Abstract
Background:
Cages have been widely used for the anterior reconstruction and fusion of cervical spine. Nonmetal cages have become popular due to prominent stress shielding and high rate of subsidence of metallic cages. This study aims to assess fusion with n-HA/PA66 cage following one level anterior cervical discectomy.
Materials and Methods:
Forty seven consecutive patients with radiculopathy or myelopathy underwent single level ACDF using n-HA/PA66 cage. We measured the segmental lordosis and intervertebral disc height on preoperative radiographs and then calculated the loss of segmental lordosis correction and cage subsidence over followup. Fusion status was evaluated on CT scans. Odom criteria, Japanese Orthopedic Association (JOA) and Visual Analog Pain Scales (VAS) scores were used to assess the clinical results. Statistically quantitative data were analyzed while Categorical data by χ2 test.
Results:
Mean correction of segmental lordosis from surgery was 6.9 ± 3.0° with a mean loss of correction of 1.7 ± 1.9°. Mean cage subsidence was 1.2 ± 0.6 mm and the rate of cage subsidence (>2 mm) was 2%. The rate of fusion success was 100%. No significant difference was found on clinical or radiographic outcomes between the patients (n=27) who were fused by n-HA/PA66 cage with pure local bone and the ones (n=20) with hybrid bone (local bone associating with bone from iliac crest).
Conclusions:
The n-HA/PA66 cage is a satisfactory reconstructing implant after anterior cervical discectomy, which can effectively promote bone graft fusion and prevent cage subsidence.
Keywords: Cervical spine, anterior fusion, discectomy, nano-hydroxyapatite/polyamide-66 cage
INTRODUCTION
Since it's initial use by Smith and Robison in the 1950s, anterior cervical discectomy and fusion (ACDF) has been the standard surgical treatment for cervical discogenic diseases associated with radiculopathy or myelopathy.1,2 This procedure allows direct decompression of the spinal canal, enlargement of stenotic neural foramen alongwith restoration of intervertebral disk height. Following anterior discectomy, various interbody implanting devices are traditionally used for reconstructing the stability of the segment involved.3,4
Autogenous tricortical iliac crest graft has been considered as the “gold standard” of anterior reconstruction due to its high fusion rate.5,6 However, it brings around 25% of donor site morbidity including hematoma, persistent donor site pain and infection.7,8 To prevent these complications, Bagby et al., had designed the first hollow cylindrical cage device (Bagby Bone Basket) made of stainless steel which allowed bone in growth.9 Subsequently, the titanium mesh cage (TMC) gradually replaced the stainless steel cages and became the most widely used device in anterior fusion due to its excellent mechanical behavior and preferable clinical outcomes.10,11 However, TMC has also been associated with prominent stress shielding, high rate of cage subsidence and noticeable influence on postoperative radiographic observation. Because of the shortages of metallic cages, various nonmetal interbody fusion devices (such as the carbon cage, polymethyl methacrylate (PMMA) cage, polyetheretherketone (PEEK) cage) became extremely popular with spine surgeons in recent times.12,13,14,15
The hollow cylindrical nano-hydroxyapatite/polyamide-66 cage (n-HA/PA66, Sichuan National Nano Technology Co., Ltd. Chengdu, Sichuan) is a nonmetal cage material made up of a composite of nanoparticle hydroxyapatite and polyamide-66 which mimicks the structure of natural bone. This cage has stiffness and elasticity similar to cortical bone and can provide satisfactory stability, high fusion rates and low subsidence rates in anterior reconstruction.16,17,18 When used in the cervical spine, this cage has a variable diameter and length for different clinical requirements and allows the surgeon to cut its ends to a given angle to match the inherent sagittal alignment of the fusion segment. The rims of this cage are designed wide (nearly 3 mm) to avoid cage penetrating into the vertebral body [Figure 1].
Figure 1.

Photograph of hollow cylinder n-HA/PA66 cage. Each cage has wide rims with several shallow recesses designed to prevent subsidence and migration
The purpose of the current study is to evaluate the clinical effects of the n-HA/PA66 cage in reconstructing and maintaining the stabilization of anterior column after single level cervical discectomy.
MATERIALS AND METHODS
68 consecutive patients were operated with cervical radiculopathy or myelopathy who had undergone single level ACDF using n-HA/PA66 cage with anterior fixation at our institute between 2008 and 2010. Forty seven patients who completed more than 2 years postoperative followup were included in this retrospective study. All these surgeries were performed by one of four attending orthopedic spine surgeons. Indications for surgery were radiculopathy or myelopathy with progressive neurologic deficit which did not respond to conservative treatment for a period of at least six weeks. The fusion level was between C3 to C7 vertebra. Informed consent for the use of the n-HA/PA cage was obtained from all patients. This study was approved by the local Ethics Committee.
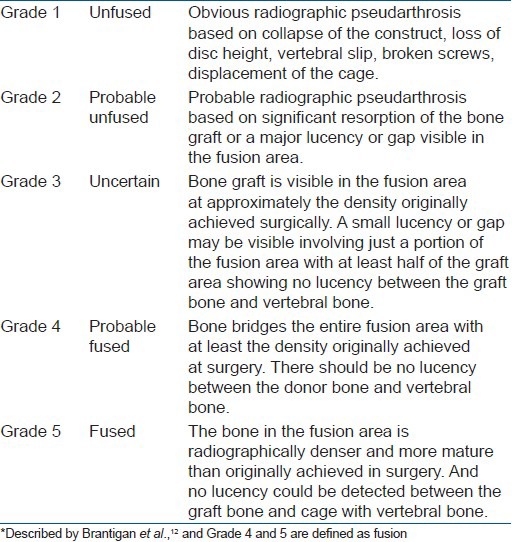
All the included patients underwent complete cervical X-rays and three-dimensional computed tomography (3DCT) before surgery and at 1 week, 3-, 6-, 9-months after surgery and finally at the last followup examination (more than 2 years). Two attending spinal surgeons, who had not joined the primary surgeries, measured each case for the segmental lordosis and intervertebral disc height on plain X-rays and their average value was adopted finally. The segmental lordosis was measured as the Cobb angle between the superior endplate of the upper vertebral body and the inferior endplate of the lower vertebral body. Intervertebral disc height was measured as the distance between the midpoint of the upper and lower endplates. The cage subsidence was calculated as the intervertebral disc height at the last followup minus the height at one week after operation. In this study, more than 2 mm loss of intervertebral disc height was defined as cage subsidence. The fusion status was evaluated on sagittal and coronal CT (3DCT) by the above two attending spinal surgeons based on the Five-grade criteria of Brantigan et al.,12 [Table 1]. The Grades 4 or 5 were defined as fused while Grade 1 or 2 as unfused and Grade 3 was unable to assess. The fusion time was estimated as the earliest followup time when we observed bone graft fusion.
Table 1.
Fusion grading criteria*
The Odom criteria was used to assess the general clinical outcome of patients at the last followup. Japanese Orthopedic Association (JOA) score and the 10-point visual analog scale (VAS) was used to evaluate the neurologic status and the body pain respectively before surgery and at the last followup.
All surgeries used the standard anterior exposure, discectomy and decompression which was performed on the lines of the technique of Smith and Robinson.1,2 The superior and inferior endplates were carefully prepared by abrading and removing the overlying cartilage using a high speed burr and curette. The intervertebral disc height of the decompression level was measured to select an appropriate n-HA/PA66 cage (If no suitable original cage was available, a saw was used to cut the cage as required). Before cage implantation, it was to be filled with bone graft. In most patients, morselized bone from local decompression was enough to fill the cage; otherwise, a part of cancellous bone would be harvested from the anterior superior iliac crest through a small incision and a cortical window. The n-HA/PA66 cage filled with local or hybrid bone grafts would be inserrted into the prepared intervertebral space [Figure 2]. To achieve immediate stabilization, a Zephir Anterior Cervical Plate System (Medtronic Sofamor Danek USA, Inc. Memphis, TN) or ATLANTIS Anterior Cervical Plate System (Medtronic Sofamor Danek USA, Inc. Memphis, TN) was used in each patient. After surgery, all patients were advised to wear a soft cervical collar for about 6 weeks.
Figure 2.
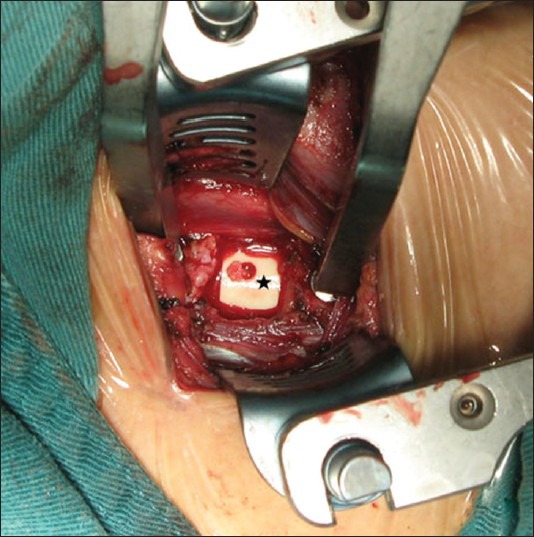
Peroperative photograph showing n-HA/PA66 cage implanting. Black asterisk denotes the cage
According to different bone grafts in the n-HA/PA66 cage, two groups were divided. Twenty seven patients who had been fused using n-HA/PA66 cage with pure local bone graft were included in Group A, while the remaining twenty patients who had been fused by n-HA/PA66 cage with hybrid bone grafts were included in Group B. The radiographic and clinical results between these two groups were compared.
SPSS 11.0 statistic software (SPSS, Inc, Chicago, Illinois, USA) was used. Quantitative data was analyzed by t test or the Mann-Whitney test as appropriate (including age, followup period, segmental lordosis, intervertebral disc height, JOA scores and VAS points). Categorical data were analyzed by χ2 test (including sexual and fusion grading). All P values were two-sided and levels of significance reaching >95% were accepted.
RESULTS
47 patients (26 men and 21 women) with a mean age of 47.7 years (range 25-66 years) who underwent one level ACDF with an n-HA/PA66 cage were included in the study. The operative level was C3-4 (n=6), C4-5 (n=13), C5-6 (n=19) and C6-7 (n=9). The mean followup was 35.9 months (range 24-45 months).
Preoperatively, the average intervertebral disc height of the pathological level was 4.7 ± 1.1 mm and the average lordosis was 1.8 ± 3.8°. After surgery, both the average intervertebral disc height and the lordosis were significantly corrected, as 7.1 ± 1.1 mm and 6.9 ± 3.0° respectively (P < 0.001). At the final followup, the average intervertebral disc height was 5.9 ± 1.1 mm and the lordosis was 5.2 ± 2.9°, while the average length of cage subsidence was 1.2 ± 0.6 mm and the loss of lordosis correction was 1.7 ± 1.9°. The rate of cage subsidence (>2 mm) was 2% (1/47). According to Brantigan's12 Five-Grade criteria, 35 patients had a Grade-5 fusion (completed fusion) and the other 12 had a Grade-4 fusion (probable fusion) at the last followup. At the last followup, the rate of satisfied osseous fusion was 100% [Figure 3]. The average fusion time was 4.2 ± 1.8 months.
Figure 3.
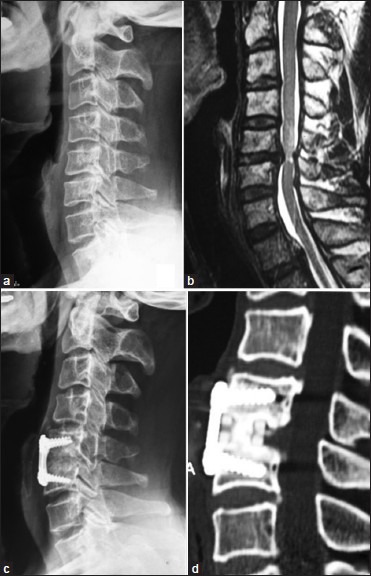
A case of cervical myelopathy due to C5/6 disc herniation. (a) Preoperative lateral radiograph of cervical spine showing narrowing of disc space between C5C6 and straightening of spine (b) Preoperative cervical T2W MRI cervidal spine sagittal cut showing prolapse of C5-6 disc associated with edema signal of the spinal cord, (c) Postoperative x-ray cervical spine lateral view three years followup showing anterior discectomy and fusion. (d) Sagittal reconstruction computed tomography scans at three years followup showing good bony fusion
There were no wound infections, allergic reactions or neurological damage in our series. Clinical outcome was assessed in all patients. According to Odom's criteria at the last followup, 22 patients (47%) presented an excellent outcome, 15 (32%) with good, 5 (11%) with fair and none of our patients had a poor outcome. The average preoperative and last followup JOA scores were 7.7 ± 2.7 and 15.0 ± 1.9 points respectively (P < 0.001). The average preoperative VAS scores were 7.0 ± 1.8 which significantly improved to 2.3 ± 1.6 points at the last followup (P < 0.001).
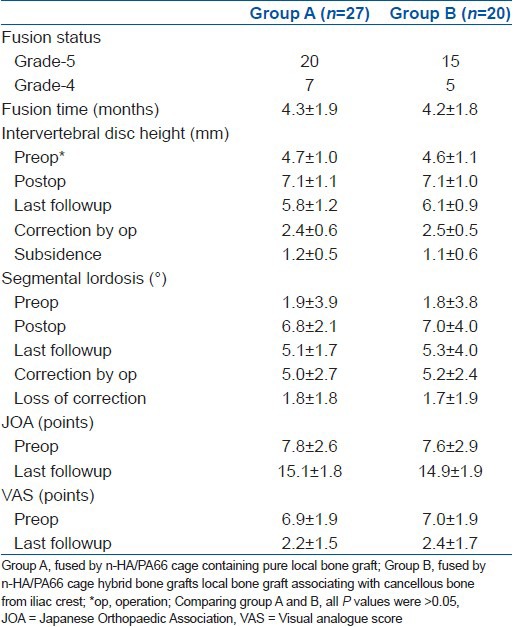
Based on different bone grafts used with the n-HA/PA66 cage, two groups (Group A and B) were formed. In Group A (pure local bone graft group), there were 15 men and 12 women with a mean age of 46.7 ± 10.6 years. While in Group B (hybrid bone grafts group), there were 11 men and 9 women with a mean age of 49.0 ± 8.8 years. In Group B, two patients (10%) developed graft site pain after surgery; however no graft site hematoma or infection was seen. No significant difference was found on gender or age criteria between these two groups (P = 0.97 and 0.428, respectively). The radiographic and clinical outcomes of these two groups are shown in Table 2. It was seen that there was no significant difference on the fusion grading (P = 0.943), subsidence (P = 0.238), JOA scores (P = 0.596) or VAS points (P = 0.991) between these two groups.
Table 2.
Radiographic and clinical outcomes of patients with different bone grafts in n-HA/PA 66 cage
DISCUSSION
Anterior reconstruction is an essential process to stabilize the cervical spine following decompressive discectomy.1,2 In order to obtain a better clinical outcome anterior reconstruction materials have been developed. Based on previous experiences, the ideal reconstruction material should be an excellent combination of great histocompatibility, sufficient mechanical strength, appropriate elasticity and good imaging characteristics.16,19,20
The design of n-HA/PA66 composite was inspired from the constituent form of the natural bone combining the apatite with collagen. The nanoparticle hydroxyapatite (nano-hydroxyapatite, n-HA) was chemically bonding with polyamide 66 (PA66) molecules in a proportion of nearly two-to-one to constitute this new biomimetic composite.21 In previous in vitro studies, the n-HA/PA66 composite exhibited excellent cytocompatibility without any obvious cytotoxicity. The mechanical test showed that the strengths of bending, tensing and compressing of n-HA/PA66 were 95, 79 and 117 MPa respectively, which matched well with human cortical bone.21,22 When implanting the n-HA/PA66 cage to reconstruct the cervical spine of adult goats, Liang et al., have found that the stability of the host vertebra was maintained and radiological and histological bone fusion was achieved within 24 weeks of surgery.23
In a study by Ou et al., the n-HA/PA66 cage was used in 42 patients with thoracic or lumbar fracture. They reported a fusion rate of 100% after a mean followup of 13 months.17 In our previous study, we had reported 51 patients who accepted n-HA/PA66 cage reconstruction after thoracic or lumbar corpectomy. More than two years after surgery, the fusion rate was 90.2% and the loss of kyphosis correction was about 1.9 degree on an average.16 In another study, Zhao et al., reported a fusion rate of 94.3% in a series of 35 patients who underwent cervical corpectomy with an n-HA/PA66 cage and anterior cervical plate-assisted reconstruction.18
In the present study, solid osseous fusion was found in all the 47 patients (100% fusion rate) 18-46 months after ACDF. These results indicated an excellent ability of the n-HA/PA66 cage in ensuring bony fusion. In fact, n-HA/PA66 cage has good mechanical properties to regain and maintain the immediate stabilization of anterior vertebral column and thus provides a suitable environment for subsequent fusion. Secondly, after implanting of the n-HA/PA66 cage into the interbody space, the micro-ion-exchange of Ca2+ and PO43 would occur between the n-HA and surrounding body fluids and then it forms a crystal layer on the cage surface.17,18,24 This layer then becomes an optimal bridge assisting in the creeping growth of bone graft. Finally, the modulus of elasticity of n-HA/PA66 cage is 5.6GPa, which is much lower than that of the bone graft (E = 12GPa). According to Wolff's law, bone grows in response to applied stress and will be absorbed if a mechanical stimulus is lacking. The lower modulus of elasticity of the n-HA/PA66 cage helps decrease stress shielding and promote early osseous fusion.
Since the application of cages, subsidence has become the most common complication in many studies.25,26,27 Cage subsidence leads to the loss of height of the fused segment that is correlated with kyphotic deformity, instrument failure or postoperative neurologic deterioration. Therefore, preventing high rate of subsidence becomes a fundamental purpose of newly developed cages. In our series, subsidence of the n-HA/PA66 cage was found in 1 of 47 patients (2%) with ACDF and the loss of height of fusion segment was 1.2 mm on an average. The appropriate elastic modulus of the n-HA/PA66 cage (similar to human bone) should be the distinguishing feature in preventing cage subsidence. In addition, wide footprints of n-HA/PA66 cage could effectively reduce the cutting of cage on endplate and reduce the possibility of subsidence. As we know, cage subsidence usually occurs before bony fusion is achieved. The n-HA/PA66 cage was able to conduct the growth of bone graft and promote the osseous fusion in an early period after surgery, which should not be an undesirable reason for the low rate of subsidence of the cage.27
Moreover, radiographic scattering was an obvious defect of metal cages, which greatly disturbed the image of bone grafting or the surrounding tissue on CT and MRI. The n-HA/PA66 cage is nonmetal and radiolucent. On one hand it allows the surgeon to evaluate the osseous fusion status on a plain radiograph or CT scans while on the other hand it does not interfere in the evaluation of spinal decompression status on an MRI.
An important advantage of the cage relative to autogenous graft is the high utilization efficiency of the bone graft. In ACDF, the morselized local bone graft harvesting were very limited and sometimes not enough to fill the cage, however a small quantity of nonstructural cancellous bone was needed to be harvested from the iliac crest rather than a mass of tricortical bone. It would markedly reduce the operative wound in the iliac and seemed to be an effective compromising way to prevent the donor site complications. At last followup, good or excellent results according to Odom's criteria were shown in 89.4% present patients under the ACDF with n-HA/PA66 cage at last followup. Particularly, no obvious difference was found in the radiographic and clinical outcomes between the patients with different bone grafts (i.e. local bone graft or hybrid bone graft) in the cage.
Several limitations exist in this study. We have evaluated only 47 patients who underwent ACDF with n-HA/PA66 cage here. Furthermore, these patients have been followed up for only 36 months on an average. Though we did not find any complications with regard to the n-HA/PA66 cage during this period, longer followups with these patients are required to evaluate the effects of this cage over a long term period after surgery.
In this study, a hollow cylindrical n-HA/PA66 cage was used to reconstruct the cervical spine following one-level anterior discectomy. This cage combines sufficient mechanical strength with appropriate elastic modulus that was similar to that of the the human cortical bone. Through the followup, a high rate of satisfactory fusion (100%) and a low rate of subsidence (2%) was seen. Additionally, this cage was radiolucent, ensuring clear images of osseous fusion or spinal decompression status on X-ray film, CT scans or MRI examination. Therefore, we concluded that the hollow cylindrical n-HA/PA66 cage is a satisfactory reconstructing device in patients undergoing for ACDF.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Robinson RA. Fusions of the cervical spine. J Bone Joint Surg Am. 1959;41-A:1–6. [PubMed] [Google Scholar]
- 2.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40-A:607–24. [PubMed] [Google Scholar]
- 3.Malloy KM, Hilibrand AS. Autograft versus allograft in degenerative cervical disease. Clin Orthop Relat Res. 2002;394:27–38. doi: 10.1097/00003086-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Zdeblick TA, Phillips FM. Interbody cage devices. Spine (Phila Pa 1976) 2003;28:S2–7. doi: 10.1097/01.BRS.0000076841.93570.78. [DOI] [PubMed] [Google Scholar]
- 5.Faldini C, Leonetti D, Nanni M, Di Martino A, Denaro L, Denaro V, et al. Cervical disc herniation and cervical spondylosis surgically treated by Cloward procedure: A 10-year-minimum followup study. J OrthopTraumatol. 2010;11:99–103. doi: 10.1007/s10195-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kao FC, Niu CC, Chen LH, Lai PL, Chen WJ. Maintenance of interbody space in one- and two-level anterior cervical interbody fusion: Comparison of the effectiveness of autograft, allograft, and cage. Clin Orthop Relat Res. 2005;430:108–16. doi: 10.1097/01.blo.0000142626.90278.9e. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui AA, Jackowski A. Cage versus tricortical graft for cervical interbody fusion. A prospective randomised study. J Bone Joint Surg Br. 2003;85:1019–25. doi: 10.1302/0301-620x.85b7.13398. [DOI] [PubMed] [Google Scholar]
- 8.Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, et al. Donor site morbidity after anterior iliac crest bone harvest for single level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2003;28:134–9. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11:931–4. doi: 10.3928/0147-7447-19880601-13. [DOI] [PubMed] [Google Scholar]
- 10.Majd ME, Vadhva M, Holt RT. Anterior cervical reconstruction using titanium cage with anterior plating. Spine (Phila Pa 1976) 1999;24:1604–10. doi: 10.1097/00007632-199908010-00016. [DOI] [PubMed] [Google Scholar]
- 11.Eck KR, Bridwell KH, Ungacta FF, Lapp MA, Lenke LG, Riew KD. Analysis of titanium mesh cages in adults with minimum two-year followup. Spine (Phila Pa 1976) 2000;25:2407–15. doi: 10.1097/00007632-200009150-00023. [DOI] [PubMed] [Google Scholar]
- 12.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine (Phila Pa 1976) 1993;18:2106–7. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 13.Faldini C, Chehrassan M, Miscione MT, Acri F, d’Amato M, Pungetti C, et al. Single level anterior cervical discectomy and interbody fusion using PEEK anatomical cervical cage and allograft bone. J Orthopaed Traumatol. 2011;12:201–5. doi: 10.1007/s10195-011-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JF, Lee ST, Wu CT. A hollow cylindrical PMMA strut for cervical spine reconstruction after cervical multilevel corpectomy. J Spinal Disord Tech. 2010;23:321–7. doi: 10.1097/BSD.0b013e3181b15bc8. [DOI] [PubMed] [Google Scholar]
- 15.Chen JF, Wu CT, Lee SC, Lee ST. Hollow cylindrical PMMA strut for spinal reconstruction after single level cervical corpectomy. J Neurosurg Spine. 2006;5:287–93. doi: 10.3171/spi.2006.5.4.287. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Song Y, Liu L, Liu H, Zeng J, Pei F. Anterior reconstruction with nano-hydroxyapatite/polyamide-66 cage after thoracic and lumbar corpectomy. Orthopedics. 2012;35:e66–73. doi: 10.3928/01477447-20111122-10. [DOI] [PubMed] [Google Scholar]
- 17.Ou Y, Jiang D, Quan Z, An H, Liu B. Application of artificial vertebral body of biomimetic nano-hydroxyapatite/polyamide 66 composite in anterior surgical treatment of thoracolumbar fractures. Zhong guo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21:1084–8. [PubMed] [Google Scholar]
- 18.Zhao Z, Jiang D, Ou Y, Tang K, Luo X, Quan Z. A hollow cylindrical nano-hydroxyapatite/polyamide composite strut for cervical reconstruction after cervical corpectomy. J Clin Neurosci. 2012;19:536–40. doi: 10.1016/j.jocn.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 19.Boakye M, Mummaneni PV, Garrett M, Baldus C, Blanke K. Anterior cervical discectomy and fusion involving a polyetheretherketone spacer and bone morphogenetic protein. J Neurosurg Spine. 2005;2:521–5. doi: 10.3171/spi.2005.2.5.0521. [DOI] [PubMed] [Google Scholar]
- 20.Niu CC, Liao JC, Chen WJ, Chen LH. Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: Titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech. 2010;23:310–6. doi: 10.1097/BSD.0b013e3181af3a84. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Li Y, Wei J, de Groot K. Development of biomimetic nano- hydroxyapatite/poly (hexamethyleneadipamide) composites. Biomaterials. 2002;23:4787–91. doi: 10.1016/s0142-9612(02)00229-6. [DOI] [PubMed] [Google Scholar]
- 22.Huang M, Feng J, Wang J, Zhang X, Li Y, Yan Y. Synthesis and characterization of nano-HA/PA66 composites. J Mater Sci Mater Med. 2003;14:655–60. doi: 10.1023/a:1024087410890. [DOI] [PubMed] [Google Scholar]
- 23.Liang Y, Jiang DM. In vivo study on intervetebral fusion cage made by nanohydroxyapatite and polyamide 66 composites [Article in Chinese] J Third Mil Med Univ. 2001;29:2333–5. [Google Scholar]
- 24.Xu Q, Lu H, Zhang J, Lu G, Deng Z, Mo A. Tissue engineering scaffold material of porous nanohydroxyapatite/polyamide 66. Int J Nanomedicine. 2010;5:331–5. doi: 10.2147/ijn.s9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmieder K, Wolzik-Grossmann M, Pechlivanis I, Engelhardt M, Scholz M, Harders A. Subsidence of the wing titanium cage after anterior cervical interbody fusion: 2-year followup study. J Neurosurg Spine. 2006;4:447–53. doi: 10.3171/spi.2006.4.6.447. [DOI] [PubMed] [Google Scholar]
- 26.Nakase H, Park YS, Kimura H, Sakaki T, Morimoto T. Complications and long term followup results in titanium mesh cage reconstruction after cervical corpectomy. J Spinal Disord Tech. 2006;19:353–7. doi: 10.1097/01.bsd.0000210113.09521.aa. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Chen D, Guo Y, Wang X, Lu X, He Z, et al. Subsidence of titanium mesh cage: A study based on 300 cases. J Spinal Disord Tech. 2008;21:489–92. doi: 10.1097/BSD.0b013e318158de22. [DOI] [PubMed] [Google Scholar]


