of stx1 had no effect on EDL933 virulence, whereas EDL933 lacking stx2 did not cause death in mice, even at doses up to 1010 CFU/animal (Fig. 3A), indicating that mortality depends on production of lethal amounts of Stx2. The much greater potency of Stx2 over Stx1 in mice has been reported previously by others (29, 30). A ΔpgaC EDL933 strain that does not produce PNAG (Fig. 1) was avirulent, and no deaths were observed (Fig. 3B), similar to the Δstx2 mutant.
FIG 3 .
Virulence of stx and pga mutants of E. coli O157 strains orally gavaged into 5-day-old mice. (A) Deletion of the stx2 gene, but not the stx1 gene, resulted in complete loss of virulence for E. coli EDL933. The challenge doses (in CFU) are indicated on the graphs, and the numbers in the parentheses are the number of survivors to the total number of infected mice. (B to D) Loss of ability to produce PNAG by deletion of the pgaC gene in strain EDL933 (B) or the entire pga locus (strains 86-24 [C] and E345 [D]) decreases virulence of these three E. coli O157 strains in infant mice. P values were determined by log rank tests comparing the values for the wild-type (WT) E. coli with the values for mutant strains.
Deletion of the entire pga locus in E. coli strains 86-24 and E345 also attenuated the virulence of these 2 STEC strains, although not to the same extent as in EDL933 (Fig. 3C and D). Together, these observations suggest that PNAG contributes to STEC pathogenicity in this infant mouse model.
Protective efficacy of antiserum raised to 9GlcNH2-Stx1b to STEC infection.
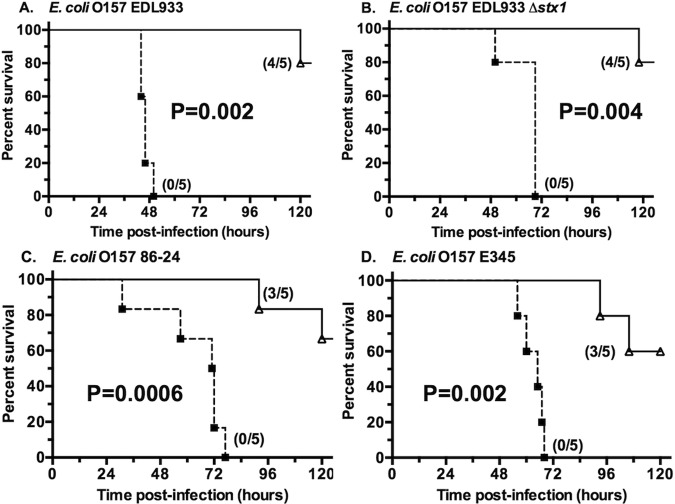
Five-day-old mice were given intraperitoneal (i.p.) injections of 80 µl of the rabbit antiserum raised to the 9GlcNH2-Stx1b conjugate vaccine 16 and 4 h prior to oral infection (Fig. 4). All mice pretreated with the control NRS became moribund or died within 76 h of STEC inoculation, whereas 60 to 80% mice pretreated with the antisera from the PNAG-Stx1b immune rabbits were alive 120 h after STEC inoculation, including mice infected with strains lacking the Stx1 target antigen. Three additional experiments with infant mice challenged with strain EDL933 also showed efficacy ranging from 50 to 100% protection in the immune mice (see Fig. S3 in the supplemental material). The variability in protective efficacy likely reflects the use of outbred CD1 mice and inability to precisely control some experimental factors such as the exact inoculum size, comparability of challenge inocula prepared on different days, and factors that naturally vary within an animal’s environment. Nonetheless, immunization with the 9GlcNH2-Stx1b conjugate vaccine elicited protective antibodies against lethal STEC infection as demonstrated in multiple experiments.
FIG 4 .
Protective efficacy of antibody raised to the 9GlcNH2-Stx1b synthetic oligosaccharide-conjugate vaccine against three O157:H7 STEC strains. Kaplan-Meier survival curves analyzed by the log rank test were used to generate the indicated P values. The numbers in parentheses are the numbers of survivors to the total number of mice challenged. The challenge strains are indicated above each graph. The challenge doses were 8 × 108 CFU of O157:H7 strain EDL933 (A), 8 × 108 CFU of strain EDL933 Δstx1 (B), 5 × 108 CFU of strain 86-24 (C), and 9 × 108 CFU of strain E345 (D).
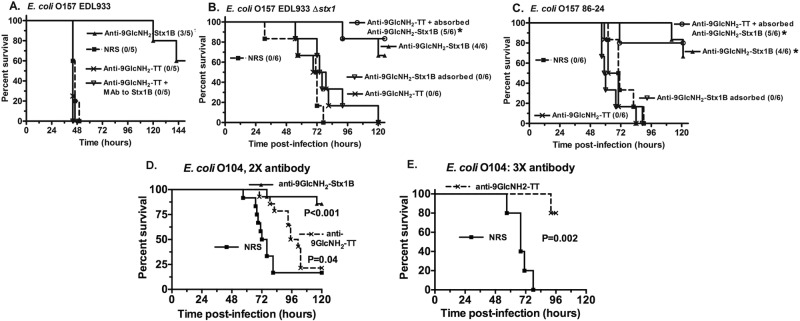
Antibodies to either PNAG or Stx present in the antisera elicited by the 9GlcNH2-Stx1b vaccine could individually confer protection from lethal STEC infection or they might both be needed for full efficacy. We thus compared protective efficacy of the antisera raised to 9GlcNH2-Stx1b with a high-titered antisera raised to 9GlcNH2-TT, which has only antibody to PNAG (24). The latter antiserum had no protective efficacy in mice (Fig. 5A), even if 3 doses were given starting 16 h preinfection and at 24 and 48 h postinfection. Addition of a monoclonal antibody to Stx1 to the monospecific antiserum to PNAG also did not result in any protection (Fig. 5A). Furthermore, antibody to PNAG alone had no protective efficacy against EDL933 Δstx1 and 86-24, STEC strains that produce only Stx2 (Fig. 5B and C). Using absorption experiments, we found that absorption of the antibody to PNAG from the serum raised to 9GlcNH2-Stx1b (anti-9GlcNH2-Stx1b absorbed) removed detectable antibody to this antigen and also abrogated protection (Fig. 5B and C). Restoration of protection was achieved by addition to the anti-9GlcNH2-Stx1b absorbed serum of antibody raised to 9GlcNH2-TT (Fig. 5B and C). Thus, in this model, neither antibody to Stx1b alone or PNAG alone was protective, but together they could prevent an otherwise lethal infection. Since antibody to Stx1b was essential to the protection of infant mice challenged with strains producing only Stx2, our findings also suggest that the titer of antibody to Stx2 elicited by the Stx1b carrier protein was sufficient to mediate this protection, consistent with a prior report on cross-reactivity of some murine MAbs to both Shiga toxins (31).
FIG 5 .
Protection against E. coli O157 but not O104 requires both antibody to PNAG and Stx. (A) Five-day-old mice challenged with 5 × 108 CFU of E. coli EDL933 by oral gavage were protected following intraperitoneal (i.p.) injection of antibody to 9GlcNH2-Stx1b, but protection could not be achieved with antibody to a different PNAG oligosaccharide conjugate vaccine, 9GlcNH2-TT. Adding a monoclonal antibody to Stx1 to the PNAG-only antibody (9GlcNH2-TT) did not achieve protection. (B and C) Protection against E. coli O157 EDL933 Δstx1 (B) and 86-24 (C) obtained with antibody to 9GlcNH2-Stx1b was lost after absorption (9GlcNH2-Stx1b absorbed) with S. aureus MN8M to remove antibody to PNAG. Similarly, there was no protection after administering antibody to PNAG only (anti-9GlcNH2-TT). Addition of antibody to 9GlcNH2-Stx1b absorbed with S. aureus MN8M to the PNAG-only antiserum 9GlcNH2-TT restored protective efficacy, indicating that antibodies to both PNAG and Stx are required for protection. Kaplan-Meier survival curves were analyzed by log rank tests. An asterisk indicates an P value of <0.01 compared to survival in mice given NRS. (D) Survival of infant mice (12 to 14 mice/group; results from two identical experiments combined) challenged orally after two injections of the indicated antiserum (2X antibody) 16 and 4 h before infection. (E) Survival of infant mice (5 mice/group) injected with antisera raised to 9GlcNH2-TT given 3 times at 16 h before infection and at 24 and 48 h postinfection. The survival curves were analyzed by log rank test.
Protection against Stx-producing E. coli O104:H4.
We next tested whether antibodies to PNAG with or without antibody to Stx protected mice against lethal oral infection with enteroaggregative E. coli (EAEC) O104:H4. We i.p. injected two or three doses of the antiserum containing antibody only to PNAG (anti-9GlcNH2-TT) or twice with the antiserum raised against 9GlcNH2-Stx1b. Two injections of serum with antibody to both PNAG and Stx1b protected infant mice against EAEC O104:H4 infection, whereas two injections of antibody to PNAG significantly (P = 0.04) extended survival, but final mortality was the same as in mice given NRS (Fig. 5E). However, three doses of antiserum containing only antibody to PNAG administered 16 h prior to infection as well as 24 and 48 h postinfection was protective against lethality caused by the E. coli O104 strain (Fig. 5F).
Protection against non-Stx-producing E. coli.
To assess whether antibody raised to the 9GlcNH2-Stx1b conjugate could protect against a non-Stx-producing, PNAG-positive E. coli isolate as previously shown for antibody raised to the 9GlcNH2-TT conjugate (24), adult mice were given 0.2 ml of antisera 24 h prior to i.p. infection with 1.5 × 108 CFU of E. coli urinary tract infection (UTI) strain J and sacrificed 48 h later when the bacterial levels in the livers were determined (see Fig. S4 in the supplemental material). This antiserum significantly (P = 0.03 [Fig. S4]) lowered bacterial levels compared with mice given NRS, and 5 of 8 of the immune animals had sterile livers as opposed to 1 of 8 mice given NRS (P = 0.059 by one-tailed Fisher’s exact test). Thus, the 9GlcNH2 oligosaccharide conjugated to TT (24) or Stx1b elicited comparable, broadly reactive, and protective antibody to the conserved PNAG antigen.
DISCUSSION
In this study, we evaluated the ability of antibody raised to a systemically delivered conjugate of a glucosamine nonasaccharide, 9GlcNH2, and Stx1b to mediate in vitro bacterial killing, Stx holotoxin neutralization, and in vivo protection against STEC. We demonstrated that cell surface-associated PNAG was detected in all 9 STEC isolates representing 6 STEC serogroups that we tested and found that antibody to the 9GlcNH2-Stx1b conjugate neutralized Stx1 potently and Stx2 modestly. For the O157 STEC strains, antibodies elicited by the 9GlcNH2-Stx1b conjugate possessed opsonic killing and bactericidal activity and protected infant mice in a lethal GI infection model. Antibodies to both PNAG and Stx were needed for infant mouse protection against O157 STEC. These antibodies also mediated killing and protection against the Stx2-producing EAEC O104:H4 strain that caused the recent HUS outbreak centered in Germany (3, 7) but with this strain, mice were protected with three doses of antibody to PNAG alone. Collectively, our observations suggest that vaccination against both PNAG and Stx, using a construct such as 9GlcNH2 conjugated to Stx would be useful against a broad range of STEC serogroups.
PNAG-based vaccines have already been shown to have the potential to induce full or partial protection against a large range of microbial pathogens (24, 25); our demonstration here that they can contribute to immunity to STEC, potent pathogens confined to the GI tract, extends the range of microbes and host tissues wherein these vaccines might contribute to protective efficacy. Additionally, we demonstrated that PNAG is required for E. coli O157 virulence in the orogastric infant mouse model of STEC lethality. These findings suggest that additional PNAG-producing GI pathogens such as Salmonella enterica and Listeria monocytogenes (25) might also be targeted by vaccination against PNAG.
Investigational vaccines to Stx-producing enteric pathogens target the toxins themselves and often an additional component such as the LPS O157 antigen, adherence factors, or other bacterial antigens (16, 17). In some experimental mouse infection models, immunity to Stx alone is sufficient for protection against E. coli GI infection (17). Numerous studies to decrease carriage of E. coli O157 in farm animals have shown effects following immunization with a variety of antigens (14). Whether Stx and other antigens targeted by these vaccine strategies would be applicable to human immunity to E. coli O157 is not established, although clinical use of MAbs to Stx to prevent HUS in children has been initiated (14). Our findings do indicate that augmenting immunity to Stx with immunity to PNAG might have the potential to reduce diarrhea or shedding of STEC in either animals or humans.
Immunity to pathogens confined to the GI tract requires that immune effectors such as antibodies, complement, and phagocytes gain access to the infecting organisms. While mucosal IgA is often evoked as a main mediator of this immunity, there is little substantive evidence in humans linking IgA levels to immunity to GI pathogens that is independent of the presence of concomitant serum IgM or IgG antibody to the pathogen (32). Furthermore, the observation that only about 10% of IgA-deficient humans have clinically significant manifestation of their condition encompassing increased susceptibility to infections (32) provides a powerful argument that mucosal IgA is not critical for protecting against GI pathogens. In contrast, serum vibriocidal antibody correlates with LPS O-antigen-specific immunity to Vibrio cholerae (33), and many examples of successful vaccination by systemic immunization and/or induction of serum IgG antibodies against mucosal pathogens such as influenza virus, poliovirus, and rotavirus (34), along with reductions in carriage of pathogens that colonize mucosal surfaces (35), are known.
Serum IgG transits onto and off the GI epithelium via the neonatal Fc receptor (FcRn) (36) expressed throughout life in humans on antigen-presenting cells as well as epithelial and endothelial cells. The FcRn contains both the major histocompatibility complex (MHC) class I β-2-microglobulin component and the MHC class II invariant chain, whose synthesis is increased during infection-mediated inflammation. The transport of serum antibody to the inflamed GI mucosa may be due to increased FcRn expression or it might also gain access along with additional mediators of immunity such as phagocytes, complement, and other factors via inflammation-induced leakage through the disrupted epithelium caused by the infecting pathogen. In this context, concern has been raised about whether antibody to PNAG could disrupt the normal GI microbial flora, and we have addressed this issue in a prior publication (25), citing among many other factors, that in the absence of inflammation there is unlikely to be an accumulation of sufficient mediators of PNAG antibody activity to impact the normal GI microbial constituents. Overall, with human trials of an IgG1 MAb to PNAG initiated and an oligosaccharide-based vaccine being developed for clinical testing in humans and economically important animals (24), prospects are bright that immunity to PNAG can provide a major component of host resistance to infection against a broad range of pathogens (25), which might need to be augmented by immunization against a limited number of additional microbial virulence factors such as Stx.
MATERIALS AND METHODS
Details are provided in Text S1 in the supplemental material.
Bacteria, plasmids, antibodies, and toxins.
The bacterial strains and plasmids used in this study, along with their relevant characteristics and sources, are listed in Table S1 in the supplemental material.
Detection of PNAG on bacterial surfaces.
Confocal scanning laser microscopy was used to detect poly-N-acetylglucosamine (PNAG) on the bacterial surface as described previously (25). Images depicted in the figures are representative of multiple fields viewed.
Preparation of the conjugate vaccine.
The thiol-derivatized 9GlcNH2-S-S (9GlcNH2 is a synthetic nonamer of nonacetylated PNAG) oligosaccharide was prepared from synthetic precursor (37) as previously described (24).
Antibody production and analysis.
Antibodies to purified 9GlcNH2 conjugated to the Shiga toxin 1b subunit (9GlcNH2-Stx1b) were raised in New Zealand White rabbits. Binding of antibodies to PNAG and Shiga toxin (Stx) was analyzed by enzyme-linked immunosorbent assay (ELISA) as described previously (21).
Antibody-dependent OPK assays.
The four key components for opsonophagocytic killing (OPK) assays, including cells with phagocytic activity, complement, sera, and bacteria were prepared as previously described (21) except differentiated HL-60 (American Type Culture Collection, Manassas, VA) promyelocytic cells were used as the phagocytes (38).
Vero cell toxicity assay.
The sera were analyzed as described previously (39) using a Vero cell cytotoxicity assay for the presence of toxin-neutralizing antibody.
Animal model of lethal STEC infection.
Animal experiments complied with institutional and federal guidelines for animal care and use and were approved by the Harvard Medical Area Institutional Animal Care and Use Committee. A moribund/lethal infection model was developed using 5-day-old CD-1 mice (Charles River Laboratories, Wilmington, MA). The infant mice were housed with and fed by their mothers; the mothers were fed a standard diet. Groups of mice were orally challenged with E. coli strains indicated in the text or the figure legends in 500 µl of phosphate-buffered saline (PBS), and survival was monitored for 1 week. For evaluation of the passive protection conferred by antibodies to 9GlcNH2-Stx1b, 5-day-old mice received 80 µl of either normal rabbit serum (NRS) or antisera to 9GlcNH2-Stx1b injected intraperitoneally (i.p.). In addition, antibodies to 9GlcNH2-TT (9GlcNH2 conjugated to tetanus toxoid) (25), monoclonal antibody (MAb) to Stx1b as well as a combined cocktail of this MAb and antibody to PNAG were evaluated against strain EDL933 infection.
Serum adsorption and reconstitution experiments.
The antiserum to 9GlcNH2-Stx1b was adsorbed with heat-killed S. aureus strain MN8M, a PNAG overproducer to remove antibody to this antigen or with S. aureus MN8Δica, which does not produce PNAG, as a control.
Mouse systemic infection model.
A previously described adult mouse systemic infection model (40) was used to investigate passive protection provided by antibodies to 9GlcNH2-Stx1b against a non-Stx-producing E. coli strain.
Statistical analysis.
Kaplan-Meier survival cures were analyzed by a log rank test incorporating Bonferroni’s correction for multiple comparisons. Pairwise comparisons of bacterial tissue burdens and overall survival between immune and control groups were evaluated using a nonparametric unpaired t test and Fisher’s exact test, respectively. P values for the latter analyses were determined from one-sided tests, as previous data and common sense indicated that the effect of the immune serum could go in only one direction, we could predict ahead of time that mice receiving nonimmune sera will have the higher tissue bacterial burdens before we collected any data and if the mice receiving the immune sera had ended up with the higher tissue bacterial levels, we would have attributed that difference to chance and called the difference “not statistically significant.”
SUPPLEMENTAL MATERIAL
Supplemental Materials and Methods. Download
Binding of antibody to 9GlcNH2-Stx1b to wells on ELISA plates coated with PNAG (A), Stx1 (B), or Stx2 (C). Values are means of three replicate values, and error bars show standard deviations (SD). Download
Determination of STEC CFU in mouse tissues at various time points postinfection of 5-day-old CD-1 mice via oral gavage with E. coli EDL933 (5 × 108 CFU/mouse). Each symbol represents the value for an individual mouse. The short black lines show the geometric means for groups of mice. No bacteria were detected outside the gastrointestinal (GI) tract (limit of detection, 2 CFU/g [rightmost panel]). Download
Protective efficacy of antibody raised to the synthetic oligosaccharide-conjugate vaccine (9GlcNH2-Stx1b) against E. coli EDL933. Infant mice were given 80 µl of the indicated antisera 24 and 4 h prior to challenge with ~8 × 108 to 9 × 108 CFU of strain EDL933/mouse. P values were generated by log rank analysis of the survival curves. Download
Protective efficacy of antiserum raised to 9GlcNH2-Stx1B against systemic infection due to E. coli strain J. Strain J, a UTI isolate, was injected i.p. 24 h after i.p. injection of 200 µl of the indicated serum. After 48 h, the mice were euthanized, their livers were removed and homogenized, and bacterial levels were enumerated. Each symbol represents the value (CFU/gram) for an individual mouse. The black horizontal lines represent the median values for the groups of mice. The P value was determined by nonparametric t test. The lower limit of detection was 2 CFU/g. Download
Bacterial strains and plasmids used in this study
ACKNOWLEDGMENTS
This research project was supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID) grants AI46706 and AI057159, a component of award U54 AI057159 (to G.B.P.), and AI042347 and HHMI (to M.K.W.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
The following reagents were obtained through BEI Resources, NIAID, NIH: monoclonal anti-Shiga toxin 1 subunit B (produced in vitro) (similar to 13C4) (catalog no. NR-844; BEI Resources), and polyclonal anti-Shiga toxin 2 subunit B (IgG, rabbit) (catalog no. NR-9352; BEI Resources). We thank Anne Kane of Tufts University’s Medical School for providing Stx1 and Stx2 holotoxins and Stx1b protein.
Footnotes
Citation Lu X, Skurnik D, Pozzi C, Roux D, Cywes-Bentley C, Ritchie JM, Munera D, Gening ML, Tsvetkov YE, Nifantiev NE, Waldor MK, Pier GB. 2014. A poly-N-acetylglucosamine−Shiga toxin broad-spectrum conjugate vaccine for Shiga toxin-producing Escherichia coli. mBio 5(2):00974-14. doi:10.1128/mBio.00974-14.
REFERENCES
- 1. Page AV, Liles WC. 2013. Enterohemorrhagic Escherichia coli infections and the hemolytic-uremic syndrome. Med. Clin. North Am. 97:681–695. 10.1016/j.mcna.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 2. Gould LH, Mody RK, Ong KL, Clogher P, Cronquist AB, Garman KN, Lathrop S, Medus C, Spina NL, Webb TH, White PL, Wymore K, Gierke RE, Mahon BE, Griffin PM, Emerging Infections Program FoodNet Working Group 2013. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000–2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog. Dis. 10:453–460. 10.1089/fpd.2012.1401 [DOI] [PubMed] [Google Scholar]
- 3. Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, Paxinos EE, Sebra R, Chin CS, Iliopoulos D, Klammer A, Peluso P, Lee L, Kislyuk AO, Bullard J, Kasarskis A, Wang S, Eid J, Rank D, Redman JC, Steyert SR, Frimodt-Møller J, Struve C, Petersen AM, Krogfelt KA, Nataro JP, Schadt EE, Waldor MK. 2011. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 365:709–717. 10.1056/NEJMoa1106920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munera D, Ritchie JM, Hatzios SK, Bronson R, Fang G, Schadt EE, Davis BM, Waldor MK. 20 January 2014. Autotransporters but not pAA are critical for rabbit colonization by Shiga toxin-producing Escherichia coli. Nat. Commun. 5:3080. 10.1038/ncomms4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15. 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thorpe CM. 2004. Shiga toxin-producing Escherichia coli infection. Clin. Infect. Dis. 38:1298–1303. 10.1086/383473 [DOI] [PubMed] [Google Scholar]
- 7. Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Muller L, King LA, Rosner B, Buchholz U, Stark K, Krause G. 2011. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365:1771–1780 [DOI] [PubMed] [Google Scholar]
- 8. Paton JC, Paton AW. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimizu T, Sato T, Kawakami S, Ohta T, Noda M, Hamabata T. 2007. Receptor affinity, stability and binding mode of Shiga toxins are determinants of toxicity. Microb. Pathog. 43:88–95. 10.1016/j.micpath.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 10. Davis TK, McKee R, Schnadower D, Tarr PI. 2013. Treatment of Shiga toxin-producing Escherichia coli infections. Infect. Dis. Clin. North Am. 27:577–597 [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181:664–670. 10.1086/315239 [DOI] [PubMed] [Google Scholar]
- 12. Wagner PL, Neely MN, Zhang X, Acheson DW, Waldor MK, Friedman DI. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 183:2081–2085. 10.1128/JB.183.6.2081-2085.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930–1936. 10.1056/NEJM200006293422601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomas DE, Elliott EJ. 2013. Interventions for preventing diarrhea-associated hemolytic uremic syndrome: systematic review. BMC Public Health 13:799–817. 10.1186/1471-2458-13-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao X, Cai K, Li T, Wang Q, Hou X, Tian R, Liu H, Tu W, Xiao L, Fang L, Luo S, Liu Y, Wang H. 2011. Novel fusion protein protects against adherence and toxicity of enterohemorrhagic Escherichia coli O157:H7 in mice. Vaccine 29:6656–6663. 10.1016/j.vaccine.2011.06.106 [DOI] [PubMed] [Google Scholar]
- 16. Mohawk KL, O’Brien AD. 2011. Mouse models of Escherichia coli O157:H7 infection and Shiga toxin injection. J. Biomed. Biotechnol. 2011:258185. 10.1155/2011/258185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mejias MP, Ghersi G, Craig PO, Panek CA, Bentancor LV, Baschkier A, Goldbaum FA, Zylberman V, Palermo MS. 2013. Immunization with a chimera consisting of the B subunit of Shiga toxin type 2 and Brucella lumazine synthase confers total protection against Shiga toxins in mice. J. Immunol. 191:2403–2411. 10.4049/jimmunol.1300999 [DOI] [PubMed] [Google Scholar]
- 18. Ahmed A, Li J, Shiloach Y, Robbins JB, Szu SC. 2006. Safety and immunogenicity of Escherichia coli O157 O-specific polysaccharide conjugate vaccine in 2−5-year-old children. J. Infect. Dis. 193:515–521. 10.1086/499821 [DOI] [PubMed] [Google Scholar]
- 19. McKenney D, Pouliot KL, Wang Y, Murthy V, Ulrich M, Döring G, Lee JC, Goldmann DA, Pier GB. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523–1527. 10.1126/science.284.5419.1523 [DOI] [PubMed] [Google Scholar]
- 20. Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maira-Litrán T, Kropec A, Goldmann DA, Pier GB. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect. Immun. 73:6752–6762. 10.1128/IAI.73.10.6752-6762.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cerca N, Jefferson KK, Maira-Litrán T, Pier DB, Kelly-Quintos C, Goldmann DA, Azeredo J, Pier GB. 2007. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect. Immun. 75:3406–3413. 10.1128/IAI.00078-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelly-Quintos C, Cavacini LA, Posner MR, Goldmann D, Pier GB. 2006. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect. Immun. 74:2742–2750. 10.1128/IAI.74.5.2742-2750.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gening ML, Maira-Litrán T, Kropec A, Skurnik D, Grout M, Tsvetkov YE, Nifantiev NE, Pier GB. 2010. Synthetic β-(1 →6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect. Immun. 78:764–772. 10.1128/IAI.01093-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cywes-Bentley C, Skurnik D, Zaidi T, Roux D, Deoliveira RB, Garrett WS, Lu X, O’Malley J, Kinzel K, Zaidi T, Rey A, Perrin C, Fichorova RN, Kayatani AK, Maira-Litràn T, Gening ML, Tsvetkov YE, Nifantiev NE, Bakaletz LO, Pelton SI, Golenbock DT, Pier GB. 2013. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc. Natl. Acad. Sci. U. S. A. 110:E2209–E2218. 10.1073/pnas.1303573110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cerca N, Maira-Litrán T, Jefferson KK, Grout M, Goldmann DA, Pier GB. 2007. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proc. Natl. Acad. Sci. U. S. A. 104:7528–7533. 10.1073/pnas.0700630104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conrady DG, Flagler MJ, Friedmann DR, Vander Wielen BD, Kovall RA, Weiss AA, Herr AB. 2010. Molecular basis of differential B-pentamer stability of Shiga toxins 1 and 2. PLoS One 5:e15153. 10.1371/journal.pone.0015153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramasubbu N, Thomas LM, Ragunath C, Kaplan JB. 2005. Structural analysis of dispersin B, a biofilm-releasing glycoside hydrolase from the periodontopathogen Actinobacillus actinomycetemcomitans. J. Mol. Biol. 349:475–486. 10.1016/j.jmb.2005.03.082 [DOI] [PubMed] [Google Scholar]
- 29. Tesh VL, Burris JA, Owens JW, Gordon VM, Wadolkowski EA, O’Brien AD, Samuel JE. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wadolkowski EA, Sung LM, Burris JA, Samuel JE, O’Brien AD. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 58:3959–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donohue-Rolfe A, Acheson DW, Kane AV, Keusch GT. 1989. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect. Immun. 57:3888–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yel L. 2010. Selective IgA deficiency. J. Clin. Immunol. 30:10–16. 10.1007/s10875-009-9357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, Harris JB, LaRocque RC, Bufano MK, Yu Y, Wu-Freeman Y, Leung DT, Sarracino D, Krastins B, Charles RC, Xu P, Kovác P, Calderwood SB, Qadri F, Ryan ET. 2012. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin. Vaccine Immunol. 19:1712–1721. 10.1128/CVI.00321-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Istrate C, Hinkula J, Hammarström L, Svensson L. 2008. Individuals with selective IgA deficiency resolve rotavirus disease and develop higher antibody titers (IgG, IgG1) than IgA competent individuals. J. Med. Virol. 80:531–535. 10.1002/jmv.21101 [DOI] [PubMed] [Google Scholar]
- 35. Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, Mendelman PM, Bohidar N, Yagupsky P. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 174:1271–1278. 10.1093/infdis/174.6.1271 [DOI] [PubMed] [Google Scholar]
- 36. Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. 2004. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20:769–783. 10.1016/j.immuni.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 37. Gening ML, Tsvetkov YE, Pier GB, Nifantiev NE. 2007. Synthesis of β-(1 →6)-linked glucosamine oligosaccharides corresponding to fragments of the bacterial surface polysaccharide poly-N-acetylglucosamine. Carbohydr. Res. 342:567–575. 10.1016/j.carres.2006.08.010 [DOI] [PubMed] [Google Scholar]
- 38. Collins SJ, Gallo RC, Gallagher RE. 1977. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature 270:347–349. 10.1038/270347a0 [DOI] [PubMed] [Google Scholar]
- 39. Karmali MA, Steele BT, Petric M, Lim C. 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet i:619–620 [DOI] [PubMed] [Google Scholar]
- 40. Kropec A, Maira-Litran T, Jefferson KK, Grout M, Cramton SE, Götz F, Goldmann DA, Pier GB. 2005. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 73:6868–6876. 10.1128/IAI.73.10.6868-6876.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods. Download
Binding of antibody to 9GlcNH2-Stx1b to wells on ELISA plates coated with PNAG (A), Stx1 (B), or Stx2 (C). Values are means of three replicate values, and error bars show standard deviations (SD). Download
Determination of STEC CFU in mouse tissues at various time points postinfection of 5-day-old CD-1 mice via oral gavage with E. coli EDL933 (5 × 108 CFU/mouse). Each symbol represents the value for an individual mouse. The short black lines show the geometric means for groups of mice. No bacteria were detected outside the gastrointestinal (GI) tract (limit of detection, 2 CFU/g [rightmost panel]). Download
Protective efficacy of antibody raised to the synthetic oligosaccharide-conjugate vaccine (9GlcNH2-Stx1b) against E. coli EDL933. Infant mice were given 80 µl of the indicated antisera 24 and 4 h prior to challenge with ~8 × 108 to 9 × 108 CFU of strain EDL933/mouse. P values were generated by log rank analysis of the survival curves. Download
Protective efficacy of antiserum raised to 9GlcNH2-Stx1B against systemic infection due to E. coli strain J. Strain J, a UTI isolate, was injected i.p. 24 h after i.p. injection of 200 µl of the indicated serum. After 48 h, the mice were euthanized, their livers were removed and homogenized, and bacterial levels were enumerated. Each symbol represents the value (CFU/gram) for an individual mouse. The black horizontal lines represent the median values for the groups of mice. The P value was determined by nonparametric t test. The lower limit of detection was 2 CFU/g. Download
Bacterial strains and plasmids used in this study