Abstract
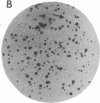
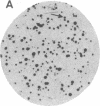
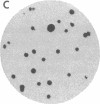
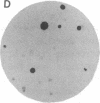
We have obtained Chinese hamster ovary cell mutants defective in the biosynthesis of glycosaminoglycans by screening replicate colonies immobilized on polyester cloth. Depending upon the strain, the mutants accumulated less 35S-labeled glycosaminoglycans per microgram of cell protein by a factor of 6-60 compared to the wild type. Some of the mutants incorporated [6-3H]glucosamine into glycosaminoglycans to the same extent as the wild type, suggesting that sulfate addition was specifically altered. In contrast, five strains failed to generate 3H-labeled glycosaminoglycans normally. In four of these, the initiation of glycosaminoglycan assembly was specifically altered, since the addition of p-nitrophenyl-beta-xyloside restored sulfation to normal. Enzymatic assay of the xylosyltransferase in extracts prepared from these mutants revealed that one of the strains, S745, contained less enzyme activity by a factor of 15 than the wild type. This mutant provides genetic evidence that the xylosyltransferase assayed in vitro is responsible for the initiation of chondroitin sulfate and heparan sulfate biosynthesis in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch D. B., Cleaver J. E., Glaser D. A. Large-scale isolation of UV-sensitive clones of CHO cells. Somatic Cell Genet. 1980 May;6(3):407–418. doi: 10.1007/BF01542792. [DOI] [PubMed] [Google Scholar]
- Campbell P., Jacobsson I., Benzing-Purdie L., Rodén L., Fessler J. H. Silk--a new substrate for UDP-d-xylose:proteoglycan core protein beta-D-xylosyltransferase. Anal Biochem. 1984 Mar;137(2):505–516. doi: 10.1016/0003-2697(84)90119-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig A. H., Slayman C. W., Adelberg E. A. Isolation of a spontaneous CHO amino acid transport mutant by a combination of tritium suicide and replica plating. Somatic Cell Genet. 1982 Jul;8(4):509–520. doi: 10.1007/BF01538711. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Autoradiographic detection of animal cell membrane mutants altered in phosphatidylcholine synthesis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5192–5196. doi: 10.1073/pnas.77.9.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Replica plating and in situ enzymatic assay of animal cell colonies established on filter paper. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1190–1193. doi: 10.1073/pnas.75.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebner E. E., Hall C. W., Neufeld E. F. Incorporation of D-xylose-C14 into glycoprotein by particles from hen oviduct. Biochem Biophys Res Commun. 1966 Mar 22;22(6):672–677. doi: 10.1016/0006-291x(66)90199-9. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Jr, Raetz C. R. Dibutyryl cAMP-inducible alkaline phosphatase in animal cell plasma membranes: fluorescence detection of mutant clones on polyester cloth. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3918–3922. doi: 10.1073/pnas.80.13.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg C. B., Perez M., Snider M., Hanneman W. L., Esko J., Raetz C. R. Autoradiographic detection and characterization of a Chinese hamster ovary cell mutant deficient in fucoproteins. J Cell Physiol. 1982 Jun;111(3):255–263. doi: 10.1002/jcp.1041110306. [DOI] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Kimata K., Barrach H. J., Brown K. S., Pennypacker J. P. Absence of proteoglycan core protein in cartilage from the cmd/cmd (cartilage matrix deficiency) mouse. J Biol Chem. 1981 Jul 10;256(13):6961–6968. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- McKeown P. J., Goetinck P. F. A comparison of the proteoglycans synthesized in Meckel's and sternal cartilage from normal and nanomelic chick embryos. Dev Biol. 1979 Aug;71(2):203–215. doi: 10.1016/0012-1606(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Okayama M., Kimata K., Suzuki S. The influence of p-nitrophenyl beta-d-xyloside on the synthesis of proteochondroitin sulfate by slices of embryonic chick cartilage. J Biochem. 1973 Nov;74(5):1069–1073. [PubMed] [Google Scholar]
- Oldberg A., Heldin C. H., Wasteson A., Busch C., Hök M. Characterization of a platelet endoglycosidase degrading heparin-like polysaccharides. Biochemistry. 1980 Dec 9;19(25):5755–5762. doi: 10.1021/bi00566a014. [DOI] [PubMed] [Google Scholar]
- Polokoff M. A., Wing D. C., Raetz C. R. Isolation of somatic cell mutants defective in the biosynthesis of phosphatidylethanolamine. J Biol Chem. 1981 Aug 10;256(15):7687–7690. [PubMed] [Google Scholar]
- Raetz C. R., Wermuth M. M., McIntyre T. M., Esko J. D., Wing D. C. Somatic cell cloning in polyester stacks. Proc Natl Acad Sci U S A. 1982 May;79(10):3223–3227. doi: 10.1073/pnas.79.10.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R. Isolation of lysosomal alpha-mannosidase mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1911–1915. doi: 10.1073/pnas.76.4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Peng S. S., Marshall J. L. Mutant Chinese hamster ovary cells pleiotropically defective in receptor-mediated endocytosis. J Cell Biol. 1983 Apr;96(4):1064–1071. doi: 10.1083/jcb.96.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N. B., Rodén L. Biosynthesis of chondroitin sulfate. Purification of UDP-D-xylose:core protein beta-D-xylosyltransferase by affinity chromatography. Carbohydr Res. 1974 Oct;37(1):167–180. doi: 10.1016/s0008-6215(00)87072-x. [DOI] [PubMed] [Google Scholar]
- Stefanini M., Reuser A., Bootsma D. Isolation of Chinese hamster ovary cells with reduced unscheduled DNA synthesis after UV irradiation. Somatic Cell Genet. 1982 Sep;8(5):635–642. doi: 10.1007/BF01542856. [DOI] [PubMed] [Google Scholar]
- Sugahara K., Schwartz N. B. Defect in 3'-phosphoadenosine 5'-phosphosulfate formation in brachymorphic mice. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6615–6618. doi: 10.1073/pnas.76.12.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H. Mutant isolation. Methods Enzymol. 1979;58:308–322. doi: 10.1016/s0076-6879(79)58147-6. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Uthne K., Westermark B. A novel assay for the biosynthesis of sulphated polysaccharide and its application to studies on the effects of somatomedin on cultured cells. Biochem J. 1973 Dec;136(4):1069–1074. doi: 10.1042/bj1361069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]