Abstract
Background:
Bone mass loss and muscle atrophy are the frequent complications occurring after spinal cord injury (SCI). The potential risks involved with these changes in the body composition have implications for the health of the SCI individual. Thus, there is a need to quantitate and monitor body composition changes accurately in an individual with SCI. Very few longitudinal studies have been reported in the literature to assess body composition and most include relatively small number of patients. The present prospective study aimed to evaluate the body composition changes longitudinally by DEXA in patients with acute SCI.
Materials and Methods:
Ninety five patients with acute SCI with neurological deficits were evaluated for bone mineral content (BMC), body composition [lean body mass (LBM) and fat mass] by dual-energy X-ray absorptiometry during the first year of SCI.
Results:
There was a significant decrease in BMC (P < 0.05) and LBM (P < 0.05) and increase in total body fat mass (TBFM) and percentage fat at infra-lesional sites. The average decrease was 14.5% in BMC in lower extremities, 20.5% loss of LBM in legs and 15.1% loss of LBM in trunk, and increase of 0.2% in fat mass in legs and 17.3% increased fat in the lower limbs at 1 year. The tetraplegic patients had significant decrease in arm BMC (P < 0.001), arm LBM (P < 0.01) and fat percentage (P < 0.01) compared to paraplegics. Patients with complete motor injury had higher values of TBFM and fat percentage, but comparable values of BMC and LBM to patients with incomplete motor injury.
Conclusions:
Our findings suggest that there is a marked decrease in BMC and LBM with increase in adiposity during the first year of SCI. Although these changes depend on the level and initial severity of lesions, they are also influenced by the neurological recovery after SCI.
Keywords: Body composition, body mass index, dual-energy X-ray absorptiometry, paraplegia, spinal cord injuries, tetraplegia
INTRODUCTION
Bone mass loss and muscle atrophy are the frequent complications occurring after spinal cord injury (SCI).1,2 Although unloading is an important factor in the pathogenesis of bone loss in SCI patients, neuronal lesion and hormonal changes also seem to be involved in this process.3 Inactivation and extreme unloading in SCI patients result in marked atrophy of the leg and thigh skeletal muscles within a few months of the injury.4 Accurate quantification of skeletal muscle is important in the assessment of nutritional status, disease risk, physical function and atrophic effects of aging and muscle wasting diseases.5 The potential risks involved with these changes in the body composition have implications for the health of the SCI individual.6 Thus, there is a need to quantitate and monitor body composition changes accurately in an individual with SCI.
Body mass is composed primarily of bone mineral, fat and fat free soft tissue [lean body mass (LBM)]. Skeletal muscle is the largest component of fat free mass, representing 50% of the non fat component in the body.7 Although dual-energy X-ray absorptiometry (DEXA) was first developed to measure bone mineral content (BMC), it has been shown to be one of the most feasible, valid and reliable measures of body composition in people with disabilities.8,9,10 Very few longitudinal studies have been reported in the literature to assess body composition and most include relatively small number of patients.4,10,11 The present prospective study aimed to evaluate the body composition changes longitudinally by DEXA in patients with acute SCI.
MATERIALS AND METHODS
This prospective, longitudinal study was carried out on 106 patients (79 males and 27 females) with acute SCI with neurological deficit who were admitted at our tertiary level health care institute. Only patients with grade A or B injury on the American Spinal Injury Association impairment scale (AIS)12 at the time of presentation to the institute and those presenting within 72 h of injury were enrolled in the study. During the initial evaluation and final followup at 1 year, 11 patients expired; therefore, the final data analysis included 95 patients (71 males and 24 females). Table 1 shows the socio-demographic profile of the study population. The mean age of the patients was 33.3 years (range 19-60 years). Majority of patients were adults below 50 years and only nine patients were aged above 50 years.
Table 1.
Socio-demographic characteristics of the study population (N =95)

Written consent was obtained from all patients. Complete general physical and neurological examination was done. Body mass index (BMI) was calculated. BMC and body composition (LBM and fat mass) were measured with DEXA scan using HOLOGIC QDR-2000 explorer (Hologic Inc., Bedford, MA, USA). The patients were asked to lie on a table and whole body scanning was carried out with a congruent beam of stable dual-energy radiation. DEXA provides a three compartment partition of the body: Bone mineral, fat mass and fat free mass. After completion of scan, the body composition results (region wise and total body) are provided by the system's software (BMC, LBM, fat, lean + BMC, total mass, %fat).
One trained technician performed and analyzed all scans to ensure consistency. Followup of all the above mentioned parameters was done at 3, 6, and 12 months. The patients showing no signs of motor recovery during the course of the study were considered as motor complete (AISA A and B) (n=41), and those patients showing signs of motor recovery during the study period were considered as motor incomplete (AISA C and D) (n=54) [Table 1]. Those patients who were fit for surgery and had unstable spine were preferably stabilized with a pedicle screw fixation. Seventy four patients (77.9%) were managed conservatively and 21 (22.1%) patients were operated. All the patients were optimally rehabilitated. A standard protocol of early rehabilitation of patients like mobilization, promotion of exercises and gait training was followed. At the end of 1 year, post SCI changes in the body composition were assessed and statistical analysis was conducted.
Statistical analysis
Unpaired t-tests were performed to determine group differences and paired Student's t-tests were used to determine significant differences within the pairs. Relationship between various parameters of body composition were calculated using Pearson's correlation (r).
The procedures followed were in accordance with the ethical standards of the institutional committee on human experimentation. Ethical committee of the institute approved the study.
RESULTS
The average BMI of the patients was 23.09. The data in this section show comparison of values at the time of presentation and after 1 year of injury. As some differences in the values between the right and left sides of the body were found, the values from the right and left sides of the body were pooled to derive the mean values. BMC was found to be significantly lower (P < 0.05) in sublesional sites. There was an average of 14.5% decrease in BMC in the lower extremities [Figure 1]. In tetraplegics, arm and leg BMC were 14.2% and 12.8% less, respectively. In paraplegics, there was 15% decrease in leg BMC, but the arm BMC decreased by 3.6% only. The decrease in arm BMC was more in tetraplegics as compared to paraplegics, and it was statistically significant. In operated patients, there was 21.6% decrease (P < 0.05) in leg BMC, but the decrease of BMC in arms was not significant. There was an average of 13.3% decrease in the BMC of lower extremities in nonoperated patients, which was statistically significant, whereas upper limbs showed a decrease of 6.2% in BMC. An average decrease of 14.1% (P < 0.0001) in leg BMC and 7% in arm BMC was observed in patients with complete SCI [Table 2]. There was 14.9% decrease (P < 0.05) in leg BMC in motor incomplete patients.
Figure 1.

Bone mineral content in spinal cord injury patients (total)
Table 2.
Mean values of BMC (in grams) in tetraplegic vs. paraplegic, operated vs. nonoperated and motor complete versus motor incomplete patients at 3, 6 and 12 months followup

There was an average of 20.5% loss of LBM in legs (P < 0.05) and 15.1% loss of LBM in trunk (P < 0.05). Tetraplegic patients had significant decrease in LBM, with an average 21.5% loss of LBM in legs and 10.3% loss of LBM in trunk. Values of LBM in paraplegic patients were 20.2% less in legs (P < 0.05) and 8.2% less in arms (P < 0.05) [Figure 2]. Significant decrease was observed in the LBM of lower extremities and trunk in operated patients, with 24.7% decrease in legs and 17.3% decrease in trunk [Table 3]. Significant decrease in LBM was observed in nonoperated patients at lower extremities and trunk, with an average of 19.1% decrease in legs and 14.1% decrease in the trunk. There was 23.4% decrease in LBM of legs at 1 year (P < 0.05). Motor incomplete patients had significant decrease in LBM of lower extremities and trunk, with an average decrease of 16.6% in lower extremities and loss of 13.3% LBM in trunk [Table 3].
Figure 2.

Lean body mass in spinal cord injury patients (total)
Table 3.
Mean values of lean body mass in tetraplegic vs. paraplegic, operated vs. nonoperated, and motor complete vs. motor incomplete patients at 3, 6, and 12 months followup

There was 0.2% increase in fat mass in legs (statistically nonsignificant). Statistically significant differences were not observed at other sites also. There was significant increase in fat mass in the upper extremities in tetraplegics, with 10.6% increase in fat mass (P < 0.05) and 9.7% increase in trunk fat [Figure 3]. In paraplegics, there was 3.1% increase in fat mass in legs (nonsignificant), but trunk fat had statistically significant increase of 9.0%. Fat mass increased by 11.7% in trunk (P < 0.05) and 14% in lower extremities in operated patients [Table 4], and nonoperated patients had 2.3% increase in fat mass in trunk and 5% increase in fat mass in the lower extremities. There was 7.5% increase in fat mass in the legs of patients with complete motor injury and 4.3% increase was seen in the upper extremities; however, it was not statistically significant. There was 8% decrease in fat mass in the legs of patients with incomplete motor injuries and a decrease of 4.2% was seen in the values of trunk [Table 4].
Figure 3.

Fat in spinal cord injury patients (total)
Table 4.
Mean values of fat mass in tetraplegic vs. paraplegic, operated vs. nonoperated, and motor complete vs. motor incomplete patients at 3, 6, and 12 months followup

Significant increase (trunk P = 0.003; legs P < 0.0001) in fat percentage for all patients was observed at all sites. There was 17.3% increase in fat percentage in the lower limbs for all patients. Tetraplegic patients had significant increase in the fat percentage of arms, trunk, and legs. Significant increase was seen in the percentage fat of lower extremities in paraplegics. Difference in the percentage fat of arm, trunk, and leg in operated patients was 2.8%, 1.1%, and 6.1%, respectively. Difference in the percentage fat of arm, trunk, and leg in nonoperated patients was 2.4%, 2.1%, and 5%, respectively. In patients with complete injury, percentage fat increase was 5.7% in legs, 1.5% in trunk, and 2.4% in upper extremities. In patients of incomplete injury, percentage fat increase was 4.6% in legs, 2.4% in trunk, and 2.6% in upper extremities [Table 5].
Table 5.
Values of percentage fat in tetraplegic vs. paraplegic, operated vs. nonoperated, and motor-complete vs. motor-incomplete patients at 3, 6, and 12 months follow up

Arm BMC and arm lean tissue mass were significantly related within groups of tetraplegics (r = 0.521, P < 0.05) and paraplegics (r = 0.732, P < 0.001) [Figure 4]. Moderate to strong relationship was found between leg BMC and leg lean tissue mass in patients with incomplete injuries (r = 0.565, P < 0.001). However, the relationship was not significant in motor-complete injuries (r = 0.055, P > 0.05) [Figure 5]. Significant correlation was not observed between body fat percentage and BMI in the present study [Figure 6].
Figure 4.
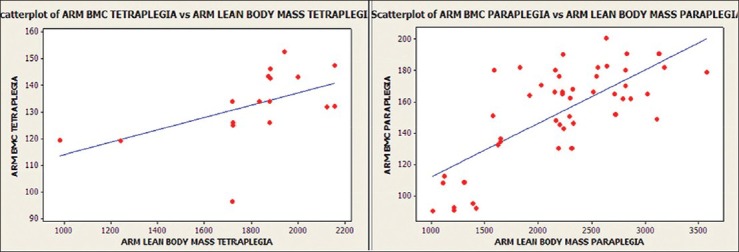
Relationship of arm bone mineral content (BMC) with arm lean tissue mass for tetraplegia and paraplegia (tetraplegia: R = 0.521, P < 0.05; paraplegia: R = 0.732, P < 0.001)
Figure 5.
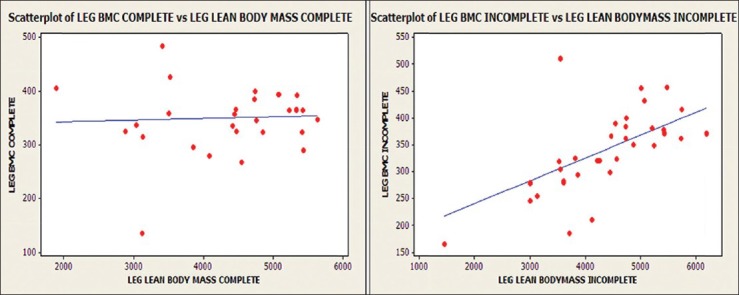
Relationship of leg BMC with leg lean tissue mass for those with complete or incomplete SCI (complete: R = 0.055, P > 0.05; incomplete: R = 0.565, P < 0.001)
Figure 6.

Relationship of total body fat percent with body mass index for spinal cord injuries at l year (SCI: R = −0.19, P > 0.05)
DISCUSSION
We found a significant decrease in BMC at sublesional sites, 1 year after SCI (up to 16.9% linear decrease in lower extremities). Biering-Sorensen et al. reported 25% lower BMC of the femoral neck and shaft and >50% lower value for the proximal tibia than the normal value.13 Patients with cervical lesions had lower BMC values in the femoral bones than those with thoracic lesions.13 In 1990, Biering-Sorensen et al. reported that BMC of the lower extremities decreased after injury, reaching new steady-state levels at 40-50% and 60-70% for proximal tibia and femoral neck, respectively, at about 2 years post injury.14 Wilmet et al. observed a rapid decrease of BMC in the paralyzed areas, of approximately 4% per month during the first year in areas rich in trabecular bone and 2% per month in areas containing mainly compact bone.10 McDonald et al. also observed 40% lower BMC in legs in paraplegic patients (P < 0.0001), while tetraplegics had significantly less BMC in the arms (−25%; P < 0.0001), legs (−46%; P < 0.0001) and trunk (−30%; P < 0.0001).11 In the present study, the tetraplegic patients had significantly lower (P < 0.05) arm BMC compared with paraplegic patients, whereas BMC in the leg, trunk, and total body was not significantly different between the two groups. These observations are similar to the findings of Spungen et al.9 and McDonald et al.11 Wilmet et al.10 reported that complete motor paralysis was associated with greater bone loss, and in patients who were likely to recover, a program of prevention of bone loss should be instituted early in the course of disease.10 This was not seen in the present study.
We observed dramatic loss of LBM below the level of the lesion in all patients (up to 22.6% loss of LBM of lower limbs). Wilmet et al. reported 15% loss of lean mass in the lower limbs in the first year after SCI.10 Jones et al. also reported significant reduction in lean tissue mass (16% less) and bone (12% less) in those with SCI, in comparison to the control group.15 In a study by McDonald et al., lean tissue mass was 38% less in legs and 11% less in trunk in the paraplegia group and 35% less in arms and 37% less in legs in the tetraplegia group.11 Similarly, Spungen et al. also reported significant loss of lean tissue mass in both the tetraplegic and paraplegic groups than in the controls.9 The possible reason for the decrease in LBM in the arms of paraplegics can be the higher amount of absolute fat mass in the arms and sacropenia observed after SCI. Castro et al. observed significantly decreased cross sectional area of the muscles of legs and thighs at 6 months of SCI on magnetic resonance images.4 Modlesky et al. also reported a disproportionate loss of muscle in the paralyzed thighs after SCI, relative to the other nonfat constituents.16 Similar to Spungen et al.'s findings, we observed significantly lower arm lean mass in tetraplegics.9 McDonald et al. also reported 36% less LBM in arms (P < 0.0001) and 16% less LBM in trunk (P < 0.05) in the tetraplegic group than in the paraplegic group.11 A potential reason for the significant difference observed between the paraplegic and tetraplegic groups could be that the subjects with paraplegia are likely to use their arms for the activities of daily living such as pushing a wheel chair, while the subjects in the tetraplegic group would not place these exercise demands on their arms. We observed up to 18.2% decrease in LBM of the lower limbs in patients with incomplete injuries, as compared to 25.9% in those with complete injuries (P < 0.05), as also reported by Spungen et al.9 Arm BMC and arm lean tissue mass were significantly related [Figure 4] within the groups of tetraplegia (r = 0.521, P < 0.05) and paraplegia (r = 0.732, P < 0.001) in the present study. Similar findings were also observed by Spungen et al.9 Moderate to strong relationship [Figure 5] was found between leg BMC and leg lean tissue mass in patients with incomplete injuries (r = 0.565, P < 0.001) in the present study; however, the relationship was not significant in motor complete injuries (r = 0.055, P > 0.05). Similar findings were observed by Spungen et al., who found a moderate to strong co-relationship between leg BMC and leg lean tissue mass in the control group (r = 0.86, P < 0.0001) and the incomplete SCI group (r = 0.74, P < 0.0001), whereas the relationship was weak in the complete SCI group (r = 0.25, P = 0.01).9 In subjects with complete SCI, a much weaker relationship was noted between these variables, as might have been expected in the absence of function and gravity bearing activity. Moreover, complete motor paralysis is associated with greater bone loss in the denervated extremities than in those with incomplete lesions.9 McDonald et al. concluded in their study that the total lean tissue was highly correlated to the total BMC in the SCI group and the control group.11 Castro et al. suggested that there was massive loss of contractile protein early after SCI, while the mechanisms responsible for loss of muscle size were not clear. It was suggested that the development of muscular imbalance as well as diminution of muscle mass would compromise the force potential early after SCI.5
We observed minor increase in the fat mass at 1 year. Bauman et al. also reported the absolute leg fat to be similar in SCI and non-SCI twin.17 We observed a significant increase (P < 0.05) in fat mass in the upper limbs of tetraplegics compared to paraplegics, whereas Spungen et al. observed significantly higher fat in the arm and leg in both tetraplegia and paraplegia groups in comparison to the control group, but the difference between tetraplegia and paraplegia groups was insignificant.9 McDonald et al. reported that paraplegia group had 28% more fat mass in legs (P < 0.02) and 35% more fat mass in trunk (P < 0.04).13 The paraplegia group had 38% more fat in legs and 39% more total body fat than the tetraplegia group.11 Similar to Spungen et al.'s findings, we also found higher absolute fat mass in groups with complete injury than in incomplete injury group.9 We observed even a decrease in fat mass in the lower extremities and the trunk in patients with motor incomplete injuries. This may be due to more active rehabilitation of patients in motor incomplete group as they had incomplete injuries. Contrary to the expected findings, we also found higher fat mass increase in the trunk and legs in operated patients compared to those managed conservatively. Several factors may explain the unpredictable nature of fat mass changes following SCI. Changes in fat mass may be variable and dependent on the interaction of different patient-specific variables, e.g. advancing age has been associated with less lean mass and increased fat mass in individuals with SCI. The activity levels may also play an important role.18
We observed 19.3% increase in the fat percentage in lower limbs and this might be related to muscle atrophy, which results in apparent increase in percentage fat in legs. Tetraplegics had 31.4% more increase in fat percentage than paraplegics in the upper extremities. In patients of complete injury, fat percentage was 16.67% more in trunk and 35.2% more in arms when compared to those with incomplete injury. McDonald et al. reported in their study that paraplegia group had 36% higher trunk fat percentage (P < 0.001), 60% higher leg fat percentage (P < 0.0001), and 37% higher total body fat percentage, as compared to the control group.11
Dopler-Nelson et al. reported that more than 90% of obese SCI subjects from a regional SCI clinic met the criteria for metabolic syndrome, which puts them at risk for type II diabetes, cardiovascular disease and stroke. Identification of obese patients at risk for metabolic syndrome using easily obtainable clinical criteria for obesity should be a high priority for clinical care.19 Recently, it has been shown that many of the disorders associated with obesity occur prematurely and at a higher prevalence in the population with SCI than in the able bodied population. Adults with SCI have higher rates of carbohydrate intolerance, insulin resistance,20,21 lipid abnormalities22,23 and heart disease24 than the able bodied population is likely to have, and these factors may contribute to the reduced lifespan of individuals with SCI. Low-energy fractures have been reported to occur in individuals with SCI during events that would not normally cause fractures, such as a transfer from a bed to chair, or being turned in bed.25 Complications related to fractures in SCI population present additional source of morbidity.1 By estimating the body composition in the patients with SCI, appropriate steps can be taken up early to minimize the effects and risks of the diseases, thus reducing the morbidity and mortality in this subset of the population.
Not much significant correlation was found between the body fat percentage and BMI in the present study [Figure 6]. Spungen et al. demonstrated in their study that correlation between BMI and total body fat percentage was statistically significant; however, in their study, the duration of injury was around 10 years and fat mass had increased with increased duration of injury.9 McDonald et al. reported that changes in the body composition observed in the subjects with SCI had profound effects on the relationship between BMI and total fat percentage, as compared to the control subjects.11 They suggested that due to these alterations in body composition, BMI significantly underestimates the level of obesity in individuals. Findings of the present study may be explained on the basis of low BMI, dietary habits and low fat mass in the Asian population. Further research is needed in this field to define new standards of obesity in the SCI population.
Our study has some limitations. The age may have effect on the outcomes in the present study. Only nine patients were aged more than 50 years. Majority of patients were adults below 50 years and this might have negated this confounding variable to some extent. A standard protocol was followed for rehabilitation of the patients. However, some patients might not have optimal rehabilitation. The inclusion of large number of patients in the present study might have negated this confounding variable to some extent.
We conclude that patients with SCI not only lose motor and/or sensory functions, but also may experience dramatic muscle and bone changes during the first year of SCI. These changes usually depend on the level and severity of lesions. These adverse body composition and bone changes may negatively impact body metabolism, which may increase the risks of microvascular diseases and fractures. It will be prudent to take measures like early mobilization, rehabilitation and specific interventions to prevent bone loss and deterioration in body composition early in the course of the SCI.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: Epidemiology, fracture prediction and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon T, Mao J. Muscle atrophy and procedures for training after spinal cord injury. Phys Ther. 1994;74:50–60. doi: 10.1093/ptj/74.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Jiang SD, Jiang LS, Dai LY. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol (oxf) 2006;65:555–65. doi: 10.1111/j.1365-2265.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- 4.Castro MJ, Apple DF, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–8. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 5.Forbes GB. London: Springer-Verlag; 1987. Human body composition: Growth, aging, nutrition and activity; pp. l53–95. [Google Scholar]
- 6.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24:266–77. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- 7.Clarys JP, Martin AD, Drinkwater DT. Gross tissue weighs in the human body by cadaver dissection. Hum Biol. 1984;56:459–73. [PubMed] [Google Scholar]
- 8.Chow YW, Inman C, Pollintine P, Sharp CA, Haddaway MJ, el Massy W, et al. Ultrasound bone densitometry and dual energy x-ray absorptiometry in patients with spinal cord injury: A cross-sectional study. Spinal Cord. 1996;34:736–41. doi: 10.1038/sc.1996.134. [DOI] [PubMed] [Google Scholar]
- 9.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: A cross-sectional study. J Appl Physiol. 2003;95:2398–407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- 10.Wilmet E. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–7. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 11.McDonald CM, Abresch-Meyer AL, Nelson MD, Widman LM. Body mass index and body composition measures by dual x-ray absorptiometry in patients aged 10 to 21 years with spinal cord injury. J Spinal Cord Med. 2007;30(Suppl 1):S97–104. doi: 10.1080/10790268.2007.11754612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maynard FM. International standards 1 for neurological and functional classification of spinal cord injury. American spinal injury association. Spinal Cord. 1997;35:266–74. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- 13.Biering-Sorensen F, Bohr H, Schaadt O. Bone mineral content of the lumbar some and lower extremities years after spinal cord lesion. Paraplegia. 1988;26:293–301. doi: 10.1038/sc.1988.44. [DOI] [PubMed] [Google Scholar]
- 14.Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–5. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones LM, Goulding A, Gerrard DF. DEXA: A practical and accurate tool to demonstrate total and regional bone loss, lean tissue loss and fat mass gain in paraplegia. Spinal Cord. 1998;36:637–40. doi: 10.1038/sj.sc.3100664. [DOI] [PubMed] [Google Scholar]
- 16.Modlesky CM, Majumdar S, Narasimhan A, Dudley G. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Min Res. 2004;19:48–55. doi: 10.1359/JBMR.0301208. [DOI] [PubMed] [Google Scholar]
- 17.Bauman WA, Spungen AM, Wang J, Pierson RN, Jr, Schwartz E. Continuous loss of bone during chronic immobilization: A monozygotic twin study. Osteoporos Int. 1999;10:123–7. doi: 10.1007/s001980050206. [DOI] [PubMed] [Google Scholar]
- 18.Olle MM, Pivarnik JM, Klish WJ, Morrow JR., Jr Body composition of sedentary and physically active spinal cord injured individuals estimated from total body electrical conductivity. Arch Phys Med Rehabil. 1993;74:706–10. doi: 10.1016/0003-9993(93)90030-e. [DOI] [PubMed] [Google Scholar]
- 19.Dopler-Nelson M, Widman LM, Abresch RT, Stanhope K, Havel PJ, McDonald CM. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007;30:S130–42. doi: 10.1080/10790268.2007.11754591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: A model of premature aging. Metabolism. 1994;43:749–56. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson AK. Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord. 1999;37:494–500. doi: 10.1038/sj.sc.3100844. [DOI] [PubMed] [Google Scholar]
- 22.Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia. 1992;30:697–703. doi: 10.1038/sc.1992.136. [DOI] [PubMed] [Google Scholar]
- 23.Brenes G, Dearwater S, Shapera R, LaPorte RE, Collins E. High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil. 1986;67:445–50. [PubMed] [Google Scholar]
- 24.Bauman WA, Raza M, Spungen AM, Machac J. Cardiac stress testing with thallium-201 imaging reveals silent ischemia in individuals with paraplegia. Arch Phys Med Rehabil. 1994;75:946–50. [PubMed] [Google Scholar]
- 25.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36(suppl 11):790–6. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]


