Abstract
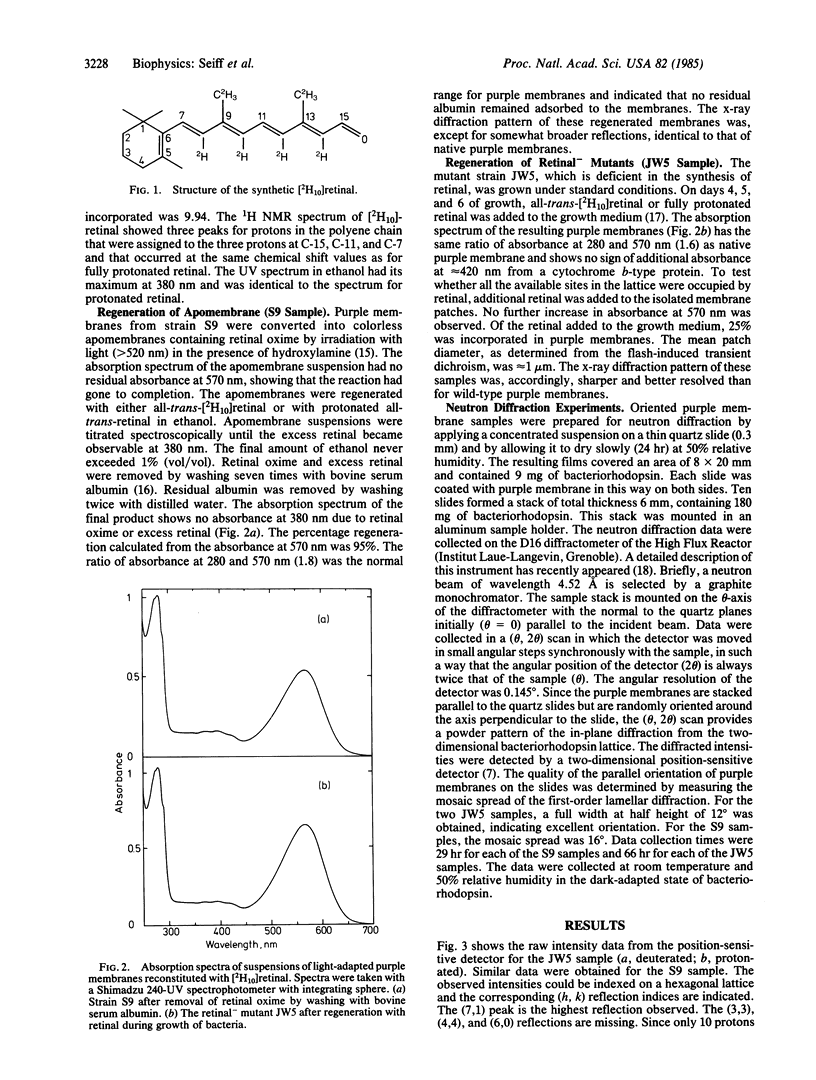
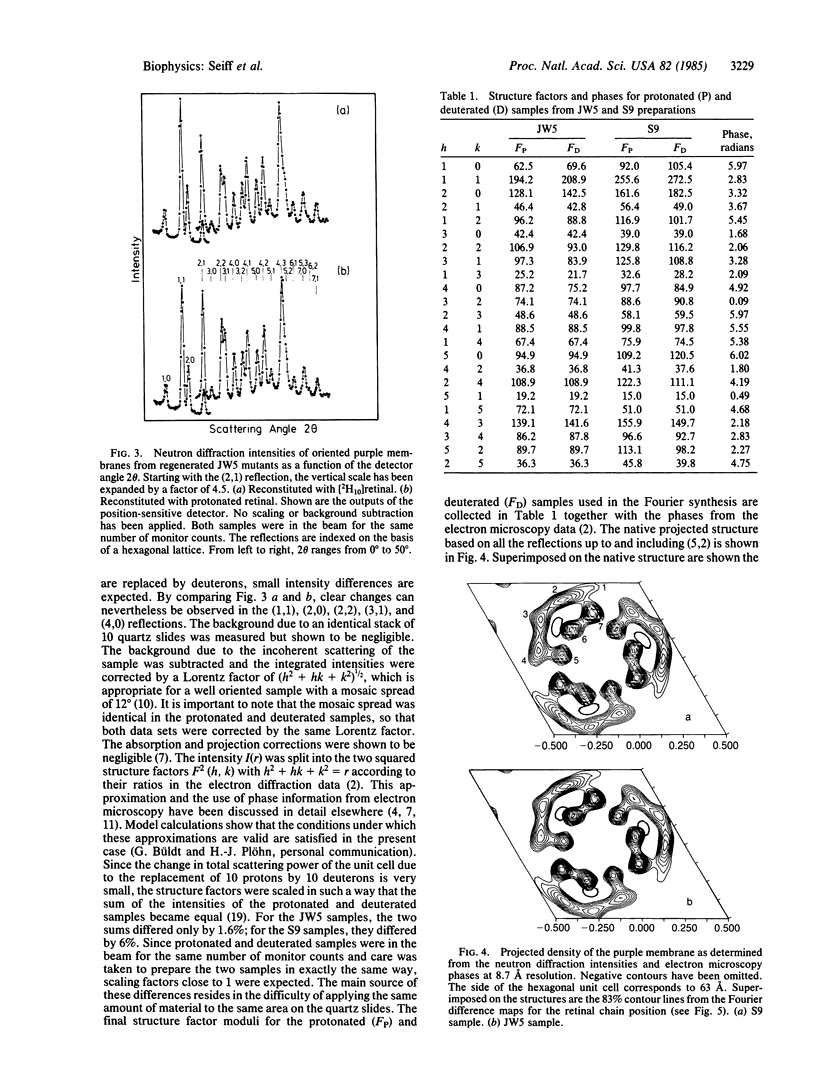
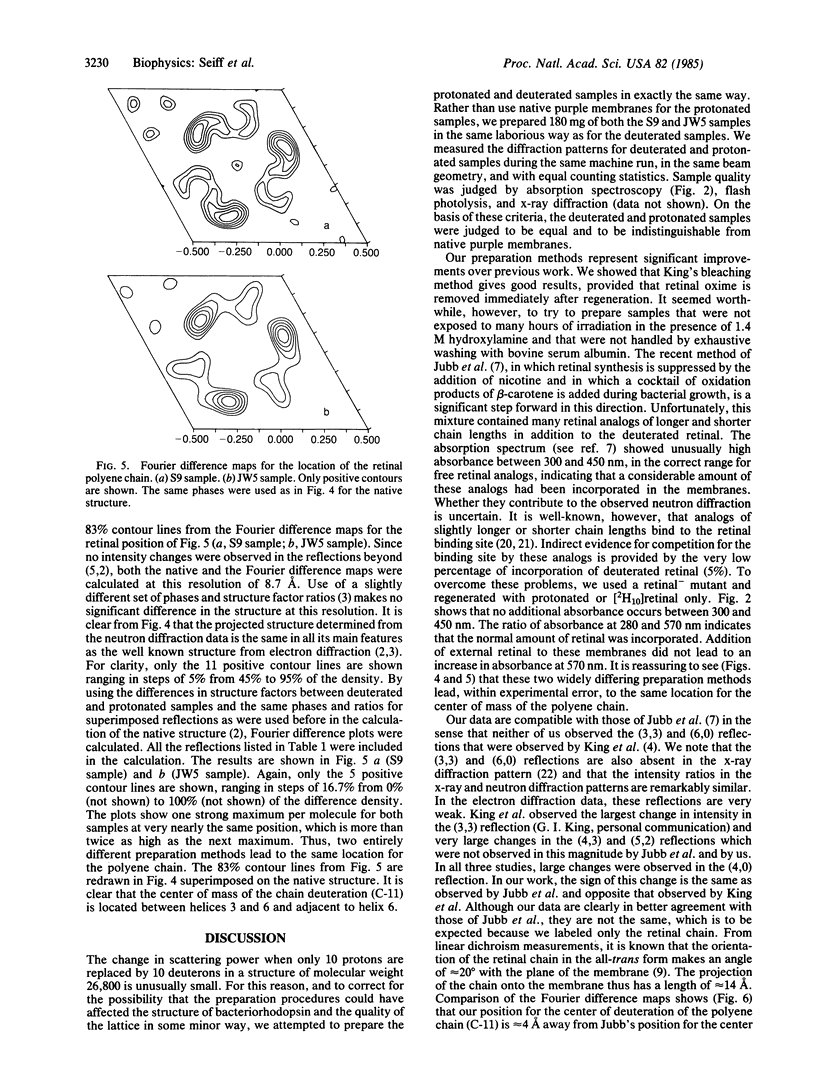
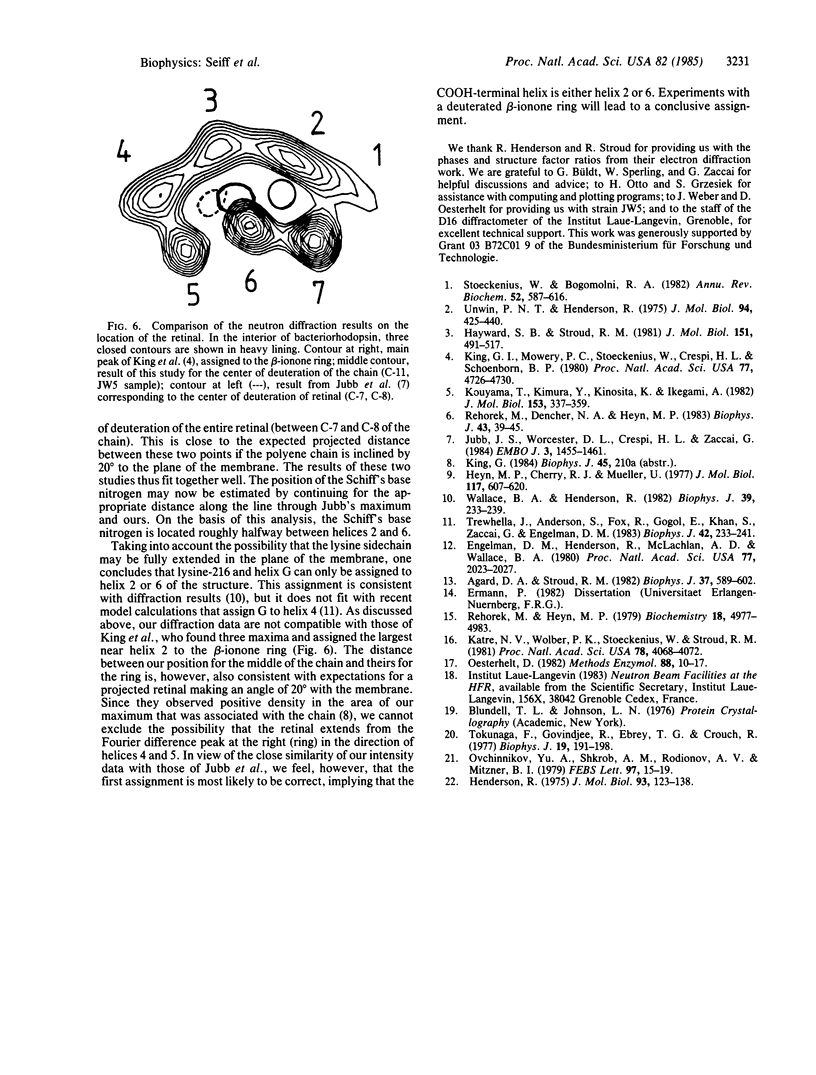
We report on the location of the chain part of the retinylidene chromophore in the projected density of bacteriorhodopsin as determined by neutron diffraction from the two-dimensional purple membrane lattice. For this purpose, partially deuterated retinal was synthesized containing 10 deuterons at positions C-8, C-10, C-12, C-14, C-19(3), and C-20(3) of the polyene chain. Two sets of dark-adapted samples were prepared in entirely different ways: (i) Deuterated retinal was incorporated biosynthetically during growth of the bacteria by using the mutant JW5, which is deficient in the synthesis of retinal. (ii) The chromophore was converted to retinal oxime, the resulting colorless apomembrane was regenerated with deuterated retinal, and the residual retinal oxime was removed by washing with bovine serum albumin. Characterization of these samples by x-ray diffraction, absorption, and flash spectroscopy showed that they were identical to native purple membrane samples as judged by these criteria. Fourier difference maps were calculated from the differences in inplane diffraction from the deuterated membranes and from protonated samples that were prepared in exactly the same way. At 8.7 A resolution, both maps show a single major peak at the same position with the center of mass of the labeled part of the chain (C-11) between helices 6 and 3 but closer to helix 6. It appears likely that the COOH-terminal helix G, to which retinal is attached at lysine-216, is either helix 2 or 6.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Stroud R. M. Linking regions between helices in bacteriorhodopsin revealed. Biophys J. 1982 Mar;37(3):589–602. [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Jubb J. S., Worcester D. L., Crespi H. L., Zaccaï G. Retinal location in purple membrane of Halobacterium halobium: a neutron diffraction study of membranes labelled in vivo with deuterated retinal. EMBO J. 1984 Jul;3(7):1455–1461. doi: 10.1002/j.1460-2075.1984.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katre N. V., Wolber P. K., Stoeckenius W., Stroud R. M. Attachment site(s) of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4068–4072. doi: 10.1073/pnas.78.7.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. I., Mowery P. C., Stoeckenius W., Crespi H. L., Schoenborn B. P. Location of the chromophore in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4726–4730. doi: 10.1073/pnas.77.8.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama T., Kimura Y., Kinosita K., Jr, Ikegami A. Location and orientation of the chromophore in bacteriorhodopsin. Analysis by fluorescence energy transfer. J Mol Biol. 1981 Dec 5;153(2):337–359. doi: 10.1016/0022-2836(81)90282-5. [DOI] [PubMed] [Google Scholar]
- Rehorek M., Dencher N. A., Heyn M. P. Fluorescence energy transfer from diphenylhexatriene to bacteriorhodopsin in lipid vesicles. Biophys J. 1983 Jul;43(1):39–45. doi: 10.1016/S0006-3495(83)84321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehorek M., Heyn M. P. Binding of all-trans-retinal to the purple membrane. Evidence for cooperativity and determination of the extinction coefficient. Biochemistry. 1979 Oct 30;18(22):4977–4983. doi: 10.1021/bi00589a027. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Govindjee R., Ebrey T. G. Synthetic pigment analogues of the purple membrane protein. Biophys J. 1977 Aug;19(2):191–198. doi: 10.1016/S0006-3495(77)85580-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewhella J., Anderson S., Fox R., Gogol E., Khan S., Engelman D., Zaccai G. Assignment of segments of the bacteriorhodopsin sequence to positions in the structural map. Biophys J. 1983 Jun;42(3):233–241. doi: 10.1016/S0006-3495(83)84391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Wallace B. A., Henderson R. Location of the carboxyl terminus of bacteriorhodopsin in purple membrane. Biophys J. 1982 Sep;39(3):233–239. doi: 10.1016/S0006-3495(82)84513-X. [DOI] [PMC free article] [PubMed] [Google Scholar]



