Abstract
Background
Chest X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) each have characteristic advantages and disadvantages that need to be considered in clinical decision-making. This point is discussed in reference to the main types of lung disease that are encountered in practice.
Methods
A selective literature search was performed in the PubMed and Google Scholar databases. Existing clinical guidelines on the main types of lung disease and studies concerning radiological diagnosis were also considered in this review.
Results
There have been no more than a few large-scale, controlled comparative trials of different radiological techniques. Chest X-ray provides general orientation as an initial diagnostic study and is especially useful in the diagnosis of pneumonia, cancer, and chronic obstructive pulmonary disease (COPD). Multi-detector CT affords nearly isotropic spatial resolution at a radiation dose of only 0.2–5 mSv, much lower than before. Its main indications, according to current guidelines, are tumors, acute pulmonary embolism, pulmonary hypertension, pulmonary fibrosis, advanced COPD, and pneumonia in a high-risk patient. MRI is used in the diagnosis of cystic fibrosis, pulmonary embolism, pulmonary hypertension, and bronchial carcinoma. The positive predictive value (PPV) of a chest X-ray in outpatients with pneumonia is only 27% (gold standard, CT); in contrast, an initial, non-randomized trial of MRI in nosocomial pneumonia revealed a PPV of 95%. For the staging of mediastinal lymph nodes in bronchial carcinoma, MRI has a PPV of 88% and positron emission tomography with CT (PET/CT) has a PPV of 79%, while CT alone has a PPV of 41% (gold standard, histology).
Conclusion
The choice of radiologicalal technique for the detection, staging, follow-up, and quantification of lung disease should be based on the individual clinical options, so that appropriate treatment can be provided without excessive use of diagnostic testing.
In the diagnosis of lung disease, assessing the pattern, location, and regional distribution of involvement is the domain of radiology. Complementing classical chest radiography, the tomographic imaging techniques of computed tomography (CT, today in the form of multidetector CT, MDCT) and magnetic resonance imaging (MRI) can demonstrate and quantify, not just morphology, but increasingly also functional processes such as perfusion, respiration, and metabolism, region by region. MDCT and MRI are also often used in treatment monitoring or disease monitoring to identify progression, to allow appropriate treatment alterations or further interventions to be initiated.
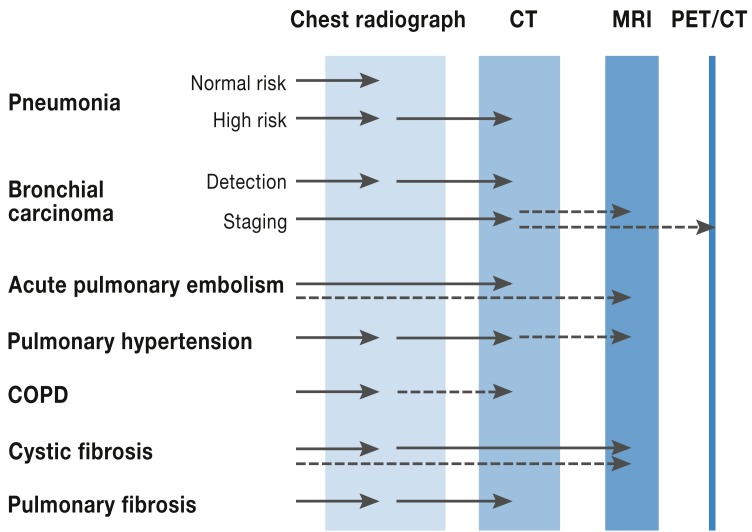
This review article will present both established and recommended radiological techniques and also newly introduced techniques used in the management of the most important lung diseases, indicating their advantages and disadvantages, how often they are used, remuneration details, and associated radiation dose (Figure 1, Table).
Figure 1.
Disease-specific choice of imaging modality. CT, computed tomography; MRI, magnetic resonance imaging; PET/CT positron emission tomography/CT; COPD, chronic obstructive pulmonary disease
Tabelle. Comparison of the most important diagnostic imaging techniques for lung disease.
| Chest radiograph | Computed tomography | Magnetic resonance imaging | |
|---|---|---|---|
| Advantages | Widely available Exploratory first study |
High spatial resolution High sensitivity High speed Workflow |
Intermediate spatial resolution High contrast resolution High temporal resolution Function No radiation exposure |
| Disadvantages | Low sensitivity Low specificity |
Allergy to contrast agent Contraindications: impaired renal function, thyroid function |
Availability Study acquisition time Allergy to contrast agent Contraindications: implants |
| Indications | Community-acquired + uncomplicated pneumonia, bronchial carcinoma (detection) Pulmonary hypertension COPD Cystic fibrosis Fibrosis |
Complicated pneumonia, pneumonia in at-risk patients Bronchial carcinoma (staging) Acute pulmonary embolism Pulmonary hypertension COPD Fibrosis |
Bronchial carcinoma Pulmonary hypertension Cystic fibrosis |
| Remuneration EBM points GöA rate (basic) |
430 €26.23 |
1865; + 645 with contrast agent €134.06; with contrast agent €151.55 + additional consumables |
3430; + 1260 with contrast agent €250.64; with contrast agent €326.42 + additional consumables |
| Dose | 0.1 mSv *1 | Low-dose-CT 0.2–1 mSv Routine now 1–5 mSv Routine 10 years ago 10 mSv *1 | None |
| Number performed | 15 million/year *2 | Total estimated 2 million/year *2 Inpatient 830000/year *3 | Inpatient 12000/year*3 |
Sources: *1Mettler FA, et al. (38); *2Federal Office for Radiation Protection (39); *3Federal Statistical Office (40)
COPD, chronic obstructive lung disease; EBM, uniform evaluation standard within the statutory health insurance scheme (Einheitlicher Bewertungsmassstab); GoÄ, medical fee schedule (Gebührenordnung für Ärzte); CT, computed tomography
Methods
A selective literature search of the PubMed and Google Scholar databases was used to identify current German and European medical society guidelines on the most important lung diseases and reports of large controlled studies of diagnostic radiology. In addition, international review papers and original articles on smaller studies from the past 10 years were included for cases where the guidelines were not sufficiently up to date or detailed from the diagnostic radiology viewpoint.
Large controlled studies comparing different radiological techniques are rare, since technical innovations are introduced very quickly and the added value of a new technique often appears obvious. Comparison of two techniques involving radiation exposure can often not be ethically justified in the setting of a clinical study, or patients would not take part in a randomized study in which one study arm is intended to undergo the slower technique with the higher radiation exposure.
Technical aspects
Projection radiography: the chest radiograph
The chest radiograph is the oldest radiological procedure and the one most frequently used for diagnostic purposes in the lung. Chest radiography is generally carried out in two planes or projections (Table). In the projection image, in which all anatomical structures located in the path of the X-rays are superimposed on each other, about 70% of the lung volume can be freely seen in at least one projection. The sensitivity for the detection of lung nodules, infiltrates, and interstitial changes is therefore limited and the differential diagnosis of abnormal findings complicated. Modern X-ray equipment, both fixed and mobile, is now entirely digital, replacing the classical film–foil combinations.
Computed tomography
Multidetector CT (MDCT) is widely available and is characterized by high, almost isotropic resolution (pixel size 0.5 to 1 mm in every spatial direction), allowing the image dataset to be viewed in any plane desired. Acquisition time ranges from 1 to 10 seconds, meaning that virtually artifact-free images can be obtained even of dyspneic patients or those unable to cooperate. The once classical incremental “high-resolution CT” (HRCT) has been replaced by MDCT, because it provides continuous imaging for the same radiation exposure, greatly facilitating disease monitoring (1). Indication-specific protocols for airway and lung parenchymal disease, and for lung nodules found during the follow-up of extrathoracic primary tumors, require studies without the use of intravenous contrast agent. For the staging of bronchial carcinoma, assessment of blood vessels, and cardiopulmonary interaction, on the other hand, intravenous contrast studies are required (1).
The disadvantage of CT is the radiation exposure (Table), although technical innovations have allowed this to be much reduced. These innovations include:
Low tube current (e.g., 40 mAs): e.g., in disease monitoring, dose reduction (up to 80%) without any loss of diagnostic yield/validity
Automatic dose modulation according to anatomy: low dose in the middle of the chest, higher dose for the shoulder girdle; dose saving approx. 30%
Modern fixed-array detectors and interactive image reconstruction algorithms: reduced image noise; dose can be reduced by approx. 25% for the same image quality.
The newest MDCT scanners are equipped with dual-energy technology, meaning that two different energies/tube voltages are used at the same time. Because of the energy dependence of absorption, this allows particular tissue characteristics to be emphasized, e.g., iodine distribution after contrast administration as a surrogate for regional perfusion (2).
Magnetic resonance imaging
MRI combines structural and functional information (3). Clinically informative MRI studies of the lung are obtained by fast sequences, parallel imaging, and respiratory and ECG triggering (3). Most lung diseases are accompanied by an increase in tissue and/or blood and displacement of air, resulting in higher signal on MRI (“plus pathology”). In addition to morphology, MRI enables complementary functional study of perfusion, ventilation, respiratory mechanics, and cardiac action and blood flow (3). With growing awareness about cardiopulmonary interaction, a chest MRI should cover both the lung and the heart, e.g., by means of “one-stop” diagnostic imaging in patients with pulmonary hypertension. Disadvantages of MRI are study acquisition time (15 to 30 min) and the spatial resolution (pixel size approx. 2 mm). The advantage is the absence of radiation exposure (Table).
Disease-specific imaging
Pneumonia
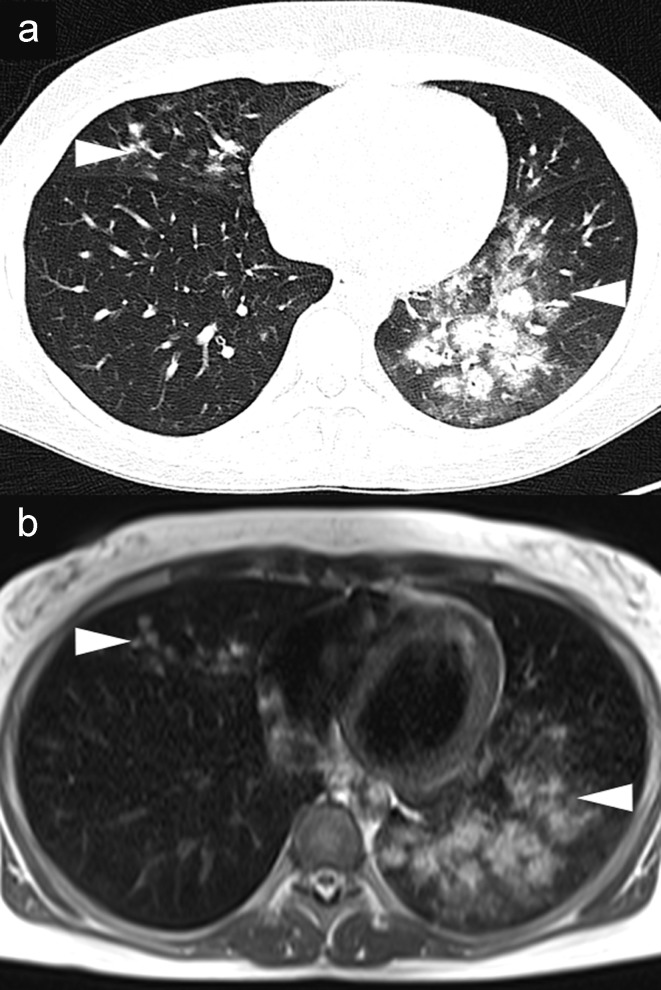
For the diagnosis of pneumonia, in addition to history, clinical findings, and laboratory test results, radiological proof of the presence of infiltrate is essential (4). According to German guidelines, in outpatient-acquired pneumonia, chest radiography in two planes is the procedure of choice (recommendation grade B) (4). This also applies for the initial diagnostic procedure for hospital-acquired pneumonia (≥48 h after hospital admission) in patients with immunocompetent status (strong recommendation, evidence level C) (5). Since the positive predictive value of chest radiography in comparison to CT is only 27% (6), for patients at higher risk—e.g., those with ventilation-associated pneumonia or the immune-compromised—non-contrast low-dose MDCT is indicated for detection and characterization of infiltrates (fungal?) (weak recommendation, evidence level C) (1, 5). In a non-randomized study in this patient group, MRI achieved a sensitivity of 95%, a specificity of 88%, and a positive predictive value of 95% in comparison to MDCT (Figure 2) (7).
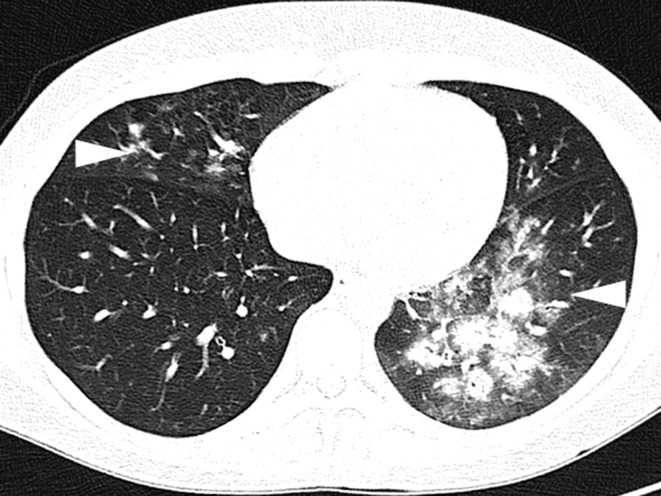
Figure 2.
Diagnosis of infiltrates in an immunosuppressed patient. A 34-year-old woman after high-dose chemotherapy for B-cell lymphoma with neutropenic fever. Axial non-contrast computed tomography shows extensive peribronchial infiltrates (arrowheads)
Bronchial carcinoma
Contrast-enhanced thoracic MDCT is the most important technique in the staging of bronchial carcinoma, according to the 2010 German guidelines (recommendation grade A) (8). In experienced centers, MRI delivers essentially similar results (evidence level 4) (8). Because of the high soft tissue contrast, MRI can better distinguish central tumor parts from poststenotic atelectasis, which is important for example in defining the target volume of radiation therapy (8). To assess infiltrations of the chest wall or mediastinum, MRI—including as a dynamic study—is likewise recommended by the guidelines (8, 9). In cases of superior sulcus tumor (Pancoast tumor) and cases where the tumor is in contact with the spine, MRI should even explicitly be carried out as part of the preoperative planning (evidence level 2a) (8).
In N-staging, the CT assessment relates to tumor stage only in terms of the short-axis diameter of the lymph nodes, leading to low sensitivity and specificity (52% to 62% and 62% to 69%, respectively) (evidence level 2a) (8). For this reason, for non-small-cell bronchial carcinoma, positron emission tomography (PET)/CT (sensitivity 74% to 85% and specificity 85% to 92%), combined with histological verification of relevant findings, is recommended (evidence level 1a) (8). Alternatively, MRI with special “diffusion-weighted” sequences can be used, in which metastatic lymph nodes appear bright and fat-containing, healthy lymph nodes appear dark. Using these sequences, a sensitivity of 90%, specificity of 93%, and positive predictive value of 88% for MRI were obtained, and for PET/CT values of 77%, 88%, and 79%, respectively (with histology as gold standard) (10); whereas the values for CT alone, in less recent studies, were much lower (63%, 57%, 41%) (11).
For detection of distant metastases (M staging), whole-body PET/CT shows a sensitivity of 93% and a specificity of 96% (evidence level 2a, recommendation grade A); unexpected metastases were found in 15% of cases in comparison to diagnostic CT alone. In several comparison studies, dedicated whole-body MRI has achieved similar values to PET/CT (positive predictive values 53% versus 44%) (12)—in particular, distant metastases in brain and bones were better identified with MRI than with PET/CT (8)—and can, according to the guidelines, be carried out as an alternative, e.g., in patients with diabetic metabolic status (8). In every case, in addition to PET/CT, cranial MRI should be carried out to exclude brain metastases (recommendation grade A) (8).
It has long been known that chest radiography is not suitable as a screening technique for bronchial carcinoma (13). In contrast to this, however, in the first large, controlled, prospectively randomized screening study of 53 454 smokers, low-dose MDCT showed a significant survival advantage. There were 20% fewer deaths from bronchial carcinoma in the MDCT group than in the control group (screening chest radiograph) (14). However, this was associated with many false-positive findings (17 702 = 96.4% in the CT group versus 4764 = 94.5% in the control group), which lead to follow-up investigations (12 757 = 72% versus 4211 = 85%) and complications due to invasive diagnostic procedures (61 = 0.4% versus 16 = 0.3%). It is still unclear how many indolent tumors are being treated that would have been irrelevant to the patient’s survival (“overdiagnosis”). Moreover, results from the US cannot be simply extrapolated to Germany, and the results of the ongoing European studies must be awaited (15, 16).
Vascular diseases
Contrast-enhanced MDCT, which has the highest sensitivity (83% to 100%) and specificity (89% to 97%) and is easily available, has become universally established in the diagnosis of acute pulmonary embolism (APE), and has also replaced scintigraphy and invasive angiography as the primary imaging procedure in the current German guidelines (17, 18).
In patients with contraindications for MDCT (contrast allergy or pregnancy), MRI can be performed. In large, non-randomized studies (PIOPED III), it has been shown that MRI can be almost equivalent to MDCT, with sensitivity and specificity rates of 78% to 90% and 99% to 100%. Its high negative predictive value of 97% for a follow-up period of 3 months (19) has clinical relevance. This investigation should be carried out first without contrast administration, using contrast-rich fast imaging sequences, to show a central pulmonary embolism within a few seconds (Figure 3). If no central pulmonary embolism is shown, the MRI is continued as a perfusion study and MR angiography, with administration of gadolinium-based contrast agent (20).
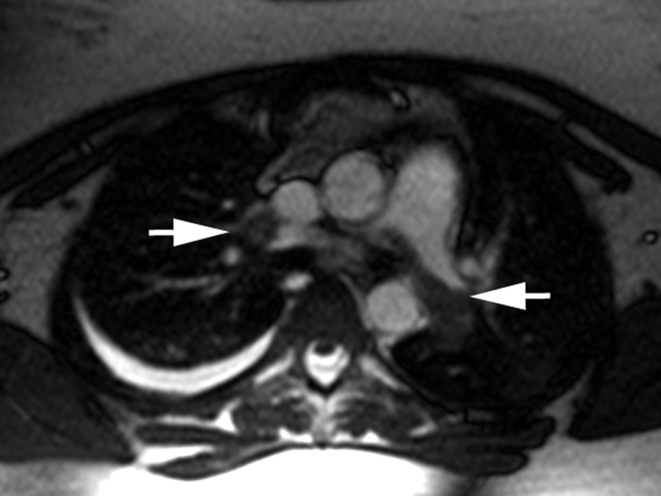
Figure 3.
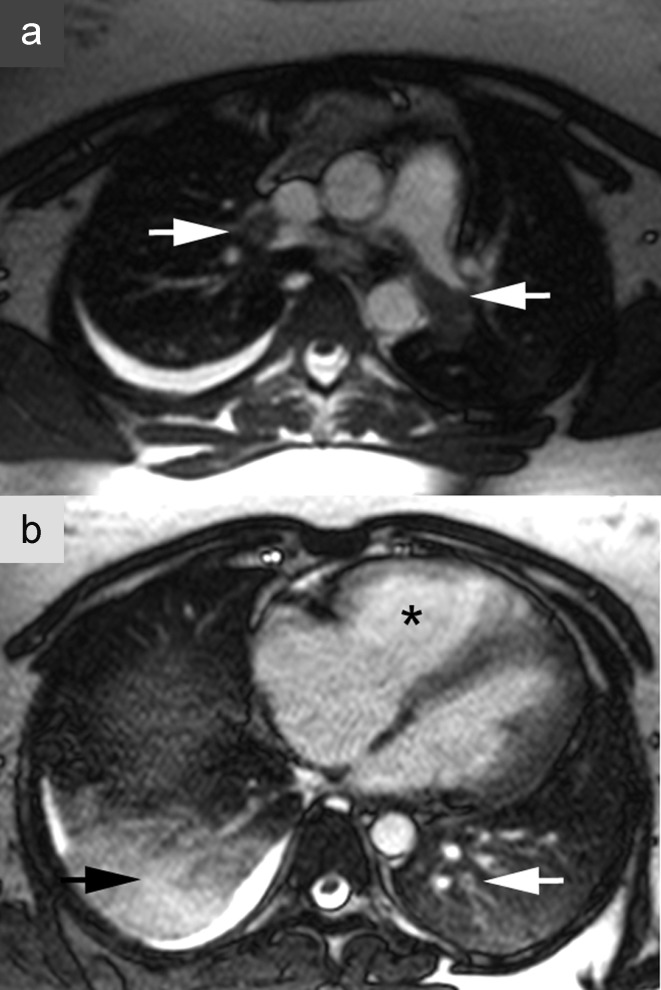
Magnetic resonance imaging (MRI) of pulmonary arterial embolism. A 23-year-old woman with a 2-week history of respiratory infection; current symptoms include hemoptysis, exercise dyspnea, and chest pain on breathing. Axial MRI without contrast administration shows central and peripheral filling defects, some of them partial, in the pulmonary artery (arrows), corresponding to extensive pulmonary embolism
According to the European guidelines, contrast-enhanced MDCT angiography is used to rule out causative diseases in patients with pulmonary hypertension, e.g., interstitial lung disease, emphysema, or chronic thromboembolic pulmonary hypertension (CTEPH); and to identify dilated bronchial arteries and right heart dilatation and hypertrophy (21). If signs of CTEPH are found, according to the 2010 German consensus conference, the findings of ventilation perfusion scintigraphy, MDCT, and invasive pulmonary artery angiography should be interpreted together to decide whether surgery (curative pulmonary thrombendarterectomy) is possible (recommendation grade 2a, evidence level C) (22). Morphological MRI (central thrombus burden, webs and bands, vascular stenosis) and functional MRI (peripheral perfusion deficits) achieved a sensitivity, specificity, and positive predictive value of 98%, 94%, and 96% in comparison with MDCT (23), and 93% sensitivity in comparison to pulmonary arterial angiography (gold standard) in imaging the subsegmental pulmonary arteries (24). For comprehensive diagnostic investigations, functional MRI techniques that quantify cardiac action, blood flow in the great vessels, and lung perfusion with high reproducibility are available (25).
Obstructive airways disease
Cystic fibrosis is the most common life-limiting autosomal recessive disease of fair-skinned people, and its prognosis is determined by the severity of lung involvement (26). From what age and at what intervals routine diagnostic imaging should be performed has not yet become firmly established. According to the German guidelines, chest radiograph, MDCT, and MRI should be performed depending on local expertise and the clinical situation (recommendation grade B) (27). MDCT is more sensitive than the chest radiograph in showing typical lung changes (28). In patients identified at neonatal screening, MDCT shows typical bronchiectasis in 27% of cases, before they become clinically manifest or show any changes in lung function tests (29). MRI is increasingly replacing MDCT as the technique for diagnosing complications or monitoring the disease (30). It shows the typical changes of bronchiectasis, wall thickening, mucus plugging, and infiltrates (“plus pathologies” on MRI) sensitively and with comparable clinical relevance to MDCT (31). In addition, MR perfusion imaging making use of hypoxic pulmonary vasoconstriction (Euler–Liljestrand reflex) shows potentially reversible perfusion and ventilation impairment (Figure 4) (30). The necessity of radiation-free diagnosis using MRI is particularly significant given the potentially high cumulative lifetime radiation exposure of cystic fibrosis patients. At present, MRI is being used for the first time in a German multicenter study in the framework of the neonatal cystic fibrosis screening program, providing a secondary surrogate endpoint for preventive treatment strategies.
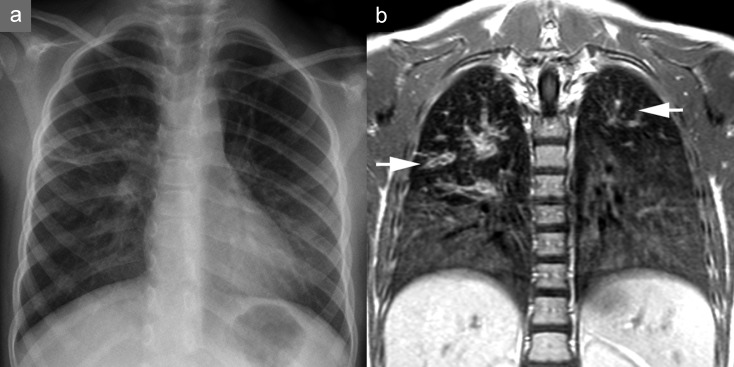
Figure 4.
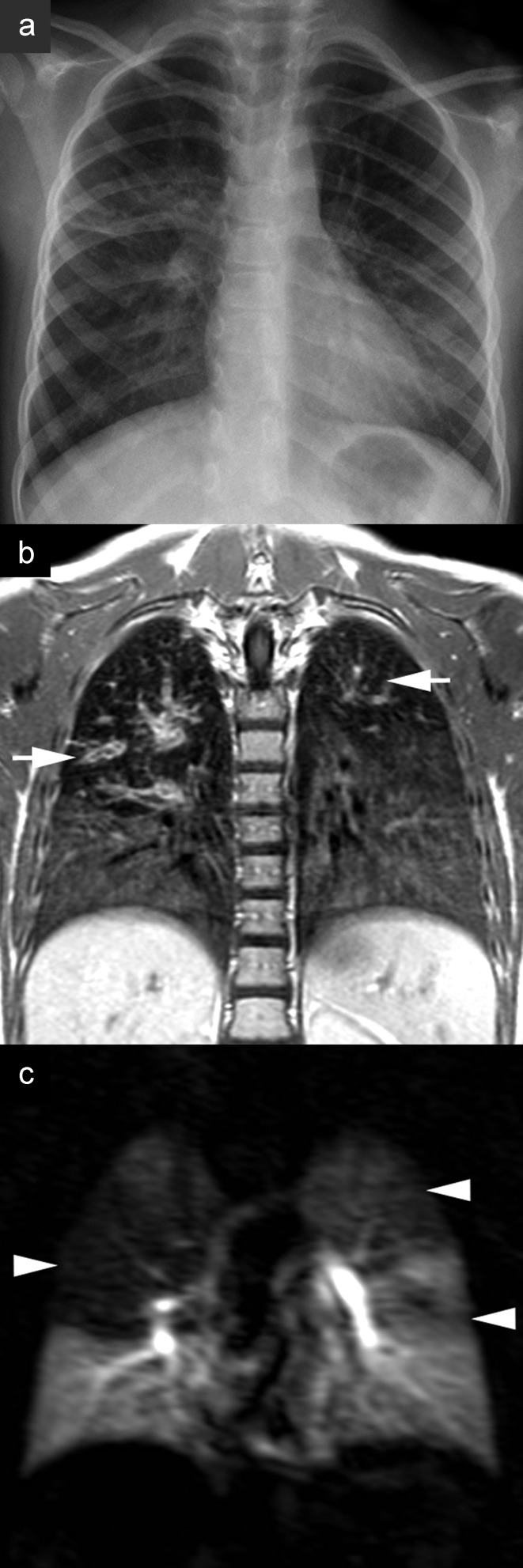
Magnetic resonance imaging (MRI) in cystic fibrosis. A 7-year-old girl in long-term treatment for cystic fibrosis at a pediatric pneumology center. a) Chest radiograph. b) MRI on the same day; the patient was sedated but free breathing. The T1-weighted contrast studies show clear bronchial wall thickening and varicose bronchiectasis, most pronounced in the upper lobes (arrows), and reduced signal apically compared to basally
In patients with smoking-related COPD, the guideline states that at first diagnosis chest radiography should be carried out as the initial study (32). To investigate the indication for endoscopic or operative treatment of severe pulmonary emphysema, and to plan these, thin-slice, non-contrast MDCT is recommended (32). This allows the severity and distribution of the emphysema to be quantified and the integrity of the lobar fissures to be determined (Figure 5) (33, 34). MDCT is also desirable when bronchial carcinoma is suspected (35). The question of whether functional MRI can yield clinically relevant functional information in COPD patients is currently being investigated in a nationwide multicenter study in Germany (“COSYCONET”) (www.asconet.net).
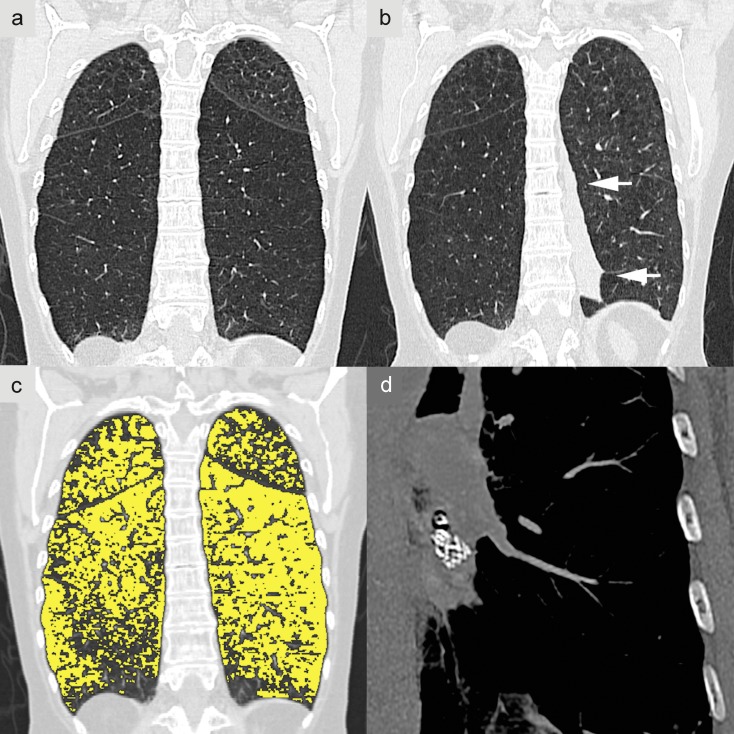
Figure 5.
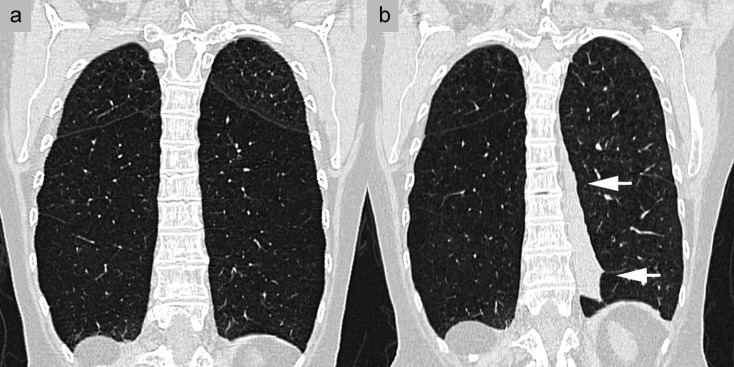
Phenotyping in chronic obstructive pulmonary disease (COPD). A 60-year-old woman with a long history of nicotine abuse. a) Multidetector computed tomography (MDCT) without contrast administration and with coronary reconstruction, showing very severe centrilobular pulmonary emphysema, most pronounced in the lower lobe. The left lower lobe, with an intact lobar fissure, was identified as the target structure for endoscopic pulmonary volume reduction. b) Outcome 3 months after the intervention, with atelectasis of treated segments 8–10 on the left side (arrows)
Diffuse interstitial lung disease—pulmonary fibrosis
In the diagnosis of idiopathic pulmonary fibrosis (IPF), since 2011 in parallel with the revision of international and national guidelines, the single gold standard—histology—has been abandoned in favor of interdisciplinary diagnostic procedures (pneumology, radiology, and pathology) mainly based on the predominant pattern on MDCT (non-contrast, slice thickness = 2 mm) (36, 37). The pattern of usual interstitial pneumonia (UIP) with a honeycomb structure and traction bronchiectasis dominating in the dorsobasal periphery is the main indicator. If this is seen together with typical clinical presentation and other causes are ruled out, IPF can be reliably diagnosed without histological confirmation.
Key Messages.
Chest radiography is the first diagnostic step in patients with pneumonia, cancer, and chronic obstructive pulmonary disease (COPD).
Among the subsequent targeted radiological and pneumological diagnostic techniques that proceed according to risk and clinical significance, and are also used for treatment planning and monitoring, tomographic imaging techniques (CT and MRI) predominate.
CT should be carried out in patients with tumors, acute pulmonary embolism, pulmonary hypertension, pulmonary fibrosis, and advanced COPD, and in at-risk patients with pneumonia.
MRI of the lungs yield clinically relevant extra information and avoids radiation exposure. It is most clinically useful in diagnosing cystic fibrosis, acute pulmonary embolism, pulmonary hypertension, and bronchial carcinoma.
The positive predictive value (PPV) for diagnosing community-acquired pneumonia by chest radiography is 27% in comparison with CT as the gold standard.
For lymph node staging in bronchial carcinoma, MRI achieves a PPV of 88% and PET/CT a PPV of 79% compared to histology.
eFigure 1.
Diagnosis of infiltrate in an immunosuppressed patient. A 34-year-old woman after high-dose chemotherapy for B-cell lymphoma with neutropenic fever. a) Axial non-contrast computed tomography shows extensive peribronchial infiltrates (arrowheads). b) The infiltrate morphology is shown identically by non-contrast T2-weighted magnetic resonance imaging
eFigure 2.
Magnetic resonance imaging (MRI) of pulmonary embolism. A 23-year-old woman with a 2-week history of respiratory infection; current symptoms include hemoptysis, exercise dyspnea, and chest pain on breathing. Axial MRI without contrast administration shows central and peripheral filling defects, some of them partial, in the pulmonary artery (white arrows), corresponding to extensive pulmonary artery embolism. b) Dilatation of the right atrium and ventricle (*), signs of right heart strain. In addition, infarction pneumonia of the right upper lobe accompanied by pleural effusion (black arrow)
eFigure 3.
Magnetic resonance imaging (MRI) in cystic fibrosis. A 7-year-old girl in long-term treatment for cystic fibrosis at a pediatric pneumology center. a) Chest radiograph.
b) MRI on the same day; the patient was sedated but free breathing. The T1-weighted contrast studies show clear bronchial wall thickening and varicose bronchiectasis, most pronounced in the upper lobes (arrows), and reduced signal apically compared to basally.
c) Perfusion imaging subtraction map shows the corresponding perfusion defects of the upper fields in both lungs (arrowheads)
eFigure 4.
Phenotyping in chronic obstructive pulmonary disease (COPD). A 60-year-old woman with a long history of nicotine abuse. a) Multidetector computed tomography (MDCT) without contrast administration and with coronal reconstruction, showing very marked centrilobular pulmonary emphysema, most pronounced in the lower lobe. The left lower lobe, with an intact lobar fissure, was identified as the target structure for endoscopic pulmonary volume reduction. b) Outcome 3 months after the intervention, with atelectasis of treated segments 8–10 on the left side (arrows). c) Results of densitometry performed as image postprocessing (Oliver Weinheimer, Heidelberg) with emphysema map overlaid on the MDCT image. The emphysema (48% of lung volume) is colored yellow. d) Visualization of a valve at the take-off of the S9/10 bronchus
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
This work was supported by the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung) (82DZL00401, 82DZL00402, 82DZL00404).
Footnotes
Conflict of interest statement
Dr. Wielpütz has received lecture fees from Activaero GmbH, Gmünden.
Professor Heussel owns shares in Stada and GSK, and patent shares in Method and Device for Representing the Microstructure of the Lungs. IPC8, Class: AA61B5055FI, PAN: 20080208038. He has received consultancy fees from Schering-Plough, Pfizer, Basilea, Boehringer Ingelheim, Novartis, Roche, Astellas, Gilead, MSD, Lilly, and Intermune. He has received research funding (third-party account) from Siemens, Pfizer, and MeVis. He has received lecture fees from Gilead, Essex, Schering-Plough, AstraZeneca, Lilly, Roche, MSD, Pfizer, Bracco, MEDA Pharma, Intermune, Chiesi, Siemens, Covidien, Pierre Fabre, Boehringer Ingelheim, Grifols, and Novartis.
Professor Kauczor has received consultancy fees from Siemens. He has received lecture fees from Boehringer Ingelheim, Bracco, Siemens, and Bayer.
Professor Herth declares that no conflict of interest exists.
References
- 1.Biederer J, Wildberger J, Bolte H, et al. Protokollempfehlungen für die Computertomographie der Lunge: Konsensus der Arbeitsgemeinschaft Thoraxdiagnostik der DRG. Rofo. 2008;180:471–479. [Google Scholar]
- 2.Ko JP, Brandman S, Stember J, Naidich DP. Dual-energy computed tomography: Concepts, performance, and thoracic applications. J Thorac Imaging. 2012;27:7–22. doi: 10.1097/RTI.0b013e31823fe0e9. [DOI] [PubMed] [Google Scholar]
- 3.Wielputz M, Kauczor HU. MRI of the lung: State of the art. Diagn Interv Radiol. 2012;18:344–353. doi: 10.4261/1305-3825.DIR.5365-11.0. [DOI] [PubMed] [Google Scholar]
- 4.Höffken G, Lorenz J, Kern W, et al. Epidemiologie, Diagnostik, antimikrobielle Therapie und Management von erwachsenen Patienten mit ambulant erworbenen unteren Atemwegsinfektionen sowie ambulant erworbener Pneumonie-update 2009. Pneumologie. 2009;63:e1–e68. doi: 10.1055/s-0029-1215037. [DOI] [PubMed] [Google Scholar]
- 5.Dalhoff K, Abele-Horn M, Andreas S, et al. Epidemiologie, Diagnostik und Therapie erwachsener Patienten mit nosokomialer Pneumonie. Pneumologie. 2012;66:707–765. doi: 10.1055/s-0032-1325924. [DOI] [PubMed] [Google Scholar]
- 6.Self WH, Courtney DM, McNaughton CD, Wunderink RG, Kline JA. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: Implications for diagnosing pneumonia. The American Journal of Emergency Medicine. 2013;31:401–405. doi: 10.1016/j.ajem.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eibel R, Herzog P, Dietrich O, et al. Pulmonary abnormalities in immunocompromised patients: Comparative detection with parallel acquisition MR imaging and thin-section helical CT. Radiology. 2006;241:880–891. doi: 10.1148/radiol.2413042056. [DOI] [PubMed] [Google Scholar]
- 8.Goeckenjan G, Sitter H, Thomas M, et al. Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms. Pneumologie. 2010;64:e1–e164. doi: 10.1055/s-0029-1243837. [DOI] [PubMed] [Google Scholar]
- 9.Seo JS, Kim YJ, Choi BW, Choe KO. Usefulness of magnetic resonance imaging for evaluation of cardiovascular invasion: Evaluation of sliding motion between thoracic mass and adjacent structures on cine MR images. J Magn Reson Imaging. 2005;22:234–241. doi: 10.1002/jmri.20378. [DOI] [PubMed] [Google Scholar]
- 10.Ohno Y, Koyama H, Nogami M, et al. Stir turbo SE MR imaging vs. Coregistered FDG-PET/CT: Quantitative and qualitative assessment of N-stage in non-small-cell lung cancer patients. J Magn Reson Imaging. 2007;26:1071–1080. doi: 10.1002/jmri.21106. [DOI] [PubMed] [Google Scholar]
- 11.Gdeedo A, van Schil P, Corthouts B, van Mieghem F, van Meerbeeck J, van Marck E. Prospective evaluation of computed tomography and mediastinoscopy in mediastinal lymph node staging. Eur Respir J. 1997;10:1547–1551. doi: 10.1183/09031936.97.10071547. [DOI] [PubMed] [Google Scholar]
- 12.Ohno Y, Koyama H, Nogami M, et al. Whole-body MR imaging vs. FDG-PET: Comparison of accuracy of M-stage diagnosis for lung cancer patients. J Magn Reson Imaging. 2007;26:498–509. doi: 10.1002/jmri.21031. [DOI] [PubMed] [Google Scholar]
- 13.Eddy DM. Screening for lung cancer. Ann Intern Med. 1989;111:232–237. doi: 10.7326/0003-4819-111-3-232. [DOI] [PubMed] [Google Scholar]
- 14.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogelmeier C, Worth H, Pfeifer M, et al. Gemeinsame Stellungnahme der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin und der Deutschen Röntgengesellschaft zur Lungenkrebsfrüherkennung mit Niedrigdosis-CT. Pneumologie. 2011;65:5–6. doi: 10.1055/s-0030-1256112. [DOI] [PubMed] [Google Scholar]
- 16.Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol. 2012;138:1475–1486. doi: 10.1007/s00432-012-1228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fachgesellschaften AdWM. Diagnostik und Therapie der Venenthrombose und der Lungenembolie. AWMF-Leitlinien-Register Nr. 065/002. 2010 [Google Scholar]
- 18.Schellhaaß A, Walther A, Konstantinides S, Böttiger BW. The Diagnosis and treatment of acute pulmonary embolis. Dtsch Arztebl Int. 2010;107:589–595. doi: 10.3238/arztebl.2010.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein PD, Chenevert TL, Fowler SE, et al. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: A multicenter prospective study (PIOPED III) Ann Intern Med. 2010;152 434(43):W142–W143. doi: 10.1059/0003-4819-152-7-201004060-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biederer J, Mirsadraee S, Beer M, et al. MRI of the lung (3/3)-Current applications and future perspectives. Insights Imaging. 2012;3:373–386. doi: 10.1007/s13244-011-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeper M, Ghofrani H, Gorenflo M, Grünig E, Rosenkranz S, Schranz D. Diagnostik und Therapie der pulmonalen Hypertonie. Der Kardiologe. 2010;4:189–207. doi: 10.1055/s-0029-1244112. [DOI] [PubMed] [Google Scholar]
- 22.Wilkens H, Lang I, Behr J, et al. Chronisch thromboembolische pulmonale Hypertonie. Dtsch Med Wochenschr. 2010;135:125–130. doi: 10.1055/s-0030-1263319. [DOI] [PubMed] [Google Scholar]
- 23.Rajaram S, Swift AJ, Capener D, et al. Diagnostic accuracy of contrast-enhanced MR angiography and unenhanced proton MR imaging compared with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Eur Radiol. 2012;22:310–317. doi: 10.1007/s00330-011-2252-x. [DOI] [PubMed] [Google Scholar]
- 24.Kreitner KF, Ley S, Kauczor HU, et al. Chronic thromboembolic pulmonary hypertension: Pre- and postoperative assessment with breath-hold MR imaging techniques. Radiology. 2004;232:535–543. doi: 10.1148/radiol.2322030945. [DOI] [PubMed] [Google Scholar]
- 25.Schiebler ML, Bhalla S, Runo J, et al. Magnetic resonance and computed tomography imaging of the structural and functional changes of pulmonary arterial hypertension. J Thorac Imaging. 2013;28:178–193. doi: 10.1097/RTI.0b013e31828d5c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 27.Muller FM, Bend J, Rietschel E, et al. S3-Leitlinie „Lungenerkrankung bei Mukoviszidose“, Modul 1: Diagnostik und Therapie nach dem ersten Nachweis von Pseudomonas aeruginosa. 2013 [Google Scholar]
- 28.Demirkazýk FB, Arýyürek OM, Özçelik U, Göçmen A, Hassanabad HK, Kiper N. High resolution CT in children with cystic fibrosis: Correlation with pulmonary functions and radiographic scores. European Journal of Radiology. 2001;37:54–59. doi: 10.1016/s0720-048x(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 29.Sly PD, Gangell CL, Chen L, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 30.Wielputz MO, Eichinger M, Puderbach M. Magnetic resonance imaging of cystic fibrosis lung disease. J Thorac Imaging. 2013;28:151–159. doi: 10.1097/RTI.0b013e31828d40d4. [DOI] [PubMed] [Google Scholar]
- 31.Puderbach M, Eichinger M, Haeselbarth J, et al. Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients: Comparison to thin-section CT and chest x-ray. Invest Radiol. 2007;42:715–725. doi: 10.1097/RLI.0b013e318074fd81. [DOI] [PubMed] [Google Scholar]
- 32.Vogelmeier C, Buhl R, Criee C, et al. Leitlinie der Deutschen Atemwegsliga und der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin zur Diagnostik und Therapie von Patienten mit chronisch obstruktiver Bronchitis und Lungenemphysem (COPD) Pneumologie. 2007;61:e1–e40. doi: 10.1055/s-2007-959200. [DOI] [PubMed] [Google Scholar]
- 33.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363 1233(44 82):2365–2370. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 34.Koenigkam-Santos M, de Paula WD, Owsijewitsch M, et al. Incomplete pulmonary fissures evaluated by volumetric thin-section CT: Semi-quantitative evaluation for small fissure gaps identification, description of prevalence and severity of fissural defects. Eur J Radiol. 2013;82:2365–2370. doi: 10.1016/j.ejrad.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 35.Kardos P, Berck H, Fuchs K, et al. Leitlinie der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin zur Diagnostik und Therapie von erwachsenen Patienten mit akutem und chronischem Husten. Pneumologie. 2010;64:336–373. doi: 10.1055/s-0029-1244083. [DOI] [PubMed] [Google Scholar]
- 36.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behr J, Günther A, Ammenwerth W, et al. S2K-Leitlinie zur Diagnostik und Therapie der idiopathischen Lungenfibrose. Pneumologie. 2013;67:81–111. doi: 10.1055/s-0032-1326009. [DOI] [PubMed] [Google Scholar]
- 38.Mettler FA, Jr., Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 39.Bundesamt für Strahlenschutz. www.bfs.de/en/ion/medizin/Medizin.pdf. (Stand 12/2013) Strahlenexposition durch Medizinische Maßnahmen. (last accessed on 17 January 2014) [Google Scholar]
- 40.Statistisches Bundesamt. www.gbe-bund.de/gbe10/owards.prc_show_pdf?p_id=16204&p_sprache=d&p_uid=&p_aid=&p_lfd_nr=1. Gesundheit - Fallpauschalenbezogene Krankenhausstatistik (DRG-Statistik), Operationen und Prozeduren der vollstationären Patientinnen und Patienten in Krankenhäusern. (last accessed on 24 October 2013) [Google Scholar]