Abstract
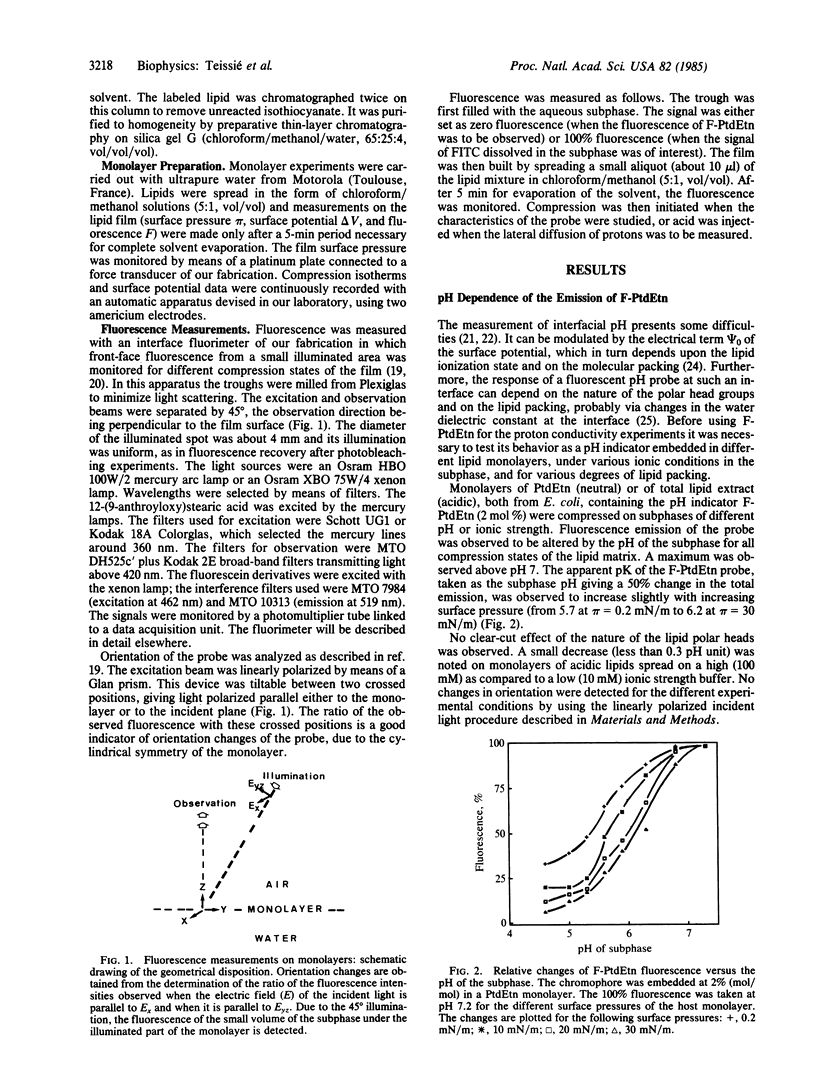
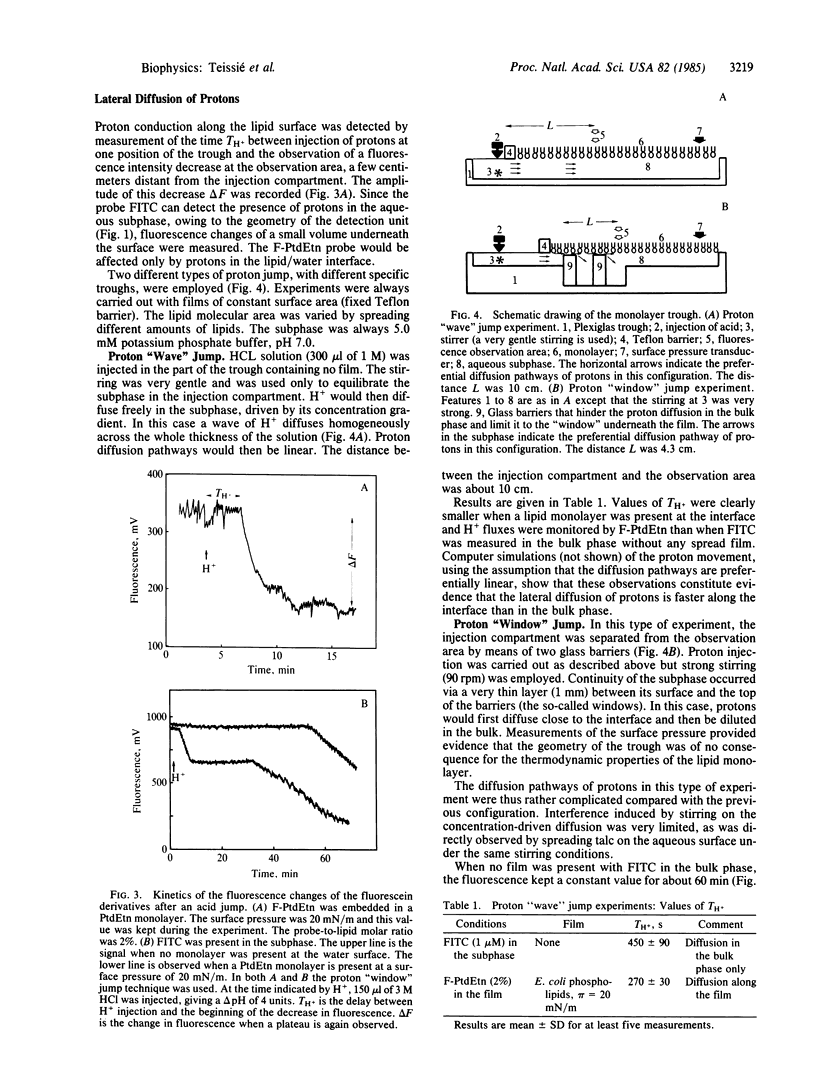
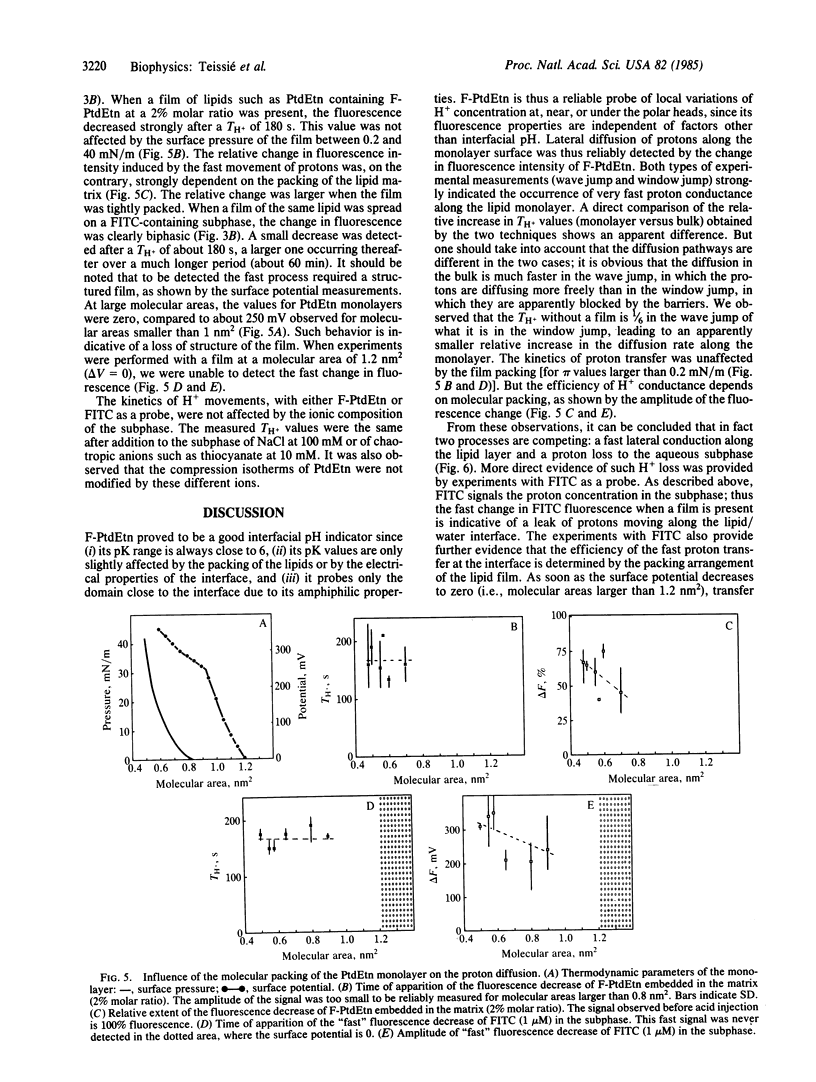
Movements of H+ along the polar heads of phospholipids spread in monolayers were compared to movements of H+ in the aqueous subphase. The probe for detecting H+ movement along the monolayer was a pH-sensitive fluorescein chromophore covalently bound to the head group of phosphatidylethanolamine. The behavior of this probe was not affected by the electrical properties of the lipid/water interface. Lateral diffusion of H+ along the phospholipid/water interface was then studied by acid-jump experiments in which advantage was taken of the large size of the monolayer. H+ was injected a few centimeters away from the probe observation area. The time needed for H+ diffusion to the probe was monitored by the change in the fluorescence signal, fluorescein being nonfluorescent in an acid medium. Diffusion of H+ in the bulk phase was monitored by the fluorescence change of water-soluble fluorescein isothiocyanate. Diffusion along the lipid monolayer was found to be 20 times faster than in the bulk water phase and required a structured monolayer in order to occur, as revealed by variation of the molecular area occupied by the lipid molecules. The molecular basis of rapid H+ transfer along the lipid monolayer may be the existence of a hydrogen-bond network along the polar heads, capable of supporting a rapid "hop and turn" of H+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausländer W., Junge W. The electric generator in the photosynthesis of green plants. II. Kinetic correlation between protolytic reactions and redox reactions. Biochim Biophys Acta. 1974 Aug 23;357(2):285–298. doi: 10.1016/0005-2728(74)90067-x. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Marvin D. A. A model for membrane transport through alpha-helical protein pores. J Theor Biol. 1978 May 8;72(1):9–16. doi: 10.1016/0022-5193(78)90015-2. [DOI] [PubMed] [Google Scholar]
- Eibl H., Woolley P. Electrostatic interactions at charged lipid membranes. Hydrogen bonds in lipid membrane surfaces. Biophys Chem. 1979 Nov;10(3-4):261–271. doi: 10.1016/0301-4622(79)85015-2. [DOI] [PubMed] [Google Scholar]
- Fromherz P. A new method for investigation of lipid assemblies with a lipoid pH indicator in monomolecular films. Biochim Biophys Acta. 1973 Oct 11;323(2):326–334. doi: 10.1016/0005-2736(73)90155-7. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Relationship between oxygen-induced proton efflux and membrane energization in cells of Escherichia coli. J Biol Chem. 1977 Aug 25;252(16):5875–5882. [PubMed] [Google Scholar]
- Gould J. M. Respiration-linked proton transport, changes in external pH, and membrane energization in cells of Escherichia coli. J Bacteriol. 1979 Apr;138(1):176–184. doi: 10.1128/jb.138.1.176-184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Krulwich T. A. Bioenergetic problems of alkalophilic bacteria. Biochem Soc Trans. 1984 Jun;12(3):411–412. doi: 10.1042/bst0120411. [DOI] [PubMed] [Google Scholar]
- Haines T. H. Anionic lipid headgroups as a proton-conducting pathway along the surface of membranes: a hypothesis. Proc Natl Acad Sci U S A. 1983 Jan;80(1):160–164. doi: 10.1073/pnas.80.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D. B. On the functional proton current pathway of electron transport phosphorylation. An electrodic view. Biochim Biophys Acta. 1979 Jul 3;549(1):55–99. doi: 10.1016/0304-4173(79)90018-1. [DOI] [PubMed] [Google Scholar]
- Lakhdar-Ghazal F., Tichadou J. L., Tocanne J. F. Effect of pH and monovalent cations on the ionization state of phosphatidylglycerol in monolayers. An experimental (surface potential) and theoretical (Gouy-Chapman) approach. Eur J Biochem. 1983 Aug 15;134(3):531–537. doi: 10.1111/j.1432-1033.1983.tb07599.x. [DOI] [PubMed] [Google Scholar]
- Lange Y., Ralph E. K., Redfield A. G. Observation of the phosphatidyl ethanolamine amino proton magnetic resonance in phospholipid vesicles: inside/outside ratios and proton transport. Biochem Biophys Res Commun. 1975 Feb 17;62(4):891–894. doi: 10.1016/0006-291x(75)90406-4. [DOI] [PubMed] [Google Scholar]
- Melandri B. A., Venturoli G., de Santis A., Baccarini-Melandri A. The induction kinetics of bacterial photophosphorylation. Threshold effects by the phosphate potential and correlation with the amplitude of the carotenoid absorption band shift. Biochim Biophys Acta. 1980 Aug 5;592(1):38–52. doi: 10.1016/0005-2728(80)90112-7. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Tristram-Nagle S. Hydrogen bonded chain mechanisms for proton conduction and proton pumping. J Membr Biol. 1983;74(1):1–14. doi: 10.1007/BF01870590. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Dilley R. A., Good N. E. Photophosphorylation as a function of illumination time. II. Effects of permeant buffers. Biochim Biophys Acta. 1976 Oct 13;449(1):108–124. doi: 10.1016/0005-2728(76)90011-6. [DOI] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Pagano R. E. Insertion of fluorescent phospholipids into the plasma membrane of a mammalian cell. J Biol Chem. 1980 Jun 10;255(11):5404–5410. [PubMed] [Google Scholar]
- Teissie J. A fluorescence study with polarised incident light of the compression of phospholipid monolayers spread at the air/water interface: orientation processes in the glycerol region. Chem Phys Lipids. 1979 Dec;25(4):357–368. doi: 10.1016/0009-3084(79)90074-4. [DOI] [PubMed] [Google Scholar]
- Teissie J. Interaction of cytochrome c with phospholipid monolayers. Orientation and penetration of protein as functions of the packing density of film, nature of the phospholipids, and ionic content of the aqueous phase. Biochemistry. 1981 Mar 17;20(6):1554–1560. doi: 10.1021/bi00509a023. [DOI] [PubMed] [Google Scholar]
- Teissie J., Tocanne J. F., Baudras A. A fluorescence approach of the determination of translational diffusion coefficients of lipids in phospholipid monolayer at the air-water interface. Eur J Biochem. 1978 Feb 1;83(1):77–85. doi: 10.1111/j.1432-1033.1978.tb12070.x. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Melandri B. A., Venturoli G., Azzone G. F., Kell D. B. Mosaic protonic coupling hypothesis for free energy transduction. FEBS Lett. 1984 Jan 2;165(1):1–5. doi: 10.1016/0014-5793(84)80002-2. [DOI] [PubMed] [Google Scholar]
- Williams R. J. The multifarious couplings of energy transduction. Biochim Biophys Acta. 1978 Sep 21;505(1):1–44. doi: 10.1016/0304-4173(78)90007-1. [DOI] [PubMed] [Google Scholar]



