Abstract
Background:
Various anterior lumbar surgical approaches, including the minimally invasive approach, have greatly improved in recent years. Vascular complications resulting from ALIF are frequently reported. Little information is available about the safety of large blood vessel stretch. We evaluated the right side stretch limit (RSSL) of the abdominal aorta (AAA) and the inferior vena cava (IVC) without blood flow occlusion and investigated stretch-induced histological injury and thrombosis in the iliac and femoral arteries and veins and the stretched vessels.
Materials and Methods:
The RSSL of blood vessels in five adult goats was measured by counting the number of 0.5-cm-thick wood slabs that were inserted between the right lumbar edge and the stretch hook. Twenty seven adult goats were divided into three groups to investigate histological injury and thrombosis under a stretch to 0.5 cm (group I) 1.5 cm (group II) for 2 h, or no stretch (group III). Blood vessel samples from groups I and II were analyzed on postsurgical days 1, 3, and 7. Thrombogenesis was examined in the iliac and femoral arteries and veins.
Results:
The RSSL of large blood vessels in front of L4/5 was 1.5 cm from the right lumbar edge. All goats survived surgery without complications. No injury or thrombosis in the large blood vessels in front of the lumbar vertebrae and in the iliac or femoral arteries and veins was observed. Under light microscopy, group I showed slight swelling of endothelial cells in the AAA and no histological injury of the IVC. The AAA of group II showed endothelial cell damage, unclear organelles, and incomplete cell connections by electron microscopy.
Conclusions:
The AAA and IVC in a goat model can be stretched by ≤0.5 cm, with no thrombosis in the AAA, IVC, iliac or femoral arteries and veins.
Keywords: Adult goat, large blood vessel stretch, surgical approach, anterior lumbar approach
INTRODUCTION
As a commonly used technique with a long surgical history, anterior lumbar interbody fusion (ALIF) has several advantages, including its minimal invasiveness, reduced bleeding, simple operative technique, and fast postsurgical recovery.1,2 Various anterior lumbar surgical approaches, including the minimally invasive approach, have greatly improved in recent years.3,4 Nevertheless, vascular complications resulting from ALIF are frequently reported. In Baker's series et al.,5 15.6% of ALIF patients had vascular injury to the inferior vena cava (IVC, 4 cases) or the common iliac vein (11 cases). Among 212 ALIF cases, Gary et al.6 found 1 case of major arterial injury and 12 cases of venous injuries. Three case studies of artery embolization after ALIF have been independently reported.7,8,9
Little information is available about the safety of large blood vessel stretch. Here, we evaluated the right side stretch limit (RSSL) of the abdominal aorta (AAA) and the IVC without blood flow occlusion. We also investigated stretch induced histological injury and thrombogenesis in the iliac and femoral arteries and veins and the stretched blood vessels, in an adult goat model.
MATERIALS AND METHODS
The study was conducted in Youyang black goats. Each experiment used blood-vessel hooks (3 cm wide, with a blunt edge), wood blocks (0.5 × 2 × 4 cm), and different individuals. The wood block was placed between the hook and the right edge of the lumbar spine [Figure 1].
Figure 1.
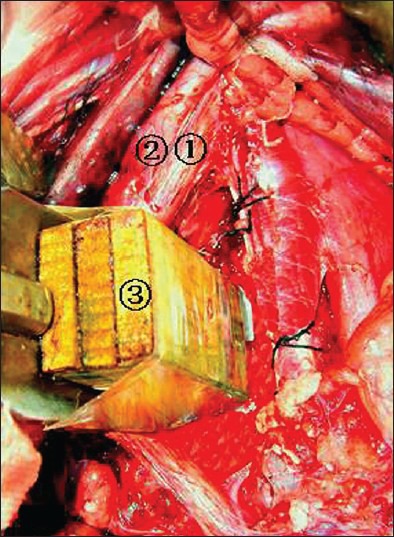
Peroperative photograph showing (1) Abdominal aorta; (2) inferior vena cava; (3) 0.5-cm-thick wood slabs
Blood vessel stretch limit
Five 10-month-old goats (average weight: 23.0 ± 1.2 kg) received anesthesia through intramuscular injection of 0.06 mL/kg sumianxin II (Veterinary Research Institute, Changchun PLA Quartermaster University, China) and 2 mg/kg ketamine hydrochloride (Jiangsu Hengrui Medicine Co., Ltd, China).
Goats were placed in a supine position, prepped and draped. To perform the L4/5 anterior lumbar surgery, an incision was made at the ventral midline and the intraperitoneal approach was used. The AAA and IVC were isolated at the L4/5 level and their transverse diameters between L4 and L5 were measured. The surgeon determined whether the abdominal aortic blood flow is obstructed by investigating the pulse at 2 cm to the start point of the bilateral iliac arteries. If three surgeons could not reach a concensus, the experiment was reperformed. Vessels were stretched to the right side with a special 3 cm wide vessel hook. Then, 0.5 cm thick slabs of wood were inserted into the cleft between the hook and the right edge of the lumbar spine until the iliac artery pulse disappeared [Figure 1]. One piece of wood was removed to reduce the stretch intensity slightly and to restore the iliac artery pulse.
Methylene blue solution (10 mL) was injected into the iliac vein. Blood was sampled upstream of the stretched IVC. The result was considered negative if the blood was blue; otherwise, it was considered positive. If the extracted blood was not blue, then the stretch intensity was gradually reduced (i.e. the 0.5 cm thick wood slabs were removed individually) until the blood became blue. The RSSL was determined as the distance between the vessel hook and the right edge of the lumbar spine, which was the product of the number of wood slabs and the thickness of wood slabs (0.5 cm).
Histological injury and thrombogenesis in the AAA and IVC under different stretch intensities
Twenty seven 10 month old goats (average weight: 24.1 ± 2.3 kg) were divided into three groups. Group I underwent blood vessel stretch to 0.5 cm (i.e. 1 wood slab was inserted between the hook and the right lumbar edge) for 2 hours. Group II underwent blood vessel stretch to 1.5 cm (i.e. 3 wood slabs were inserted) for 2 hours. Group III was employed as control (neither the AAA nor the IVC was stretched after isolation). The surgical process was the same as described above.
The blood vessel sampling sites after stretching are shown in Figure 2. Ring-shaped blood vessel samples were collected from three goats in groups I, II, and III on postsurgical days 1, 3, and 7 after sacrificing the animals. Blood vessel samples were investigated by general observation (of injury or thrombosis in the vessel wall), light microscopy (hematoxylin and eosin [HE] and elastic fiber staining) and transmission electron microscopy (TEM). The iliac and femoral arteries and veins were opened to investigate thrombus formation.
Figure 2.
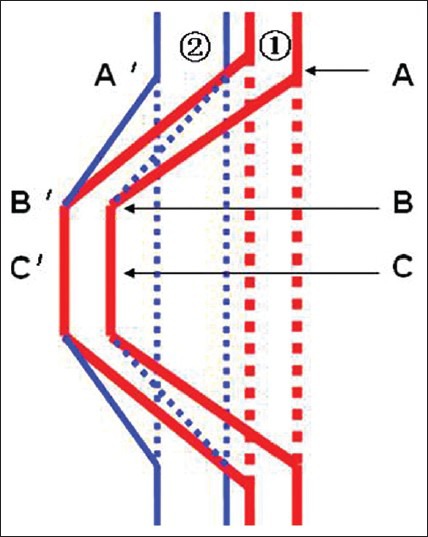
A schematic diagram showing sampling sites of the abdominal aorta (A, B, C) and the inferior vena cava (A’, B’, C’) after stretching
The intimal layer, middle smooth muscle layer and outer layer at points A, B, and C of the AAA and points A’, B’ and C’ of the IVC were observed under light microscopy. Observation included the morphology of the endothelial cells, smooth muscle cells and elastic fibers. Injury was determined on the basis of organizational continuity, completeness, and uniformity. The degree of injury was determined as follows: intact = vascular ring on the slide was set as 100%; score = 0 represented no injury, score = 1 represented < 10% injury, score = 2 represented 10% to 25% injury, score = 3 represented 25% to 50% injury and score = 4 represented > 50% injury. Scoring was performed by a single pathologist. The ultrastructure of cells was observed with TEM.
All goats were provided by the experimental animal center of Third Military Medical University. The animal experimental design and sacrificing methods were approved by the experimental animal ethics committee of Third Military Medical University.
Statistical analysis
Blood vessel diameters are reported as 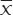 ± S. Quantitative data acquired by light microscopy were analyzed with factorial variance analysis in the SPSS 18.0 software package. Intragroup analysis was performed using repeated measures analysis of variance. Differences with P < 0.05 were considered statistically significant.
± S. Quantitative data acquired by light microscopy were analyzed with factorial variance analysis in the SPSS 18.0 software package. Intragroup analysis was performed using repeated measures analysis of variance. Differences with P < 0.05 were considered statistically significant.
RESULTS
Blood vessel stretch limit
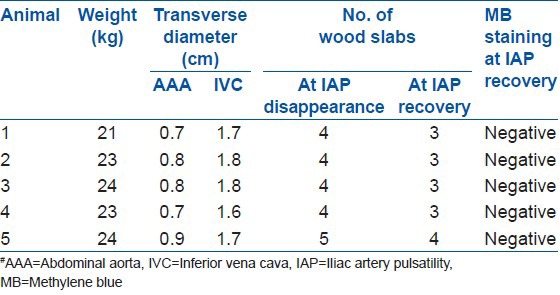
The results for the blood vessel stretch limit are shown in Table 1. The blood vessel transverse diameters were 0.78 ± 0.08 cm for the AAA and 1.72 ± 0.08 cm for the IVC. The lumbar spine was divided into three parts: left, middle, and right. The AAA was in the middle part and the IVC was adjacent to the AAA on the right side. In four goats, when the AAA was stretched to 1.5 cm from the right lumbar edge, blood in the AAA and IVC still flowed. However, if the distance was > 2 cm, then the blood flow in the AAA was blocked. In the fifth goat, when the AAA was stretched to 2 cm from the right lumbar edge, the blood in the AAA and IVC still flowed. However, if the distance was > 2.5 cm, then the blood flow in the AAA was blocked. These results indicate that the RSSL for large blood vessels in front of the lumbar spine was 1.5 cm from the right lumbar edge.
Table 1.
Blood vessel stretch limit
Histological injury and thrombogenesis in the AAA and IVC under different stretch intensities
All goats survived surgery without developing complications. General observation revealed no injury or thrombosis in the vascular wall or in the iliac or femoral arteries and veins.
According to light microscopy, the AAA of group I specimens showed only slight swelling of the endothelial cells without stripping off and the middle and outer membranes showed no histological injury. The IVC showed no injury. On postoperative days 1 and 3, TEM revealed that the group I specimens showed many organelles, such as mitochondria, vesicles, and some endoplasmic reticulum, in the AAA.
For group II, light microscopy revealed obvious injuries in the two types of blood vessels. On postoperative days 1 and 3, the AAA showed endothelial cell shedding, endothelial denudation and swelling, inflammatory cell infiltration and intimal thickening, but no injury in the middle and outer membranes [Figure 3]. These results were in sharp contrast to observations in the control group. On postoperative day 7, the AAA showed obvious intimal thickening and the IVC showed occasional endothelial cell swelling. The TEM results indicated that the AAA of group II showed endothelial cell damage, unclear organelles, and incomplete cell connections, with some endothelial cells attached to red blood cells [Figure 4]. For group III, light microscopy revealed no injuries to the blood vessels.
Figure 3.
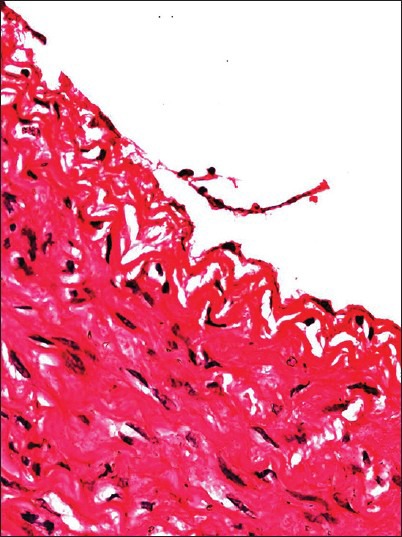
Sample image of point C in group II at 1 postsurgical day. Some endothelial cells have fallen off (HE ×400)
Figure 4.

Transmission electron microscopy image of point A in group II at 1 postsurgical day. Endothelial cells are attached to red blood cells and incomplete cell connections can be observed (TEM ×8000)
Comparing the injuries at points A, B and C of the AAA, groups I, II, and III showed significant differences (P = 0.000). Injuries at points A, B and C of the AAA in group I showed no differences (P = 0.296); the same result was obtained in group II (P = 0.216).
DISCUSSION
Anterior lumbar spinal surgery requires the AAA and IVC to be stretched to the right side without blockade of blood flow. However, blockade of the IVC can reduce back flow and result in systemic hemodynamic disorders, which cannot be tolerated by some patients.10 Due to its large stretch intensity, the AAA can be easily blocked. A “safe” time limit of 30 min for blockade of the AAA has been determined.11 Within this safe limit, the hemodynamics, pH, blood biochemical indicators and ultrastructure of the major organs (e.g. brain, lungs, liver, kidneys, pancreas, intestines and spinal cord) show only mild, reversible changes. However, if the safe limit is exceeded, then irreversible changes could occur in the organ ultrastructure.12 Hypoxic-ischemic injury caused by blockade of the AAA, systemic inflammatory response syndrome and clamp withdrawal induced reperfusion injury can result in multiple organ failure and endanger life. Anterior lumbar spinal surgery requires that the blood vessels be stretched for no less than 1 hour. Therefore, it is important to maintain blood flow in the AAA and IVC during surgery. In the present study, we found that blood flow in the large blood vessels in front of the lumbar spine was not blocked when the vessels were stretched by ≤1.5 cm. When stretched by >1.5 cm, the blood flow was blocked in the AAA but not in the IVC, probably because, compared to the AAA, the IVC was closer to the right lumbar edge. Hence was less stretched and its blood flow is easier to maintain.
Thrombosis is a severe postsurgical complication of lumbar surgery. A thrombus can partially or completely occlude the vessel and can migrate with the blood flow to clog the blood vessels or cause an embolism. In a study of 336 cases of ALIF treatment, Kulkarni et al. 13 found 5 cases of common iliac artery thrombosis. Of these, four cases were related to the left side artery and one case was related to the right side; two cases were treated with the extraperitoneal approach and three cases were treated with an abdominal approach; one case was diagnosed during surgery and the other cases were diagnosed ≤16 h after surgery. Thomas2 reported 25 cases of ALF treatment and found 1 case of DVT. The average incidence rate for DVT after thoracolumbar spinal surgery is 0.9% to 14% and the incidence for secondary fatal pulmonary embolism is 1.7% to 2.8%.14 Thrombosis has three major risk factors, arteriovenous stasis, intimal injury and hypercoagulable state. All of which exist in patients treated with anterior lumbar spine surgery. Surgery and trauma can cause bleeding, stress can reduce blood flow and anesthesia and controlled hypotension can decelerate the blood flow and cause platelet aggregation and adhesion to the blood vessel walls. Perisurgical blood vessel isolation and stretching can injure or even destroy the vascular endothelial cells, exposing subendothelial collagen and activating the endogenous coagulation system.
The injured tissues can release large amounts of prothrombin to induce a hypercoagulable state during surgery. Platelet adhesiveness peaks at 3 postoperative days15 and the surgical area shows cell metabolic disorders, thrombin aggregation, and decreased fibrinolytic activity, which in turn can promote thrombogenesis. Wilson et al. suggested that DVT usually occurs within 1 week after trauma; for this reason, we monitored the animals for 7 postsurgical days. Our blood vessel specimens showed no obvious injury or thrombogenesis and the iliac and femoral arteries and veins did not display thrombi. Although injury to the intimal membrane of the AAA may activate the extrinsic coagulation pathway through the release of tissue factor, the endothelial cells, and various physiological factors show anticoagulant effects. In addition, the arterial blood flow is very fast. As a result, the impaired AA wound cannot form a thrombus.
Overstretching of the large blood vessels can induce obvious histological injury in the blood vessels. Although we did not observe thrombosis during the 7-day monitoring period, we cannot exclude the possibility that thrombosis can occur after 7 days. Endothelial exfoliation and shedding, as well as red blood cell adhesion, may provide a suitable environment for thrombogenesis. Therefore, we suggest that the exposed surgical field and the traction of the blood vessels be minimized during ALIF surgery, to reduce damage to the vessels, especially the endothelium. This tactic could also reduce the pressure on the blood vessels and prevent blood deposition. To minimize the occurrence of thrombosis and related complications, surgeons should maintain hypocoagulability through moderate infusion of anticoagulants, physical therapy, etc.
Lumbar surgery through the transabdominal approach can significantly affect the intestines, induce large loss of body fluids and temperature and cause postoperative intestinal paralysis.16 A left extraperitoneal approach for the surgery is common, because the spleen and aorta are more suitable for operation than the liver and IVC. L5/S1 surgery can be accomplished through the iliac vascular space without isolating the iliac vessel,17 whereas L3/4 and L4/5 intervertebral surgeries require isolation of the AAA and IVC on the right side.2,18 In these cases, the AAA withstands the major stretch force and amplitude and can be easily injured.
In the present study, we found that AAA injury was mainly focused on the endothelial cells of the intimal layer but not the middle or outer layers of the artery. The IVC was injured to a lesser degree, probably because the IVC is on the right side and thus had less stretch injury. The AAA showed only slight histological injuries under light and electron microscopy when stretched by 0.5 cm to the right lumbar edge. However, the AAA showed very significant injury when stretched by 1.5 cm. For instance, the TEM results indicated endothelial cell damage, unclear organelles, and incomplete cell connections, with some endothelial cells attached to red blood cells. We did not find any significant difference in the injury of points A, B, and C in groups I and II. This finding indicates that when the blood vessels were stretched, the free ends of the blood vessels and the contact site between the stretch equipment and the blood vessels can be injured. Hence, it is necessary to protect the whole isolated blood vessel and the stretch should be gentle.
We employed a large animal model of goats, whose lumbar spine and front vascular anatomy are similar to those of humans. Many studies have reported the successful use of goat models to study the spine.19,20,21 Therefore, although the results cannot be directly extrapolated to humans the experimental results obtained may play an important role in guiding human lumbar anterior surgery.
We conclude that during lumbar anterior surgery, the AAA and IVC should not be stretched by more than 0.5 cm to the right lumbar edge.
Footnotes
Source of Support: This study was supported by the Chongqing Key Technologies R and D Program (CSTC, 2010AB5118-4)
Conflict of Interest: None
REFERENCES
- 1.Mehren C, Korge A, Siepe C, Grochulla F, Mayer HM. Minimal invasive anterior midline approach to L2-L5. Oper Orthop Traumatol. 2010;22:573–81. doi: 10.1007/s00064-010-8053-6. [DOI] [PubMed] [Google Scholar]
- 2.Zdeblick TA, David SM. A prospective comparison of surgical approach for anterior L4-L5 fusion: Laparoscopic versus mini anterior lumbar interbody fusion. Spine (Phila Pa 1976) 2000;25:2682–7. doi: 10.1097/00007632-200010150-00023. [DOI] [PubMed] [Google Scholar]
- 3.Kleeman TJ, Michael Ahn U, Clutterbuck WB, Campbell CJ, Talbot-Kleeman A. Laparoscopic anterior lumbar interbody fusion at L4-L5: An anatomic evaluation and approach classification. Spine (Phila Pa 1976) 2002;27:1390–5. doi: 10.1097/00007632-200207010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Gumbs AA, Shah RV, Yue JJ, Sumpio B. The open anterior paramedian retroperitoneal approach for spine procedures. Arch Surg. 2005;140:339–43. doi: 10.1001/archsurg.140.4.339. [DOI] [PubMed] [Google Scholar]
- 5.Baker JK, Reardon PR, Reardon MJ, Heggeness MH. Vascular injury in anterior lumbar surgery. Spine (Phila Pa 1976) 1993;18:2227–30. doi: 10.1097/00007632-199311000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Garg J, Woo K, Hirsch J, Bruffey JD, Dilley RB. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg. 51:946–50. doi: 10.1016/j.jvs.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 7.Khazim R, Boos N, Webb JK. Progressive thrombotic occlusion of the left common iliac artery after anterior lumbar interbody fusion. Eur Spine J. 1998;7:239–41. doi: 10.1007/s005860050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsicano J, Mirovsky Y, Remer S, Bloom N, Neuwirth M. Thrombotic occlusion of the left common iliac artery after an anterior retroperitoneal approach to the lumbar spine. Spine (Phila Pa 1976) 1994;19:357–9. doi: 10.1097/00007632-199402000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Raskas DS, Delamarter RB. Occlusion of the left iliac artery after retroperitoneal exposure of the spine. Clin Orthop Relat Res. 1997:86–9. doi: 10.1097/00003086-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Smyrniotis VE, Kostopanagiotou GG, Gamaletsos EL, Vassiliou JG, Voros DC, Fotopoulos AC, et al. Total versus selective hepatic vascular exclusion in major liver resections. Am J Surg. 2002;183:173–8. doi: 10.1016/s0002-9610(01)00864-9. [DOI] [PubMed] [Google Scholar]
- 11.Sharp WJ, Bashir M, Word R, Nicholson R, Bunch C, Corson J, et al. Suprarenal clamping is a safe method of aortic control when infrarenal clamping is not desirable. Ann Vasc Surg. 2008;22:534–40. doi: 10.1016/j.avsg.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Qu D, Yan JB. An experimental study of normothermic abdominal aorta clamping in dogs: I. The safe time limit and syndromes after abdominal aorta clamping. J Tongji Med Univ. 1988;8:243–8. doi: 10.1007/BF02887900. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni SS, Lowery GL, Ross RE, Ravi Sankar K, Lykomitros V. Arterial complications following anterior lumbar interbody fusion: Report of eight cases. Eur Spine J. 2003;12:48–54. doi: 10.1007/s00586-002-0460-4. [DOI] [PubMed] [Google Scholar]
- 14.Rokito SE, Schwartz MC, Neuwirth MG. Deep vein thrombosis after major reconstructive spinal surgery. Spine (Phila Pa 1976) 1996;21:853–8. doi: 10.1097/00007632-199604010-00016. [DOI] [PubMed] [Google Scholar]
- 15.Campbell IA, Bentley DP, Prescott RJ, Routledge PA, Shetty HG, Williamson IJ. Anticoagulation for three versus six months in patients with deep vein thrombosis or pulmonary embolism, or both: Randomised trial. BMJ. 2007;334:674. doi: 10.1136/bmj.39098.583356.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sicard GA, Reilly JM, Rubin BG, Thompson RW, Allen BT, Flye MW, et al. Transabdominal versus retroperitoneal incision for abdominal aortic surgery: Report of a prospective randomized trial. J Vasc Surg. 1995;21:174–81. doi: 10.1016/s0741-5214(95)70260-1. [DOI] [PubMed] [Google Scholar]
- 17.Tribus CB, Belanger T. The vascular anatomy anterior to the L5-S1 disk space. Spine (Phila Pa 1976) 2001;26:1205–8. doi: 10.1097/00007632-200106010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Inamasu J, Kim DH, Logan L. Three-dimensional computed tomographic anatomy of the abdominal great vessels pertinent to L4-L5 anterior lumbar interbody fusion. Minim Invasive Neurosurg. 2005;48:127–31. doi: 10.1055/s-2004-830262. [DOI] [PubMed] [Google Scholar]
- 19.Brantigan JW, McAfee PC, Cunningham BW, Wang H, Orbegoso CM. Interbody lumbar fusion using a carbon fiber cage implant versus allograft bone. An investigational study in the Spanish goat. Spine (Phila Pa 1976) 1994;19:1436–44. doi: 10.1097/00007632-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Mullender MG, Krijnen MR, Helder MN, Smit TH, Everts V, Wuisman PI. Lumbar body fusion with a bioresorbable cage in a goat model is delayed by the use of a carboxymethylcellulose-stabilized collagenous rhOP-1 device. J Orthop Res. 2007;25:132–41. doi: 10.1002/jor.20285. [DOI] [PubMed] [Google Scholar]
- 21.Qin J, He X, Wang D, Qi P, Guo L, Huang S, et al. Artificial cervical vertebra and intervertebral complex replacement through the anterior approach in animal model: A biomechanical and in vivo evaluation of a successful goat model. PLoS One. 2012;7:e52910. doi: 10.1371/journal.pone.0052910. [DOI] [PMC free article] [PubMed] [Google Scholar]


