Abstract
Introduction:
We sought to determine the effects of brief exposures to low concentrations of tobacco secondhand smoke (SHS) on arterial flow-mediated dilation (FMD, a nitric oxide-dependent measure of vascular endothelial function), in a controlled animal model never before exposed to smoke. In humans, SHS exposure for 30min impairs FMD. It is important to gain a better understanding of the acute effects of exposure to SHS at low concentrations and for brief periods of time.
Methods:
We measured changes in FMD in rats exposed to a range of real-world levels of SHS for durations of 30min, 10min, 1min, and 4 breaths (roughly 15 s).
Results:
We observed a dose-response relationship between SHS particle concentration over 30min and post-exposure impairment of FMD, which was linear through the range typically encountered in smoky restaurants and then saturated at higher concentrations. One min of exposure to SHS at moderate concentrations was sufficient to impair FMD.
Conclusions:
Brief SHS exposure at real-world levels reversibly impairs FMD. Even 1min of SHS exposure can cause reduction of endothelial function.
INTRODUCTION
Even short exposures to low levels of secondhand smoke (SHS) have deleterious effects on health. Smoke-free policies in public places and workplaces have been demonstrated repeatedly to lead to reductions in hospital admissions for myocardial infarction, stroke, and other cardiovascular and respiratory outcomes (Barnoya & Glantz, 2005; Institute of Medicine, 2010; Lightwood & Glantz, 2009; Sargent, Shepard, & Glantz, 2004; Tan & Glantz, 2012). One important and rapid consequence of both active smoking and SHS exposure is the impairment of arterial flow-mediated dilation (FMD), the process by which arteries vasodilate in response to increased fluid shear stress (Flammer et al., 2012; Pyke & Tschakovsky, 2005). The endothelium modulates blood flow to peripheral tissues and the heart by producing nitric oxide (NO) and other factors that lead to vasodilation. Endothelial cells sense fluid shear stress and activate endothelial nitric oxide synthase (eNOS), leading to vasodilation. FMD is quantified clinically by ultrasound as the percent dilation of the brachial artery in response to restoration of blood flow after transient occlusion (Celermajer et al., 1992). FMD is a well-established clinical prognostic indicator of endothelial function that is concordant with other measures of cardiovascular health (Celermajer et al., 1992; Flammer et al., 2012; Nabel, Selwyn, & Ganz, 1990; Widlansky, Gokce, Keaney, & Vita, 2003). Brachial artery FMD correlates with endothelium-dependent vasodilation of the coronary arteries (Anderson et al., 1995) and with a number of adverse cardiovascular outcomes that are increased by cigarette smoke (Yeboah, Crouse, Hsu, Burke, & Herrington, 2007). Smoking and chronic exposure to SHS both impair FMD (Celermajer et al., 1993, 1996). In fact, we and others have previously shown that a 20- to 30-min exposure to SHS is sufficient to temporarily impair FMD in humans (Giannini et al., 2007), even at concentrations found in public places where smoking is permitted (Frey et al., 2012; Heiss et al., 2008a). However, the acute effects of very short (e.g., 1min) exposures have not been examined. It is important, for both public health regulatory policy and personal decision-making, to better understand the effects of exposure to SHS at very low levels or for brief exposure times.
FMD measurement in humans is confounded by genetics, lifestyle, diet, and prior exposure to tobacco. To study how SHS affects endothelial function in a physiologically consistent study population, we used a method that we previously developed to measure FMD by micro-ultrasound in the hindlimbs of living rats (Heiss et al., 2008b). FMD measured in rats is similar to human FMD with respect to factors that impair or improve the response and underlying mechanisms, and has provided us with unprecedented insight into the roles played by mediators of NO bioavailability (Chen et al., 2013; Heiss et al., 2008b).
In this study, we measured the acute effect of SHS exposure at progressively lower concentrations and for extremely short times on FMD. We report that impairment of FMD by 30min of exposure exhibits a dose response relationship through the range of SHS concentrations typically encountered in public, and saturates at higher levels. Notably, impairment of FMD is detected within 1min of exposure to SHS at respirable particle concentrations typical of a smoky restaurant.
METHODS
See Supplementary Material online for more details about the following: Arterial blood gas measurements, FMD, nitroglycerin administration, measurement of cotinine.
Animals
We used Sprague-Dawley rats (Charles River), female, 8–12 weeks old. Experiments were conducted under ketamine/xylazine anesthesia and were terminal. All experiments were approved by the UCSF Institutional Animal Care and Use Committee.
Flow-Mediated Dilation
FMD was measured in living rats as we have described (Heiss et al., 2008b) by a blinded investigator. See Supplementary Material online for details. Briefly, an arterial loop occluder was surgically positioned upstream of the femoral artery, and the artery was occluded for 5min followed by release and reperfusion of the leg. Femoral artery diameter at diastole was measured with a 35 MHz ultrasound transducer (Vevo660, VisualSonics) over 3min. FMD was calculated as % change: (peak diameterpostischemia − diameterbaseline)/diameterbaseline × 100. Rats were kept at 37°C on a thermal blanket throughout to avoid anesthesia-induced hypothermia.
Exposure to Cigarette Smoke
Cigarettes were Marlboro Red brand. The smoking regimen was based on the ISO Standard 3308:2012, one 35ml puff of 2 s duration once/min, using cigarettes of pre-equilibrated humidity. SHS is a mixture of sidestream smoke from the lit end of the cigarette and exhaled mainstream smoke; since most SHS is sidestream smoke, we will use these two terms interchangeably.
An initial smoke exposure experiment was performed using a cigarette smoking machine from Teague Enterprises. SHS was generated from three cigarettes smoked in sequence over 30min, and piped to anesthetized rats after baseline FMD measurement and before post-exposure FMD measurement.
For subsequent experiments, the machine was rebuilt to facilitate lower exposure levels (Supplementary Figure S1 online). The rebuilt machine collects SHS in a Plexiglas chamber. The wall of the SHS collection chamber contains a gasket through which the nose of an anesthetized rat is inserted to breathe the smoky air. A Sidepak AM510 personal aerosol monitor (TSI) calibrated for tobacco smoke particles and excluding those >2.5 µm, monitors the respirable suspended particle (RSP) concentration in the exposure chamber and exhausts back into the chamber. Air in the chamber is mixed with a small fan. Arterial blood gas measurements confirmed that oxygen levels did not decline over the course of the exposure (see Supplementary Methods and Results online).
For each experiment using the modified system, a cigarette was lit and smoked for 3min and extinguished, and particle concentration in the exposure chamber was adjusted until the desired starting concentration was reached. At that time, an individual anesthetized rat, after baseline FMD measurement, was exposed for the specified duration and was then returned to the ultrasound system for post-smoke FMD measurement. As summarized in Figure 1, FMD was measured in all rats before and after exposure, with a ~10min interval between end of exposure and ultrasound reading for technical reasons. FMD was measured a third time to determine recovery 30min later (i.e., 40min after the end of exposure) in all groups except for the 6,000 µg/m3 peak concentration group. (For that group only, recovery was assessed 15min after the initial post-smoke measurement, 25min after the end of exposure, so we did not combine these data with the recovery data from the other groups.)
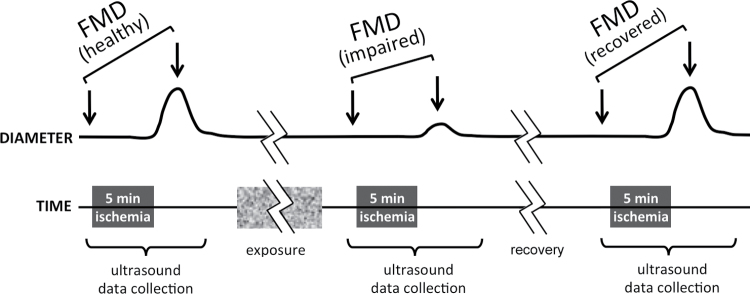
Figure 1.
Experimental design for smoke exposures. The pre-smoke FMD measurement procedure consisted of baseline ultrasound measurement of artery diameter, occlusion of the artery for 5min (ischemia), release of occlusion, and a second ultrasound measurement of peak artery diameter. Diameter returned to baseline level within 5min. This pre-smoke FMD measurement was followed by exposure to smoke or clean air, and then the entire FMD measurement procedure (ultrasound, ischemia, release, ultrasound) was repeated to determine post-smoke FMD roughly 10min after the end of exposure. A smaller post-ischemia peak diameter indicated impaired FMD after SHS exposure. In most experiments, FMD was measured again to document recovery 30min later (i.e., 40min after the end of exposure).
Statistics
For simple comparison of exposure to SHS versus clean air (i.e., Figure 2) and to assess the effects of varying SHS exposure duration at given starting concentrations (Figure 4), we used a repeated measures ANOVA followed by all pairwise multiple comparisons using a Šidák correction using Stata version 12.1 xtmixed with the REML option; sample size associated with SE calculations was the entire sample. For the experiment shown in Figure 4, because complete recovery from impaired FMD was observed within 30min after the initial post-exposure measurement, initial post-exposure was compared with the average of the other two time points via a planned contrast, with pre-exposure and recovery weighted equally (initial post-exposure − [pre-exposure + recovery]/2).
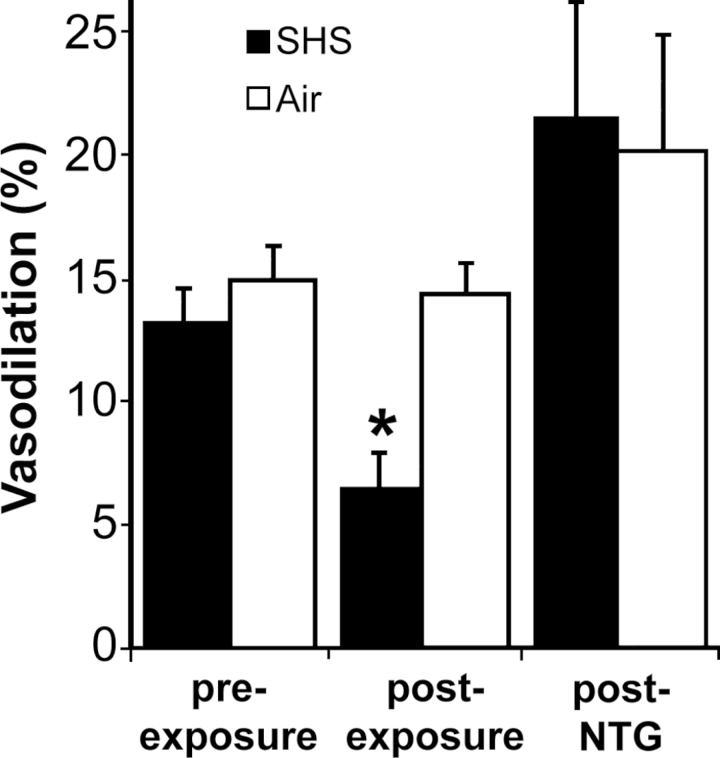
Figure 2.
Impairment of FMD, but not endothelium-independent vasodilation, after 30min of exposure to SHS. FMD was measured before and after 30min of exposure to SHS or clean air (n = 3/group). FMD was reduced after exposure to SHS, but not clean air. Nitroglycerin (NTG) administered immediately after post-exposure FMD measurement confirmed that endothelium-independent vasodilation was not impaired. Bars = SEM; *p < .0005 versus respective pre-exposure.
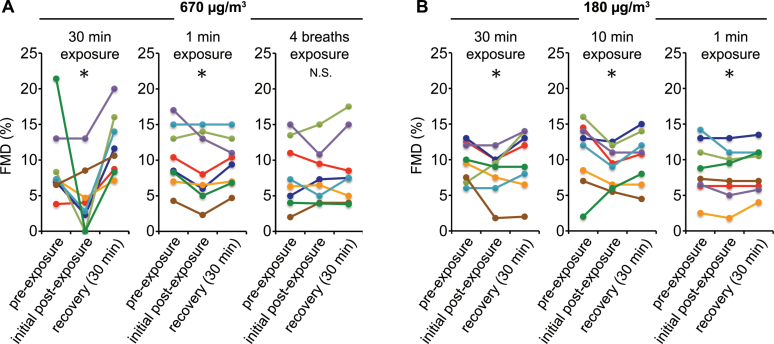
Figure 4.
FMD is impaired by 1min of SHS exposure. RSP levels listed denote starting levels, which did not change appreciably during the 1min experiment. Four breaths is estimated at roughly 15 s. n = 8/group, *Significant impairment compared to average of pre-exposure and recovery.
To identify the dose-response relationship between SHS exposure (constant duration) and FMD impairment (Figure 3), the first step that we took was to conduct a locally weighted scatterplot smoothing (LOWESS) nonparametric regression of FMD 10min after end of exposure (initial post-exposure) as a function of maximum exposure (dose). The LOWESS regression suggested a piecewise linear relationship with a break at a 670 µg/m3 peak exposure. To test whether such a relationship would provide a better description of the data than a linear regression, we fit the data with the two-component linear spline
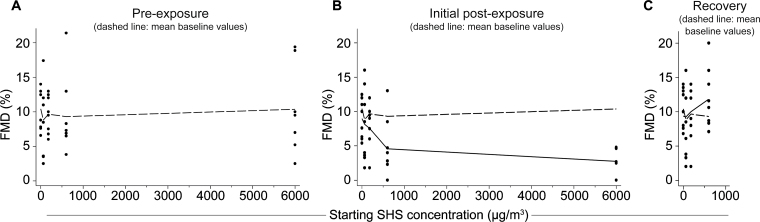
Figure 3.
Dose response of FMD impairment after varying SHS particle concentrations for 30min. Exact values are shown in Supplementary Figure S3 online. (A) Baseline FMD, before exposure to SHS at concentrations starting at 0, 67, 180, 670, and 6,000 µg/m3. Dots represent individual rats; n = 8/group except for n = 7 for 6,000 µg/m3. Dashed line connects mean FMD values at each subsequent SHS exposure level. (B) FMD measured ~10min following 30-min SHS exposure. Solid line is fit using the two-component spline at mean post-exposure FMD levels for each group and dashed line shows mean baseline levels (from panel A). The relationship between SHS concentration and FMD impairment is dose-dependent up to the 670 µg/m3 peak exposure, then the effect appears to saturate (that is, the slope higher than 670 µg/m3 is not significantly different from 0). The seven data points for the “6000” group overlap and are not all distinguishable. (C) Thirty minutes later, FMD has recovered to baseline. In the 670 µg/m3 starting group only, recovery FMD has slightly surpassed the baseline value but this difference was not significant. Solid line is fit using the two-component spline at mean recovery FMD levels for each group and dashed line is mean baseline levels (from panel A).
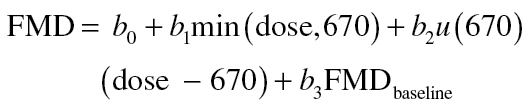 |
where u(670) is the unit step function that takes on a value of 1 at 670 µg/m3 or higher and 0 otherwise. We also included pre-exposure FMD in the equation to allow for rat-to-rat differences in baseline FMD. The spline function provided a significantly better fit to the data than a simple linear regression (p = .03) by likelihood ratio test, so the two-component spline was used to analyze all the data. Calculations were done with Stata procedures mkspline and regress; sample size for SE calculations was the entire sample.
RESULTS
Thirty-Minute Exposure to SHS Impaired FMD
In an initial experiment, we tested the effect of SHS exposure on endothelium-dependent and endothelium-independent vasodilation, using a cigarette smoking system that generates continuous streams of SHS. Baseline FMD was measured in two groups of anesthetized rats (n = 3/group). The rats were then exposed for 30min to clean air or smoke, after which FMD was measured again (for these experiments, we did not control SHS particle concentration or measure serum cotinine). Exposure to SHS led to impaired FMD (post: 6.5% ± 1.3% vs. pre: 13.2% ± 1.4% (SEM); p < .0005), whereas exposure to clean air did not lead to detectable impairment (post: 14.2% ± 1.3% vs. pre: 14.9% ± 1.4%; p > .999; Figure 2). After the post-exposure measurement, we measured vasodilation in response to the endothelium-independent vasodilator nitroglycerin. Nitroglycerin treatment caused vasodilation in excess of pre-exposure FMD (p = .028). The response to nitroglycerin was not impaired by SHS (SHS: 21.6% ± 4.5% vs. clean air: 20.1% ± 4.5%; p > .999).
Exposure Chamber Particle Concentration Kinetics Are Consistent
To achieve greater control over low particle concentrations for subsequent experiments, we modified the smoke generation system as described in the Methods section. Because the modified system requires the cigarette to be extinguished before exposure of the rat, adsorption of smoke particles to surfaces in the exposure chamber causes a progressive decrease in RSP concentration over 30min. We confirmed that the decay curves are consistent between experiments (Supplementary Figure S2 online) and across different starting particle concentrations (data not shown). Thus, the experiments described below list RSP concentrations as starting (peak) concentration; Table 1 shows the corresponding mean over time and the total cumulative exposure for each condition based on the average kinetic curve for each experimental group.
Table 1.
Mean Particle Concentration and Relative Total Exposure (Area Under the Curve) Corresponding to the Starting Particle Concentrations for Each 30-Min Duration Group
| Target starting concentration (µg/m3) | Actual starting concentration (µg/m3) | Mean concentration over time (µg/m3) | Relative total exposure (µg/m3·min) |
|---|---|---|---|
| 6,000 | 5,932±15 | 1,975±1,104 | 51,595 |
| 670 | 674±4.8 | 191±88 | 4,919 |
| 180 | 180a | 55a | 1,435a |
| 67 | 66.4±0.72 | 30±7.3 | 883 |
| 0 | 0b | – | – |
Note. aEstimated value. Due to loss of particle detector data for this set, the mean concentration over time and total exposure values are extrapolated from the target starting concentration based on the relationships in the 6,000 and 670 groups, for reference.
bExposure chamber air sampled over 30min on five separate occasions was measured as 2.9±3.1 µg/m3.
Numbers following ± are SD.
Impairment of FMD Was Dose-Dependent
The RSP concentrations associated with real-world exposure conditions are typically under 1,000 µg/m3 (California Environmental Protection Agency, 2005; Institute of Medicine, 2010), so we measured changes in FMD in groups exposed for 30min to starting levels of 6,000 µg/m3 (comparable to conditions in an unventilated residential room with multiple smokers), 670 µg/m3 (smoky bar conditions), 180 µg/m3 (mid-range of restaurants with smoking), 67 µg/m3 (low end of range in restaurants that allow smoking), and clean air. Figure 3 shows the values for all the rats, with mean values listed in Table 2 and statistical analyses summarized in Table 3.
Table 2.
Mean FMD Values (%) Corresponding to Figure 3
| Starting concentration (µg/m3) | Pre-exposure FMD | Initial post-exposure FMD | Recovery 15min FMD (25min after end of exposure) | Recovery 30min FMD (40min after end of exposure) |
|---|---|---|---|---|
| 6,000 | 10.4±2.5 | 2.7±0.8 | 3.8±1.2 | – |
| 670 | 9.3±2.0 | 4.4±1.6 | – | 12.0±1.6 |
| 180 | 9.7±1.0 | 8.2±1.1 | – | 9.8±1.5 |
| 67 | 8.8±1.9 | 7.8±1.9 | – | 8.0±1.8 |
| 0 | 10.4±1.1 | 8.9±1.0 | – | 10.7±1.0 |
Note. Numbers following ± are SEM.
Table 3.
Regression Analysis: Changes in FMD (%) Associated With 30-Min SHS Exposure
| Pre-exposure | Initial post-exposure | |||
|---|---|---|---|---|
| Effect | p value | Effect | p value | |
| Dose, b 1 | −0.0008±0.0035 | .831 | −0.0062±0.0024 | .015 |
| Incremental effect above 670 µg/m3, b 2 | 0.0002±0.0004 | .647 | −0.0004±0.0003 | .274 |
| Baseline FMD, b 3 | 0.249±0.128 | .060 | ||
| Constant, b 0 | 9.71±1.11 | 6.35±1.50 | ||
| p for regression | .897 | .001 | ||
Note. Statistical analysis refers to Figure 3. Numbers following ± are SE of respective regression coefficients.
Regression analysis showed that there were no significant differences between the baseline FMD levels (Figure 3A) for the different exposure groups (p = .897). FMD exhibited a dose-dependent drop following 30min of SHS smoke exposure (Figure 3B) up to the 670 µg/m3 starting concentration (b1, the slope of the dose-response below 670, is significant, Table 3), then the effect saturated (i.e., b2, the slope of the dose-response above 670, is not significantly different from 0). Thirty minutes after the initial post-exposure measurement, FMD had recovered to baseline for rats exposed to 670 µg/m3 starting SHS and lower (Figure 3C; b1 is not significantly different from 0; Table 3) (FMD changes for individual rats are shown in Supplementary Figure S3 online.) The 6,000 µg/m3 starting SHS group is not included in this analysis of 30-min recovery because we measured recovery at 15min in this group. In each case, higher baseline FMD was associated with higher FMD levels following SHS exposure and recovery, but this effect did not reach conventional statistical significance.
We also measured cotinine levels in serum collected from all groups after the final FMD measurement, but did not detect significant correlations between smoke particle concentration and cotinine level (not shown).
One Minute of SHS Exposure Impaired FMD
We then tested whether FMD was impaired at progressively shorter exposures of 10min, 1min, or 4 breaths (roughly 15 s; Figure 4, Table 4, and Supplementary Table S1 online). Notably, 1min at 670 µg/m3, comparable to smoky restaurant levels, caused a modest but significant impairment of FMD. One minute at 180 µg/m3 also resulted in statistically significant impairment, although the effect was extremely small (see Discussion section). We did not observe significant impairment after only four breaths.
Table 4.
Mean FMD Values (%) Corresponding to Figure 4
| Starting conc. (µg/m3) | Duration (min) | Pre-exposure FMD | Initial post- exposure FMD | Recovery FMD | Overall test (p) | Contrast (p) | FMD difference (SE) |
|---|---|---|---|---|---|---|---|
| 670 | 30 | 9.3±2.0 | 4.4±1.6 | 12.0±1.6 | <.00005 | .001 | −6.3% (1.8%) |
| 670 | 1 | 10.4±1.5 | 8.7±1.7 | 9.7±1.2 | .0141 | .008 | −1.3% (0.5%) |
| 670 | 4 breaths | 8.0±1.6 | 7.8±1.4 | 8.6±1.8 | .4542 | .396 | −0.6% (0.7%) |
| 180 | 30 | 9.7±1.0 | 8.2±1.1 | 9.8±1.5 | .0001 | <.0005 | −1.5% (0.4%) |
| 180 | 10 | 10.9±1.7 | 9.0±1.0 | 10.2±1.3 | .0042 | .003 | −1.6% (0.5%) |
| 180 | 1 | 8.7±1.4 | 8.0±1.3 | 8.6±1.2 | .0018 | .001 | −0.7% (0.2%) |
Note. Numbers following ± are SEM.
DISCUSSION
In this study, we show in a controlled in vivo model that 30min of SHS exposure causes a dose-dependent impairment of NO-dependent endothelial function, and that even 1min of exposure is sufficient to cause significant impairment.
We have developed a clinically-relevant, integrative, physiological rodent ultrasound model of FMD measurement in which hormonal and neural influences are intact (Heiss et al., 2008b), which we have used to study deleterious effects of tobacco SHS at realistic levels. In contrast, classical laboratory-based approaches toward the study of endothelium-dependent vasodilatory function, and its inhibition by factors such as cigarette smoke, have used isolated aortic segments in culture that are pre-constricted and then dilated pharmacologically, and omit most of the relevant physiology underlying vascular endothelial function. Our approach has advantages over human studies, which are clinically relevant but are confounded by many differences in genetics, diet, lifestyle, and previous exposure to SHS; differences that are minimal in laboratory rats. Studies of human exposure, tissue explants, and endothelial cell culture have yielded important insights into endothelial physiology and effects of mainstream tobacco smoke (Barua, Ambrose, Srivastava, DeVoe, & Eales-Reynolds, 2003; Barua et al., 2001; Celermajer et al., 1993, 1996; Hutchison et al., 1998), but they are unable to bridge the gap between physiological context and phenotypic consistency, and are thus limited with respect to conclusions that can be drawn about intact physiology. Our approach accomplishes what clinical and tissue explant studies cannot: that is, study of vascular functional impairment in a model that is both physiological and devoid of excessive subject variability. This rodent model of SHS exposure has shown that even 1min of exposure to realistic levels of SHS can cause decreases in FMD, and will support subsequent studies into the molecular and physiological mechanisms by which SHS affects the endothelium.
Because rats and humans are different species, we cannot draw conclusions about the health effects of specific SHS concentrations in humans based on these rat results. However, it is notable that the range of levels in which changes in smoke concentration exerted differential acute effects on vascular physiology corresponds to real-world SHS exposure conditions. The dose-response curve shown in Figure 3 for 30min exposures is consistent with effects of SHS on endothelium that occur at the lower doses and then saturate. The line breaks at 670 µg/m3 starting (~200 µg/m3 averaged over time) based on the set of exposure conditions that we used; the actual break may be lower. These results support our previous findings that increasing smoke concentration leads to increasing impairment of endothelial function (Frey et al., 2012), and suggest that maximal impairment is reached within the range of real-world SHS concentrations.
The range of exposure times from 30min down to 1min mimics typical unintentional exposure conditions in public. The rapidity with which an acute cardiovascular effect was detected (i.e., 1min of exposure) has not been previously reported to our knowledge. While the impairment detected at the relatively low peak 180 µg/m3 for 1min was modest, FMD after 1min of SHS exposure at peak 670 µg/m3 (comparable to a smoky bar or restaurant) was clearly impaired as evidenced by following FMD over time in individual rats (Figure 4). The short exposure times may explain why we did not detect significant correlations between serum cotinine levels and smoke exposure levels, highlighting the sensitivity of functional endpoint analysis for studies of acute exposure.
We conclude that exposure to real-world concentrations of SHS for as little as 1min can impair FMD in rats. The effects that we studied here in the rat, as well as related effects that we have reported in humans (Heiss et al., 2008a), were temporary; FMD was restored to healthy levels within 30min in the rat and within 2.5hr in the human study. However, it is likely that repeated brief exposures for an extended period of time will gradually reduce baseline FMD level, post-exposure FMD level, or both; based on the findings of Celermajer et al. (1996) that FMD was impaired in people frequently exposed to SHS even if they were not exposed at the time of the measurement. Whether such a progressive decline occurs after repeated temporary impairments, or even after repeated exposures to conditions that did not lead to significant impairment after one event, is a topic of continued interest and future study. Furthermore, the extent of brachial artery FMD reflects that of endothelium-dependent vasodilation of the coronary arteries (Anderson et al., 1995). Therefore, brief impairments of endothelial function may potentially contribute to increased incidence of acute cardiovascular events, consistent with the decrease in MI admissions observed in communities with smoke-free public place and workplace laws (Lightwood & Glantz, 2009; Sargent et al., 2004; Tan & Glantz, 2012). Our findings support the need to prevent even brief SHS exposure.
SUPPLEMENTARY MATERIAL
Supplementary Table 1, Figures 1–3, and Supplementary Material can be found online at http://www.ntr.oxfordjournals.org
FUNDING
This work was supported by the Flight Attendant Medical Research Foundation (Clinical Innovator Award number 072131) to M.L.S.; the William Cahan Endowment of the Flight Attendant Medical Research Institute to S.A.G.; the National Institute on Drug Abuse at the National Institutes of Health (R21 DA031699) to M.L.S.; a grant from the University of California, San Francisco (UCSF) Research Evaluation and Allocation Committee to M.L.S.; and the UCSF Helen Diller Family Comprehensive Cancer Center.
DECLARATION OF INTERESTS
None declared.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michael Matthay for help with arterial blood gas measurements, Torsten Neilands for suggesting analysis using splines, and Lejla Medžiković and Weisheng Ye for technical assistance.
REFERENCES
- Anderson T. J., Uehata A., Gerhard M. D., Meredith I. T., Knab S., …, Selwyn A. P. (1995). Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology, 26, 1235–1241 [DOI] [PubMed] [Google Scholar]
- Barnoya J., Glantz S. A. (2005). Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation, 111, 2684–2698 doi:10.1161/CIRCULATIONAHA.104.492215 [DOI] [PubMed] [Google Scholar]
- Barua R. S., Ambrose J. A., Eales-Reynolds L. J., DeVoe M. C., Zervas J. G., Saha D. C. (2001). Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation, 104, 1905–1910 doi:10.1161/hc4101.097525 [DOI] [PubMed] [Google Scholar]
- Barua R. S., Ambrose J. A., Srivastava S., DeVoe M. C., Eales-Reynolds L. J. (2003). Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: An in vitro demonstration in human coronary artery endothelial cells. Circulation, 107, 2342–2347. 10.1161/01.CIR.0000066691.52789.BE [DOI] [PubMed] [Google Scholar]
- California Environmental Protection Agency (2005). Proposed identification of environmental tobacco smoke as a toxic air contaminant. Part A: Exposure assessment. California Environmental Protection Agency. Retrieved from http://www.arb.ca.gov/toxics/id/summary/etspt_a.pdf [Google Scholar]
- Celermajer D. S., Adams M. R., Clarkson P., Robinson J., McCredie R., Donald A., Deanfield J. E. (1996). Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. New England Journal of Medicine, 334, 150–154 doi:10.1056/NEJM199601183340303 [DOI] [PubMed] [Google Scholar]
- Celermajer D. S., Sorensen K. E., Georgakopoulos D., Bull C., Thomas O., Robinson J., Deanfield J. E. (1993). Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation, 88, 2149–2155 doi:10.1161/01.CIR.88.5.2149 [DOI] [PubMed] [Google Scholar]
- Celermajer D. S., Sorensen K. E., Gooch V. M., Spiegelhalter D. J., Miller O. I., Sullivan I. D., Deanfield J. E. (1992). Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet, 340, 1111–1115 doi:10.1056/NEJM199601183340303 [DOI] [PubMed] [Google Scholar]
- Chen Q., Sievers R. E., Varga M., Kharait S., Haddad D. J., Patton A. K., Springer M. L. (2013). Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo. Journal of Applied Physiology, 114, 752–760. 10.1152/japplphysiol.01302.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer A. J., Anderson T., Celermajer D. S., Creager M. A., Deanfield J., Ganz P., Lerman A. (2012). The assessment of endothelial function: From research into clinical practice. Circulation, 126, 753–767. 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey P. F., Ganz P., Hsue P. Y., Benowitz N. L., Glantz S. A., Balmes J. R., Schick S. F. (2012). The exposure-dependent effects of aged secondhand smoke on endothelial function. Journal of the American College of Cardiology, 59, 1908–1913. 10.1016/j.jacc.2012.02.025 [DOI] [PubMed] [Google Scholar]
- Giannini D., Leone A., Di Bisceglie D., Nuti M., Strata G., Buttitta F., Balbarini A. (2007). The effects of acute passive smoke exposure on endothelium-dependent brachial artery dilation in healthy individuals. Angiology, 58, 211–217. 10.1177/0003319707300361 [DOI] [PubMed] [Google Scholar]
- Heiss C., Amabile N., Lee A. C., Real W. M., Schick S. F., Lao D., Yeghiazarians Y. (2008a). Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: Sustained vascular injury and blunted nitric oxide production. Journal of the American College of Cardiology, 51, 1760–1771 doi:10.1016/j.jacc.2008.01.040 [DOI] [PubMed] [Google Scholar]
- Heiss C., Sievers R. E., Amabile N., Momma T. Y., Chen Q., Natarajan S., Springer M. L. (2008b). In vivo measurement of flow-mediated vasodilation in living rats using high resolution ultrasound. American Journal of Physiology Heart and Circulatory Physiology, 294, H1086–H1093 doi:10.1152/ajpheart.00811.2007 [DOI] [PubMed] [Google Scholar]
- Hutchison S. J., Glantz S. A., Zhu B. Q., Sun Y. P., Chou T. M., Chatterjee K., Sudhir K. (1998). In-utero and neonatal exposure to secondhand smoke causes vascular dysfunction in newborn rats. Journal of the American College of Cardiology, 32, 1463–1467 doi:10.1016/S0735-1097(98)00217-4 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2010). Secondhand smoke exposure and cardiovascular effects: Making sense of the evidence. Washington, DC: The National Academies Press; Retrieved from http://www.nap.edu/catalog.php?record_id=12649#toc [PubMed] [Google Scholar]
- Lightwood J. M., Glantz S. A. (2009). Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation, 120, 1373–1379. 10.1161/CIRCULATIONAHA.109.870691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E. G., Selwyn A. P., Ganz P. (1990). Large coronary arteries in humans are responsive to changing blood flow: An endothelium-dependent mechanism that fails in patients with atherosclerosis. Journal of the American College of Cardiology, 16, 349–356. 0735-1097(90)90584-C [DOI] [PubMed] [Google Scholar]
- Pyke K. E., Tschakovsky M. E. (2005). The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. The Journal of Physiology, 568, 357–369. 10.1113/jphysiol.2005.089755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent R. P., Shepard R. M., Glantz S. A. (2004). Reduced incidence of admissions for myocardial infarction associated with public smoking ban: Before and after study. British Medical Journal, 328, 977–980. 10.1136/bmj.38055.715683.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. E., Glantz S. A. (2012). Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: A meta-analysis. Circulation, 126, 2177–2183. 10.1161/CIRCULATIONAHA.112.121301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky M. E., Gokce N., Keaney J. F., Jr., Vita J. A. (2003). The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology, 42, 1149–1160 doi:10.1016/S0735-1097(98)00217-4 [DOI] [PubMed] [Google Scholar]
- Yeboah J., Crouse J. R., Hsu F. C., Burke G. L., Herrington D. M. (2007). Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation, 115, 2390–2397. 10.1161/CIRCULATIONAHA.106.678276 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.