Abstract
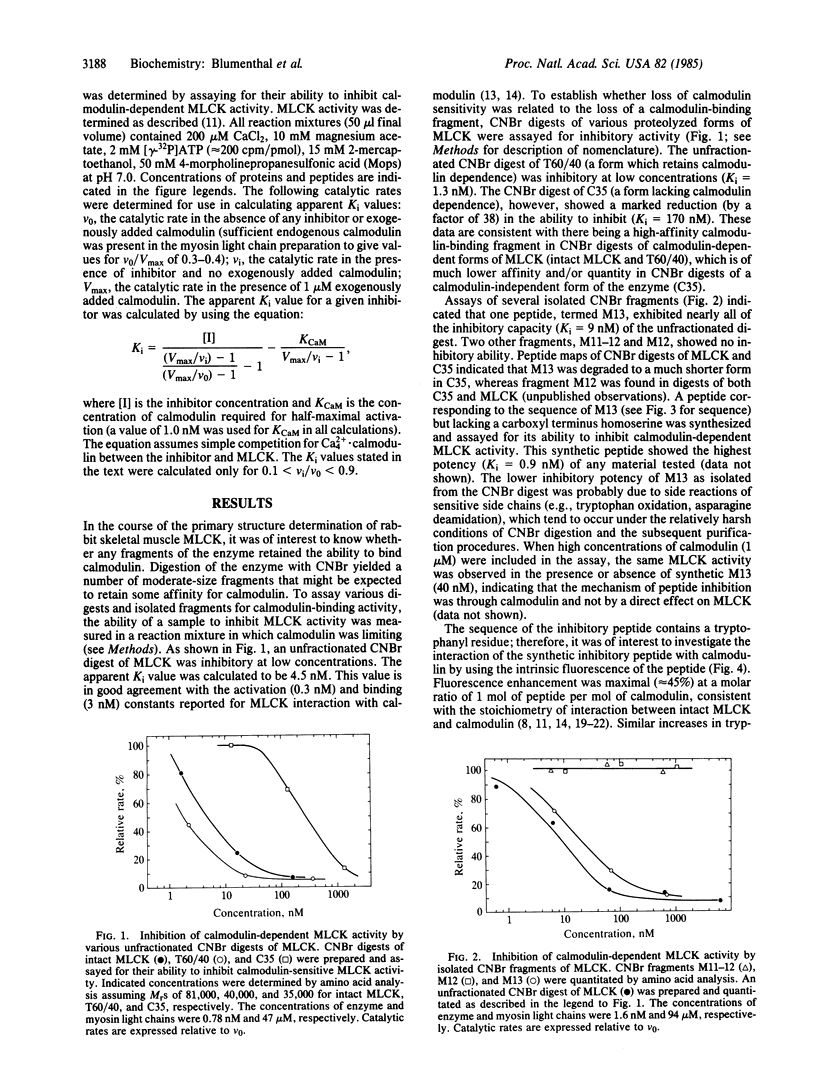
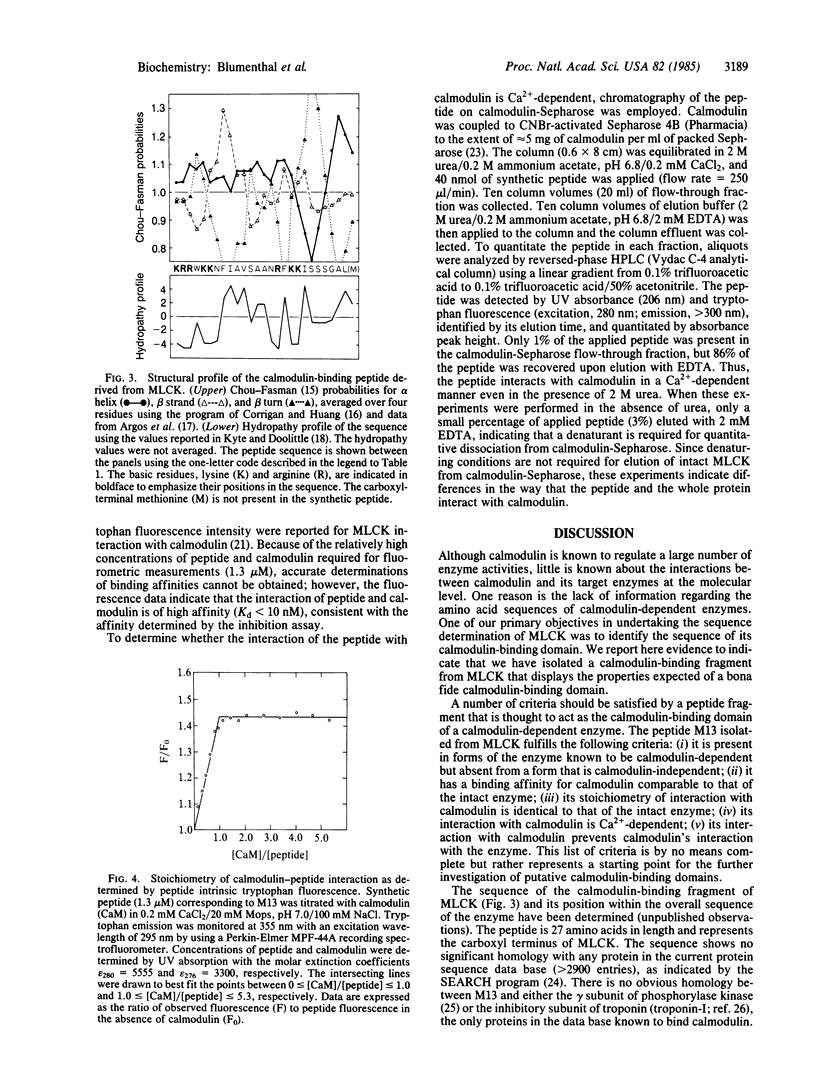
In the course of determining the primary structure of rabbit skeletal muscle myosin light chain kinase (MLCK; ATP:protein phosphotransferase, EC 2.7.1.37) a peptide fragment was obtained that appears to represent the calmodulin-binding domain of this enzyme. Low concentrations of the peptide inhibited calmodulin activation of MLCK (Ki congruent to 1 nM). The peptide was not associated with a catalytically active, calmodulin-independent form of MLCK that was obtained by limited proteolysis. The peptide is 27 residues in length and represents the carboxyl terminus of MLCK. The sequence of the peptide shows no significant homology with any known protein sequence. The peptide contains one tryptophanyl residue and a high percentage of basic and hydrophobic residues, but no acidic or prolyl residues. Much of the sequence has a high probability of forming alpha helix. A chemically synthesized peptide has been prepared to study the interactions of the peptide and calmodulin in more detail. The intrinsic tryptophan fluorescence of the synthetic peptide shows a significant enhancement (approximately equal to 45%) in the presence of Ca2+ and calmodulin; fluorescence enhancement is maximal at a peptide:calmodulin stoichiometry of 1:1. Calmodulin-Sepharose affinity chromatography in the presence of 2 M urea indicates that the interaction of peptide and calmodulin is Ca2+-dependent. The results of these studies indicate that the catalytic and calmodulin-binding domains of MLCK represent distinct and separable regions of the protein. In addition, the results provide a basis for future studies of the molecular and evolutionary details of calmodulin-dependent enzyme regulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Andreasen T. J., Keller C. H., LaPorte D. C., Edelman A. M., Storm D. R. Preparation of azidocalmodulin: a photoaffinity label for calmodulin-binding proteins. Proc Natl Acad Sci U S A. 1981 May;78(5):2782–2785. doi: 10.1073/pnas.78.5.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Hanei M., Garavito R. M. The Chou-Fasman secondary structure prediction method with an extended data base. FEBS Lett. 1978 Sep 1;93(1):19–24. doi: 10.1016/0014-5793(78)80795-9. [DOI] [PubMed] [Google Scholar]
- Barnette M. S., Daly R., Weiss B. Inhibition of calmodulin activity by insect venom peptides. Biochem Pharmacol. 1983 Oct 1;32(19):2929–2933. doi: 10.1016/0006-2952(83)90398-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal D. K., Stull J. T. Activation of skeletal muscle myosin light chain kinase by calcium(2+) and calmodulin. Biochemistry. 1980 Nov 25;19(24):5608–5614. doi: 10.1021/bi00565a023. [DOI] [PubMed] [Google Scholar]
- Blumenthal D. K., Stull J. T. Effects of pH, ionic strength, and temperature on activation by calmodulin an catalytic activity of myosin light chain kinase. Biochemistry. 1982 May 11;21(10):2386–2391. doi: 10.1021/bi00539a017. [DOI] [PubMed] [Google Scholar]
- Chan K. F., Graves D. J. Isolation and physicochemical properties of active complexes of rabbit muscle phosphorylase kinase. J Biol Chem. 1982 May 25;257(10):5939–5947. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Corrigan A. J., Huang P. C. A BASIC microcomputer program for plotting the secondary structure of proteins. Comput Programs Biomed. 1982 Dec;15(3):163–168. doi: 10.1016/0010-468x(82)90001-0. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Frearson N., Perry S. V. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975 Oct;151(1):99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedroc D. P., Ling N., Puett D. Identification of beta-endorphin residues 14-25 as a region involved in the inhibition of calmodulin-stimulated phosphodiesterase activity. Biochemistry. 1983 Nov 22;22(24):5584–5591. doi: 10.1021/bi00293a020. [DOI] [PubMed] [Google Scholar]
- Glass D. B. Synthesis of oligopeptides for the study of cyclic nucleotide-dependent protein kinases. Methods Enzymol. 1983;99:119–139. doi: 10.1016/0076-6879(83)99046-8. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Wilkinson J. M. The amino acid sequence of rabbit cardiac troponin I. Biochem J. 1976 Dec 1;159(3):633–641. doi: 10.1042/bj1590633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Holroyde M. J., Crouch T. H., Solaro R. J., Potter J. D. Fluorescence studies of the interaction of calmodulin with myosin light chain kinase. J Biol Chem. 1981 Dec 10;256(23):12194–12198. [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Krinks M. H. Purification of cyclic 3',5'-nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to Sepharose. Biochemistry. 1978 Jan 10;17(1):120–126. doi: 10.1021/bi00594a017. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Wierman B. M., Storm D. R. Calcium-induced exposure of a hydrophobic surface on calmodulin. Biochemistry. 1980 Aug 5;19(16):3814–3819. doi: 10.1021/bi00557a025. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of hormones and neuropeptides by calmodulin. Biochemistry. 1983 Apr 12;22(8):1995–2001. doi: 10.1021/bi00277a040. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of simple peptides, hormones, and neurotransmitters by calmodulin. Biochemistry. 1982 Jul 6;21(14):3480–3486. doi: 10.1021/bi00257a035. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Peptide binding by calmodulin and its proteolytic fragments and by troponin C. Biochemistry. 1984 May 22;23(11):2420–2428. doi: 10.1021/bi00306a016. [DOI] [PubMed] [Google Scholar]
- Manalan A. S., Klee C. B. Calmodulin. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:227–278. [PubMed] [Google Scholar]
- Maulet Y., Cox J. A. Structural changes in melittin and calmodulin upon complex formation and their modulation by calcium. Biochemistry. 1983 Nov 22;22(24):5680–5686. doi: 10.1021/bi00293a035. [DOI] [PubMed] [Google Scholar]
- Mayr G. W., Heilmeyer L. M., Jr Shape and substructure of skeletal muscle myosin light chain kinase. Biochemistry. 1983 Aug 30;22(18):4316–4326. doi: 10.1021/bi00287a024. [DOI] [PubMed] [Google Scholar]
- Nagamoto H., Yagi K. Properties of myosin light chain kinase prepared from rabbit skeletal muscle by an improved method. J Biochem. 1984 Apr;95(4):1119–1130. doi: 10.1093/oxfordjournals.jbchem.a134700. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Perry S. V. Calmodulin and myosin light-chain kinase of rabbit fast skeletal muscle. Biochem J. 1979 Apr 1;179(1):89–97. doi: 10.1042/bj1790089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnally M. H., Stull J. T. Mammalian skeletal muscle myosin light chain kinases. A comparison by antiserum cross-reactivity. J Biol Chem. 1984 Feb 10;259(3):1776–1780. [PubMed] [Google Scholar]
- Olwin B. B., Edelman A. M., Krebs E. G., Storm D. R. Quantitation of energy coupling between Ca2+, calmodulin, skeletal muscle myosin light chain kinase, and kinase substrates. J Biol Chem. 1984 Sep 10;259(17):10949–10955. [PubMed] [Google Scholar]
- Picton C., Klee C. B., Cohen P. Phosphorylase kinase from rabbit skeletal muscle: identification of the calmodulin-binding subunits. Eur J Biochem. 1980 Oct;111(2):553–561. doi: 10.1111/j.1432-1033.1980.tb04971.x. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Titani K., Ericsson L. H., Wade R. D., Fischer E. H., Walsh K. A. Homology of the gamma subunit of phosphorylase b kinase with cAMP-dependent protein kinase. Biochemistry. 1984 Aug 28;23(18):4185–4192. doi: 10.1021/bi00313a027. [DOI] [PubMed] [Google Scholar]
- Takio K., Smith S. B., Walsh K. A., Krebs E. G., Titani K. Amino acid sequence around a "hinge" region and its "autophosphorylation" site in bovine Lung cGMP-dependent protein kinase. J Biol Chem. 1983 May 10;258(9):5531–5536. [PubMed] [Google Scholar]
- Takio K., Wade R. D., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Guanosine cyclic 3',5'-phosphate dependent protein kinase, a chimeric protein homologous with two separate protein families. Biochemistry. 1984 Aug 28;23(18):4207–4218. doi: 10.1021/bi00313a030. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Hidaka H. Hydrophobic regions function in calmodulin-enzyme(s) interactions. J Biol Chem. 1980 Dec 10;255(23):11078–11080. [PubMed] [Google Scholar]



