Abstract
Background:
Mucoid degeneration (MD) is a rare pathological affection of the anterior cruciate ligament (ACL). Mucinous material within the substance of ACL produces pain and limited motion in the knee. This series describes the clinicoradiological presentation of patients with mucoid ACL, partial arthroscopic debridement of ACL and outcomes.
Materials and Methods:
During a period of 3 years, 11 patients were included based upon the clinical suspicion, magnetic resonance imaging (MRI) findings, arthroscopic features and histopathologic confirmation of MD of ACL.
Result:
Six patients were male and five were female with median age of 40 years (range 21-59 years). All patients complained of knee pain with median duration of 5 months (range 1-24 months). All patients had painful deep flexion with 63.6% (N = 7) reporting trivial trauma before the onset of symptoms. MRI revealed MD of ACL in all with associated cyst in three patients. Partial debridement of ACL was done in ten and complete in one patient. None of them required notchplasty. Histopathology confirmed the diagnosis in all of them. At the mean followup of 13.81 months (range 6-28 months), all patients regained complete flexion and none complained of instability.
Conclusion:
Prior knowledge of condition with high index of suspicion and careful interpretation of MRI can establish the diagnosis preoperatively. It responds well to partial debridement of ACL and mucinous material without development of instability.
Keywords: Anterior cruciate ligament, celery stalk, debulking, mucoid degeneration
INTRODUCTION
Mucoid degeneration (MD) of the anterior cruciate ligament (ACL) is a rare pathological entity with disputed theories of origin.1,2,3,4,5 It is characterized by infiltration of mucoid like substance (glycosaminoglycans) interspersed within the substance of ACL causing knee pain and limited motion. This entity was described only a decade ago by Kumar et al. in 1999.6 Since then, many authors have identified and described their experiences and suggested their own guidelines for management.3,4,6,7 Bergins's et al. review of 4221 knee magnetic resonance imaging (MRI) revealed that 1.8% (N = 74) of knees can be affected with MD (N = 17) and mucoid cyst (N = 57).8 Regarded as a rare occurrence in the past, of late many reports of MD have highlighted the fact that it is not a rare entity and possibly was under diagnosed or misdiagnosed and reported as partial or complete tear of ACL.2,3,5,7,9,10,11,12 The Purpose of this prospective study is to discuss the clinical, radiological, arthroscopic features, treatment and outcome in eleven such patients.
MATERIALS AND METHODS
11 patients were included in this prospective study conducted between March 2008 and May 2011. All patients presented with knee pain without instability. All of them had failed the trial of conservative management of at least 6-8 weeks. MRI (of 1.5 tesla strength) was done in all patients. All the patients underwent diagnostic arthroscopy of the knee under tourniquet under appropriate anesthesia. During diagnostic arthroscopy of the knee through standard anterolateral portal, the ACL appeared hypertrophied, bulbous and occupying most of the notch with absent synovial lining over ACL and ligamentum mucosum in all cases. The concomitant meniscal and cartilage lesions were treated as per standard protocol through standard anteromedial portal. Using combination of 4.0 mm shaver blade (Styrker, USA) and arthroscopic basket forceps, bulky mucoid ACL was debrided judiciously, removing mucinous material as much and carefully saving normal fibers. Mostly, the fibers that impinge upon lateral femoral condyle (LFC) are posterolateral group of fibers. Care was taken to save normal fibers with intact femoral and tibial attachment. Occasionally, small amount of mucoid material have been left behind to protect normal ACL fibers as it is impossible to remove all mucinous material without sacrificing remaining normal ACL. Notchplasty was not performed in any of the patients. Other concomitant cysts at the base of ACL or behind posterior cruciate ligament (PCL) were shaved off. The degenerated ACL and mucinous material was sent for histopathology examination. Histopathology was suggestive of MD of ACL. Postoperatively, all patients were permitted immediate weight bearing, knee mobilization and muscle strengthening exercises.
RESULT
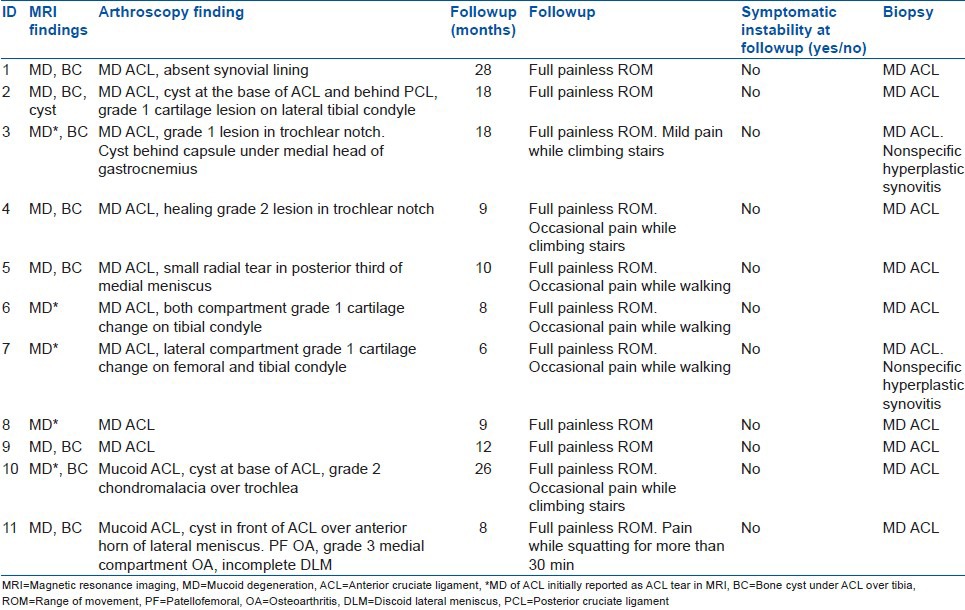
Six males and five females (Ratio of 1.2:1) were the part of the study [Table 1]. Median age of patients was 40 years (range 21-59 years). Median duration of pain was 5 months (range 1-24 months). All patients presented with knee pain and difficulty in knee flexion while extension remained unaffected. Seven patients (63.6%) reported trivial trauma prior to the onset of symptoms. Five patients (45.45%) had joint line tenderness. Five patients (45.45%) presented with full flexion but painful deep flexion beyond 100°. Remaining six had mean flexion range of motion of 109° (range 80-120°) and further movement was not possible. Plain radiographs of index knee were normal in all the patients. Three MRI from outside (four MRI were done in various outside hospitals) and two in-house MRI were initially reported as partial or complete tear of ACL, whereas remaining six were reported as MD of ACL. However when reviewed by the author and an expert institutional radiologist as no patient had reported instability, MRI revealed MD of ACL in all the patients and no tear [Figures 1 and 2]. Furthermore, 72.7% (N = 8) had associated solitary osseous cyst at the base of tibial attachment of ACL. Separate bundle involvement of ACL in MRI could not be commented upon. Arthroscopy revealed features suggestive of MD in all eleven cases with associated intraarticular cyst in three of them [Figure 3a–d]; one had it in front of ACL and behind PCL, another two had in front of ACL. Another one had an extracapsular cyst under medial head of gastrocnemius. Six patients also had grade 1-2 cartilage changes in knee; and one had grade 3 changes in the medial compartment. One patient had small radial tear in posterior third of medial meniscus and another had incomplete discoid lateral meniscus. After judicious debridement of mucoid ACL and associated cyst [Figure 3d], histopathology confirmed the diagnosis of MD of ACL in all eleven cases [Figure 4]. Two cases also revealed hyperplastic synovium. Mean followup was of 13.81 months (range 6-28 months). All patients regained complete flexion with no loss of extension. However few continued to have occasional pain while climbing stair, prolonged walking or squatting [Table 2]. All patients have resumed their normal daily activities. None complained of any instability. Seven patients (63.6%) had grade 1 Lachman positive and one had grade 2 Lachman and remainder revealed no objective laxity. Mean postoperative Lysholm scores were 89.54 (range: 85-95).
Table 1.
Clinical details of patients
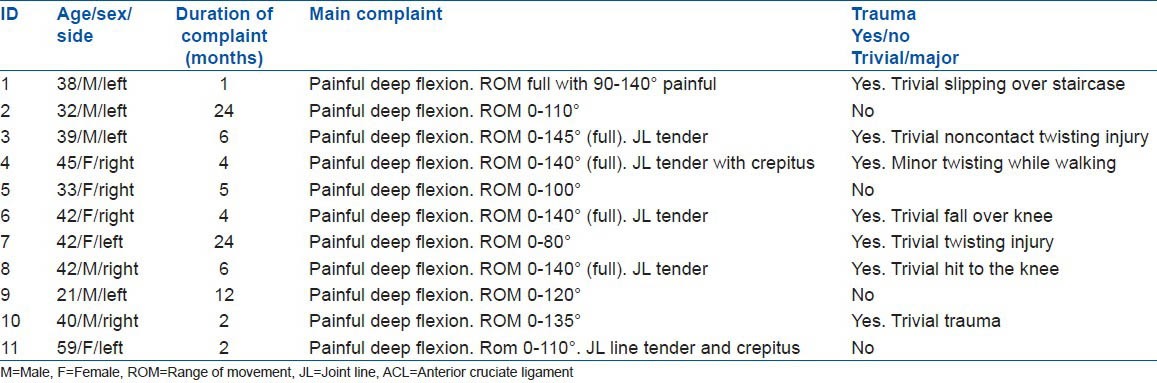
Figure 1.
The sagittal proton density weighted (a) and T1-weighted (b) Magnetic resonance images (T1 and FS T2) showing a cyst in front of the anterior cruciate ligament and multiloculated cyst behind the posterior cruciate ligament (ACL) with preserved fibers; however the ACL is bulky and has increased signal intensity and a celery stalk appearance. Intraosseous cyst present below tibial attachment of the ACL (white arrow)
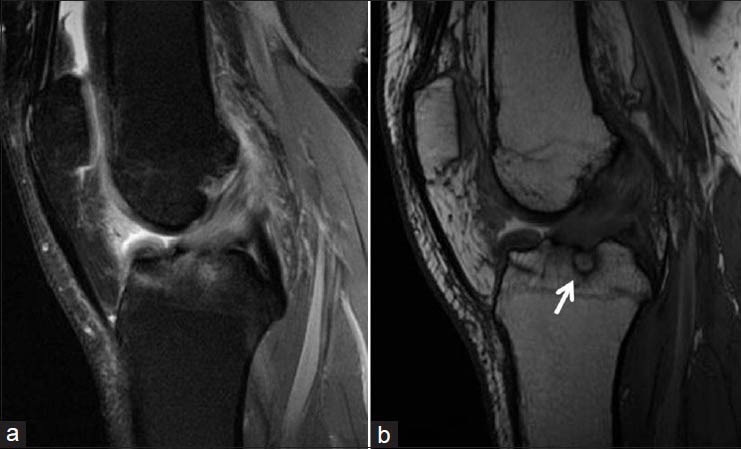
Figure 2.
Magnetic resonance images showing a multiloculated cyst behind the posterior cruciate ligament and the anterior cruciate ligament
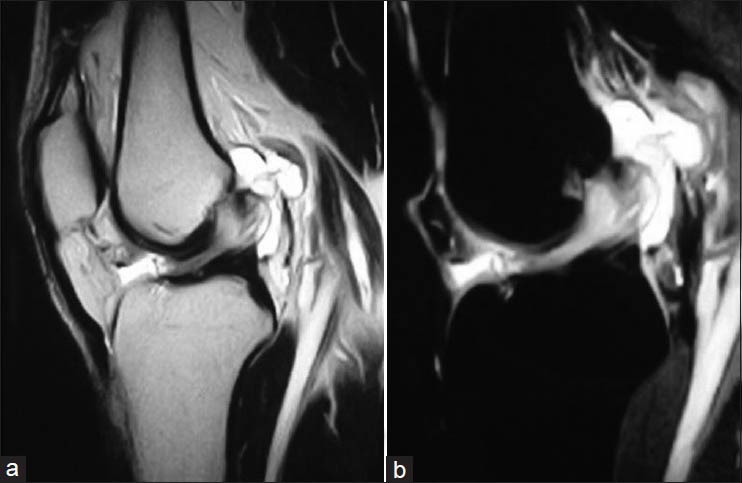
Figure 3.
Arthroscopic picture of left knee revealing (a) bulky anterior cruciate ligament (ACL) occupying the intercondylar notch with absent synovial layer. (b) Bulky fibres impinging upon the lateral femoral condyle. (c) Multiloculated cyst infront of mucoid ACL (black star). (d) Decongested intercondylar area after debulked mucoid ACL with exposed mucoid material (arrow). LFC, lateral femoral condyle
Figure 4.
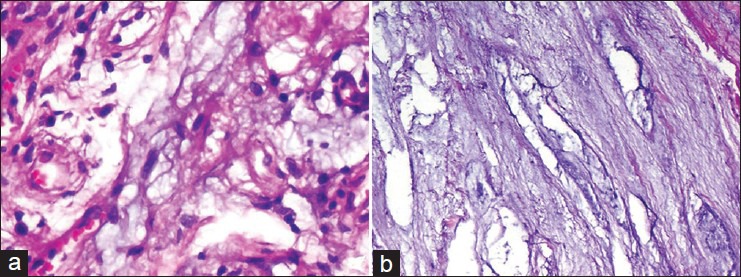
Photomicrograph revealing (a) collagen fibers admixed with faint purple-coloured mucoid material along with some fine capillaries at places (H and E, ×400). (b) loose connective tissue with extensive mucoid degeneration (H and E, ×100)
Table 2.
Diagnostic and treatment summary of patients
DISCUSSION
MD is a rare pathology of ACL1,2,3,4,5 and usually affects middle aged individuals with mean age of 42 years.2,4,5,8,9,13 Kim et al. has found it to be more common in the elderly (mean age: 61 years) with degenerative knees.1 In our study, median age was found to be 40 years, which is also reported by other authors. Male to female sex ratio had been confirmed to be 1:1 by Bergin et al.8 and 1.28:1 by Salvati et al.13 Our study had the ratio of 1.2:1. Median duration of pain was 5 months. Other authors also report that symptoms may persist for weeks or months.1,9,14
Multiple theories such as synovial, traumatic, degenerative, ectopic, altered joint mechanics, etc., have been put forth to explain the elusive pathological origin of MD of ACL.1,2,3,4 “Synovial” theory postulates accumulation of synovial fluid inside the substance of ACL in a herniated pouch of synovium.15 “Traumatic” theory highlights the fact that post injury, ACL fibroblasts secrete glycosaminoglycans that are deposited inside the substance of ACL.16 Another theory of traumatic origin says that it is the cellular response to trauma that liberates a mucin substance, hyaluronic acid. With joint and tissue motion, the mucin substance dissects the ligament fibers and gets interspersed within the fibers of the ligament, causing its fusiform dilatation.17 Other theories include “Degenerative” theory which states that MD could occur due to ageing.1,11,18 “Ectopic” theory is an interesting hypothesis wherein ectopic synovial tissue could exist inside ACL substance giving rise to microcyst formation.3,14 Other theories include proliferation from pluripotent mesenchymal stem cells4 and subtle alterations in joint kinematics due to osteoarthritis, meniscal tears and other degenerative changes leading to stretching of cruciate ligaments.1 Lancaster et al. theory of “multiple microtrauma” states that multiple micro/insignificant injuries result in microcyst formation, which eventually merges into large mucoid formations.12 He also believed that primary cause of MD could be the injury to synovia, which is supported by the fact that most cases with MD had absent or atrophic synovia over the ACL. 63.6% (N = 7) of our patients reported trivial trauma prior to the onset of symptoms. Majority of series have not reported any significant trauma prior to the onset of symptoms.1,5 Whereas few others reported traumatic event before the onset of symptom.19 Lancaster‘s’ et al. theory was also supported by Amiel et al. where he proved that injury to the synovium can result in exposure of ACL substance to the deleterious effects of haemarthrosis.20 Deie et al. proved that the synovium enveloping the ACL has a healing and a protective capacity on the ACL and this diminishes once resected or damaged.21 All of our cases did not have synovial lining over ACL. Several other authors too reported absence of synovial lining over ACL.5 Cha et al. believed that notch anatomy especially smaller and vertical notch predisposed the ACL to impingement and therefore resulting in microtrauma to the ACL.22 We did not make any attempt to measure the size of notch and correlate with MD of ACL. Therefore, most authors conclude that the primary factor for ACL MD seems to be injury to synovial lining. Jung et al. in their study have reported that there is significant correlation between increased tibial slope and MD of ACL.23
The most common and consistent symptom is knee pain, mostly posterior.1,3,4,10,24 Hypothetically, it is caused due to mechanical impingement on the PCL and posterior capsule1,23 or by causing bone erosions.3,4,6 A study of Fealy et al. mentioned that pain could also be due to intratendinous nociceptor irritation during increased knee flexion.10 Decompression of ACL relieving tension amidst fibers supports this fact. Knee pain during flexion is probably due to the tightening of the anteromedial bundle.10,25 Knee pain during terminal extension has also been described.1,3 The active role of chemical mediators like substance P and calcitonin gene related peptide has also been mentioned.10 The other clinical manifestations are mechanical block to extension; swelling and clicks are variable.3,4,10 In our patients, the nature of presentation was similar to that described in literature. All our patients had pain on deep flexion with no instability. None had extension block. At present it is difficult to point out a single lone cause for the knee pain and with the literature available, it seems to be multifactorial. We consider the possibility of pain due to impingement on soft tissues and bone in the vicinity, which was arthroscopically confirmed. However, concluding a clinical diagnosis of MD of ACL is challenging due to the multitude of nonspecific presentations in this condition.1,2,7,10,24 Furthermore, associated pathologies like cartilage damage and meniscal tear do contribute to the pain and may be the reason of residual pain after the surgery for MD of ACL.
The gold standard imaging for the diagnosis of MD of ACL is MRI, which exhibits intermediate signal intensity on T1-weighted and high signal intensity on T2-weighted images.1,4,5,7 The ACL fibers are usually thick and ill-defined, but the orientation and continuity is usually maintained.1,2,7,22 Bergin et al. in their study have reported detailed findings after retrospectively studying 4221 knee MRIs and helped differentiating MD from mucoid cyst.8 Narvekar and Gajjar describes the presence of a mass like configuration intertwined in the fibres.5 However, the most characteristic appearance is that akin to a “Celery stalk”.7 Considering the limited awareness, misdiagnosis as a tear is quiet common both clinically and on MRI.2,5,7,22,24 In our series, initially 45.5% MRI (N = 5) were erroneously reported as ACL tear. Mcintyre et al. has described ten patients who were initially mistaken for tears on MRI, but arthroscopy and probing expressed mucoid material.7 Makino et al. has described the presence of associated lesions like intraosseous tibial cyst and ganglion picked up on MRI.2 72.7% cases (N = 8) in our series had associated intraosseous cyst at tibial attachment of ACL.
The arthroscopic features include an intact but fibrillated, yellowish and hypertrophied ACL1,4,5,7 with interspersed yellowish mucinous material along the fibers exposed on probing,7 lack of smooth synovial lining7,8,14,26,27 and absent ligamentum mucosum.5 The otherwise normal ACL looks smooth with a thin synovial lining covering it and presence of ligamentum mucosum is quite a consistent finding. The ACL typically bulges anteriorly in the notch and impinges upon the LFC. In our cases, the anteriorly bulging ACL fibers were intact, bulky and impinged upon the LFC. In all the cases, ACL was devoid of synovial lining and ligamentum mucosum was absent as reported in the literature. There were associated cystic changes at the base of the ACL and behind PCL in three cases and one case had a cyst under the medial head of gastrocnemius consolidating the fact that MD and MC can coexist and share the common pathogenesis. It is also important to recognize MD of ACL otherwise it can lead to unwanted meniscectomies.26
Under the microscope, MD is typically described to have dense granular glycoproteins and mucoproteins (glycosoaminoglycans) located between thin, fragile collagen fibrils of ACL, which are detectable by hematoxylin and eosin or Alcian blue.4,10 MD can be differentiated from MC by diffuse, interstitial lesion amidst healthy ACL fibers, no cystic envelope around MD and absence of synovial lining. All 11 cases of ours revealed MD of ACL. Shelly et al. has reported a unique case of metastatic adenocarcinoma of the lung into the knee mimicking MD of the ACL.24 This fact establishes the importance of sending the excised specimen for the histopathology even though radiologically and arthroscopically it may appear to be as MD of ACL or mucoid cyst.
Kumar et al. considered total removal of ACL as a safe option, which did not result in instability.6 But most of the authors believe that debridement of mucinous substance with partial ACL debulking is an effective therapeutic option which does not cause instability.3,4,7,22 An additional notchplasty is considered essential by some authors.1,5 But Motmans and Verheyden specifically mention that notchplasty is not required, because thorough debridement by itself resolves the impingement and thereby the pathology.4 Lintz et al. performed two notchplasties out of 29 patients but not routinely.27 We performed a meticulous and judicious debridement of the mucoid ACL with the aim of reducing the volume, achieving removal of the mucoid mass and decompression of the bulky pathological ACL. We did not perform notchplasty in any of our patients, as we did not consider it as a part of primary pathology. We believe that notchplasty may be needed in some cases where notch is quite stenotic and impinged by osteophytes especially in elderly patients. However, it may not be needed in middle aged patients where notch is free of osteophytes.
Though all patients regained full flexion after debulking mucinous ACL, some of them continued to experience mild pain while walking or climbing stairs. This could be explained probably because of concomitant lesions like cartilage damage in patellofemoral or tibiofemoral joint or meniscal tear.
Post debulking, Lintz et al. demonstrated increased anterior translation (Mean: 8 mm) on Telos examination. However, only two out of 29 required ACL reconstruction 2-5 years after the index procedure.27 However, authors did not clarify that whether these two patients had developed instability due to chronic stretching after partial resection or due to subtotal or total resection of mucoid ACL. Dejour et al. demonstrated positive anterior drawer in 36% and positive pivot shift in 55% of his patients after debridement.28 In our series, postdebulking, 63.6% (N = 7) had grade 1 Lachman positive and 9% (n = 1) had grade 2 Lachman. None demonstrated a positive pivot shift. None developed instability until final followup. However, it is premature to say that these patients will not develop instability in future, as all of these were patients with sedentary activities except one who plays badminton. Whether patients whose occupation requires heavy demand from knee or athletes would not develop instability in future after partial debulking, is a matter of debate. Multicentric, long term followup of patients will determine whether judicious debulking of ACL is safe and sufficient or there is a need for complete removal of the ACL with or without reconstruction.
MD of the ACL is not that uncommon cause of knee pain with limited flexion in adults with or without a background of trivial trauma. The clinical and radiological features maybe inconclusive and may rather mislead to a diagnosis of a torn ACL tear. Awareness about this uncommon entity is important for arthroscopists as they may be confronted with a mucoid ACL intraoperatively. Furthermore, arthroscopists must educate themselves to interpret the MRI suggestive of MD of ACL as, in many instances, it may be reported as ACL tear. At present, judicious debulking and decompression of ACL with removal of mucinous material as much as possible is the preferred line of management. Notchplasty may not be required in all cases unless there is a stenotic notch impinging upon residual fibers. Definitive diagnosis is established with histopathology. Short term results after debridement are excellent. Furthermore, patient should be informed regarding mild residual pain which may arise due to concomitant lesion in cartilage and meniscus. However, long term followup is necessary to establish the credibility of partial debridement is most optimal treatment without any residual instability.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Kim TH, Lee DH, Lee SH, Kim JM, Kim CW, Bin SI. Arthroscopic treatment of mucoid hypertrophy of the anterior cruciate ligament. Arthroscopy. 2008;24:642–9. doi: 10.1016/j.arthro.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Makino A, Pascual-Garrido C, Rolón A, Isola M, Muscolo DL. Mucoid degeneration of the anterior cruciate ligament: MRI, clinical, intraoperative, and histological findings. Knee Surg Sports Traumatol Arthrosc. 2011;19:408–11. doi: 10.1007/s00167-010-1239-5. [DOI] [PubMed] [Google Scholar]
- 3.Melloni P, Valls R, Yuguero M, Sáez A. Mucoid degeneration of the anterior cruciate ligament with erosion of the lateral femoral condyle. Skeletal Radiol. 2004;33:359–62. doi: 10.1007/s00256-004-0754-1. [DOI] [PubMed] [Google Scholar]
- 4.Motmans R, Verheyden F. Mucoid degeneration of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2009;17:737–40. doi: 10.1007/s00167-008-0690-z. [DOI] [PubMed] [Google Scholar]
- 5.Narvekar A, Gajjar S. Mucoid degeneration of the anterior cruciate ligament. Arthroscopy. 2004;20:141–6. doi: 10.1016/j.arthro.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Bickerstaff DR, Grimwood JS, Suvarna SK. Mucoid cystic degeneration of the cruciate ligament. J Bone Joint Surg Br. 1999;81:304–5. doi: 10.1302/0301-620x.81b2.9243. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre J, Moelleken S, Tirman P. Mucoid degeneration of the anterior cruciate ligament mistaken for ligamentous tears. Skeletal Radiol. 2001;30:312–5. doi: 10.1007/s002560100336. [DOI] [PubMed] [Google Scholar]
- 8.Bergin D, Morrison WB, Carrino JA, Nallamshetty SN, Bartolozzi AR. Anterior cruciate ligament ganglia and mucoid degeneration: Coexistence and clinical correlation. AJR Am J Roentgenol. 2004;182:1283–7. doi: 10.2214/ajr.182.5.1821283. [DOI] [PubMed] [Google Scholar]
- 9.Chudasama CH, Chudasama VC, Prabhakar MM. Arthroscopic management of mucoid degeneration of anterior cruciate ligament. Indian J Orthop. 2012;46:561–5. doi: 10.4103/0019-5413.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fealy S, Kenter K, Dines JS, Warren RF. Mucoid degeneration of the anterior cruciate ligament. Arthroscopy. 2001;17:E37. doi: 10.1053/jars.2001.26878. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes JL, Viana SL, Mendonça JL, Freitas FM, Bezerra AS, Lima GA, et al. Mucoid degeneration of the anterior cruciate ligament: Magnetic resonance imaging findings of an underdiagnosed entity. Acta Radiol. 2008;49:75–9. doi: 10.1080/02841850701660497. [DOI] [PubMed] [Google Scholar]
- 12.Lancaster TF, Kirby AB, Beall DP, Wolff JD, Wu DH. Mucoid degeneration of the anterior cruciate ligament: A case report. J Okla State Med Assoc. 2004;97:326–8. [PubMed] [Google Scholar]
- 13.Salvati F, Rossi F, Limbucci N, Pistoia ML, Barile A, Masciocchi C. Mucoid metaplastic-degeneration of anterior cruciate ligament. J Sports Med Phys Fitness. 2008;48:483–7. [PubMed] [Google Scholar]
- 14.Hensen JJ, Coerkamp EG, Bloem JL, De Schepper AM. Mucoid degeneration of the anterior cruciate ligament. JBR-BTR. 2007;90:192–3. [PubMed] [Google Scholar]
- 15.Diard F, Chateil JF, Hauger O, Moinard M. Para-articular and intraosseous synovial cysts and articular mucoid cysts. J Radiol. 1999;80:679–96. [PubMed] [Google Scholar]
- 16.Rolf C, Watson TP. Case report: Intratendinous ganglion of the anterior cruciate ligament in a young footballer. J Orthop Surg Res. 2006;1:11. doi: 10.1186/1749-799X-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bui-Mansfield LT, Youngberg RA. Intraarticular ganglia of the knee: Prevalence, presentation, etiology, and management. AJR Am J Roentgenol. 1997;168:123–7. doi: 10.2214/ajr.168.1.8976934. [DOI] [PubMed] [Google Scholar]
- 18.Krudwig WK, Schulte KK, Heinemann C. Intraarticular ganglion cysts of the knee joint: A report of 85 cases and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2004;12:123–9. doi: 10.1007/s00167-003-0372-9. [DOI] [PubMed] [Google Scholar]
- 19.Kakutani K, Yoshiya S, Matsui N, Yamamoto T, Kurosaka M. An intraligamentous ganglion cyst of the anterior cruciate ligament after a traumatic event. Arthroscopy. 2003;19:1019–22. doi: 10.1016/j.arthro.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Amiel D, Billings E, Jr, Harwood FL. Collagenase activity in anterior cruciate ligament: Protective role of the synovial sheath. J Appl Physiol (1985) 1990;69:902–6. doi: 10.1152/jappl.1990.69.3.902. [DOI] [PubMed] [Google Scholar]
- 21.Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66:28–32. doi: 10.3109/17453679508994634. [DOI] [PubMed] [Google Scholar]
- 22.Cha JH, Lee SH, Shin MJ, Choi BK, Bin SI. Relationship between mucoid hypertrophy of the anterior cruciate ligament (ACL) and morphologic change of the intercondylar notch: MRI and arthroscopy correlation. Skeletal Radiol. 2008;37:821–6. doi: 10.1007/s00256-008-0527-3. [DOI] [PubMed] [Google Scholar]
- 23.Jung KH, Cho SD, Park KB, Youm YS. Relation between mucoid degeneration of the anterior cruciate ligament and posterior tibial slope. Arthroscopy. 2012;28:502–6. doi: 10.1016/j.arthro.2011.08.315. [DOI] [PubMed] [Google Scholar]
- 24.Shelly MJ, Dheer S, Kavanagh EC. Metastatic adenocarcinoma of the lung mimicking mucoid degeneration of the anterior cruciate ligament. Ir J Med Sci. 2010;179:309–11. doi: 10.1007/s11845-009-0311-y. [DOI] [PubMed] [Google Scholar]
- 25.Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res. 1975;106:216–31. doi: 10.1097/00003086-197501000-00033. [DOI] [PubMed] [Google Scholar]
- 26.Lintz F, Pujol N, Boisrenoult P, Bargoin K, Beaufils P, Dejour D. Anterior cruciate ligament mucoid degeneration: A review of the literature and management guidelines. Knee Surg Sports Traumatol Arthrosc. 2011;19:1326–33. doi: 10.1007/s00167-011-1433-0. [DOI] [PubMed] [Google Scholar]
- 27.Lintz F, Pujol N, Dejour D, Boisrenoult P, Beaufils P. Anterior cruciate ligament mucoid degeneration: Selecting the best treatment option. Orthop Traumatol Surg Res. 2010;96:400–6. doi: 10.1016/j.otsr.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Dejour D, Cohn J, Tavernier T. La dégénérescence spontanée du ligament croisé antérieur: étude radio clinique et anatomo-pathologique. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:67. doi: 10.1016/s0035-1040(05)84535-0. [DOI] [PubMed] [Google Scholar]


