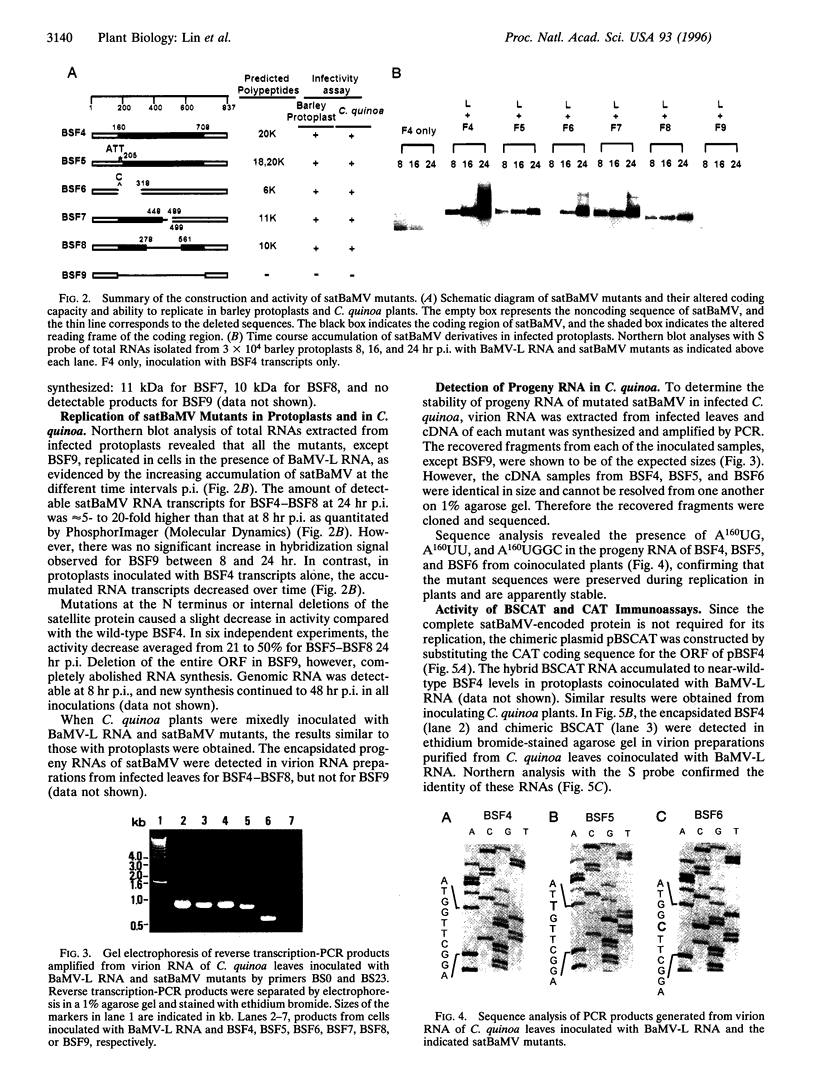
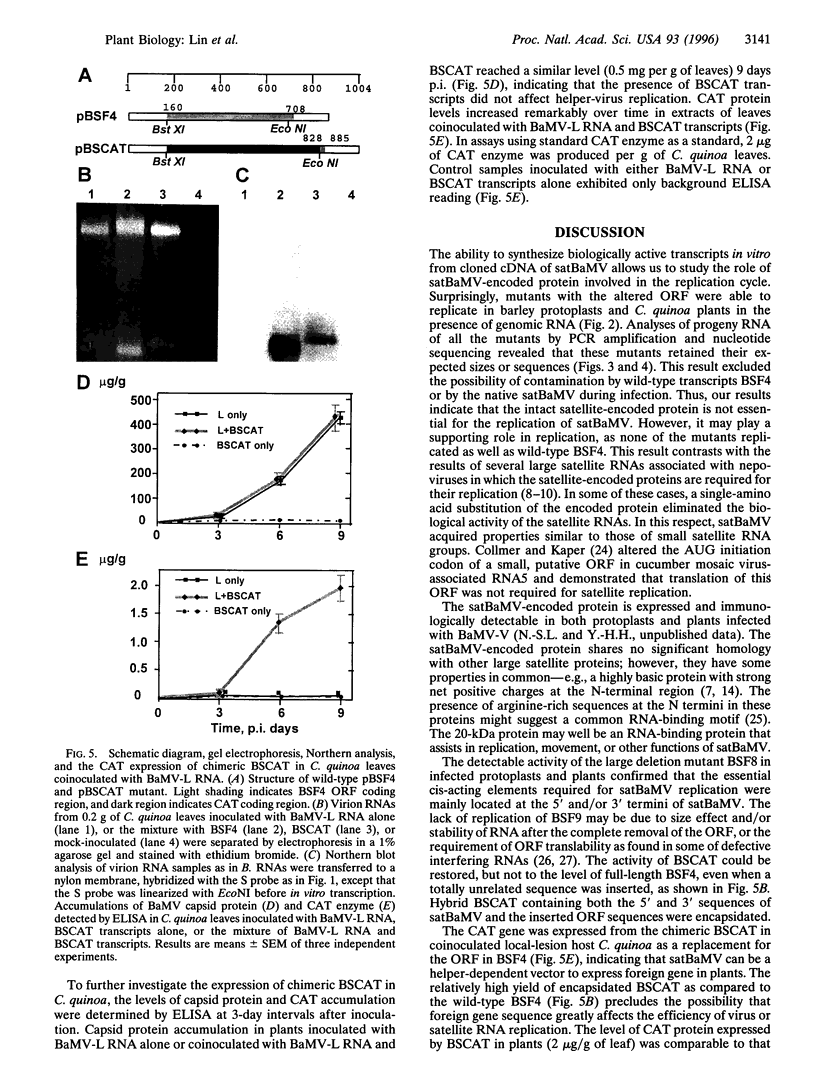
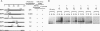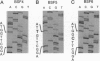Abstract
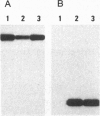
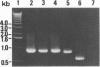
A satellite RNA of 836 nt depends on the bamboo mosaic potexvirus (BaMV) for its replication and encapsulation. The BaMV satellite RNA (satBaMV) contains a single open reading frame encoding a 20-kDa nonstructural protein. A full-length infectious cDNA clone has been generated downstream of the T7 RNA polymerase promoter. To investigate the role of the 20-kDa protein encoded by satBaMV, satBaMV transcripts containing mutations in the open reading frame were tested for their ability to replicate in barley protoplasts and in Chenopodium quinoa using BaMV RNA as a helper genome. Unlike other large satellite RNAs, mutants in the open reading frame did not block their replication, suggesting that the 20-kDa protein is not essential for satBaMV replication. Precise replacement of the open reading frame with sequences encoding chloramphenicol acetyltransferase resulted in high level expression of chloramphenicol acetyltransferase in infected C. quinoa, indicating that satBaMV is potentially useful as a satellite-based expression vector.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft J. B., Rouleau M., Johnston R., Prins L., Mackie G. A. The entire nucleotide sequence of foxtail mosaic virus RNA. J Gen Virol. 1991 Sep;72(Pt 9):2173–2181. doi: 10.1099/0022-1317-72-9-2173. [DOI] [PubMed] [Google Scholar]
- Beames B., Braunagel S., Summers M. D., Lanford R. E. Polyhedrin initiator codon altered to AUU yields unexpected fusion protein from a baculovirus vector. Biotechniques. 1991 Sep;11(3):378–383. [PubMed] [Google Scholar]
- Burd C. G., Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994 Jul 29;265(5172):615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Chapman S., Kavanagh T., Baulcombe D. Potato virus X as a vector for gene expression in plants. Plant J. 1992 Jul;2(4):549–557. doi: 10.1046/j.1365-313x.1992.t01-24-00999.x. [DOI] [PubMed] [Google Scholar]
- Collmer C. W., Kaper J. M. Site-directed mutagenesis of potential protein-coding regions in expressible cloned cDNAs of cucumber mosaic viral satellites. Virology. 1988 Apr;163(2):293–298. doi: 10.1016/0042-6822(88)90269-3. [DOI] [PubMed] [Google Scholar]
- Donson J., Kearney C. M., Hilf M. E., Dawson W. O. Systemic expression of a bacterial gene by a tobacco mosaic virus-based vector. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7204–7208. doi: 10.1073/pnas.88.16.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Ahlquist P. Intercistronic as well as terminal sequences are required for efficient amplification of brome mosaic virus RNA3. J Virol. 1987 May;61(5):1457–1465. doi: 10.1128/jvi.61.5.1457-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch C., Mayo M., Hemmer O. Properties of the satellite RNA of nepoviruses. Biochimie. 1993;75(7):561–567. doi: 10.1016/0300-9084(93)90062-w. [DOI] [PubMed] [Google Scholar]
- Gendloff E. H., Bowen B., Buchholz W. G. Quantitation of chloramphenicol acetyl transferase in transgenic tobacco plants by ELISA and correlation with gene copy number. Plant Mol Biol. 1990 Apr;14(4):575–583. doi: 10.1007/BF00027503. [DOI] [PubMed] [Google Scholar]
- Hans F., Pinck M., Pinck L. Location of the replication determinants of the satellite RNA associated with grapevine fanleaf nepovirus (strain F13). Biochimie. 1993;75(7):597–603. doi: 10.1016/0300-9084(93)90066-2. [DOI] [PubMed] [Google Scholar]
- Hemmer O., Oncino C., Fritsch C. Efficient replication of the in vitro transcripts from cloned cDNA of tomato black ring virus satellite RNA requires the 48K satellite RNA-encoded protein. Virology. 1993 Jun;194(2):800–806. doi: 10.1006/viro.1993.1321. [DOI] [PubMed] [Google Scholar]
- Huisman M. J., Linthorst H. J., Bol J. F., Cornelissen J. C. The complete nucleotide sequence of potato virus X and its homologies at the amino acid level with various plus-stranded RNA viruses. J Gen Virol. 1988 Aug;69(Pt 8):1789–1798. doi: 10.1099/0022-1317-69-8-1789. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lin N. S., Hsu Y. H. A satellite RNA associated with bamboo mosaic potexvirus. Virology. 1994 Aug 1;202(2):707–714. doi: 10.1006/viro.1994.1392. [DOI] [PubMed] [Google Scholar]
- Lin N. S., Lin B. Y., Lo N. W., Hu C. C., Chow T. Y., Hsu Y. H. Nucleotide sequence of the genomic RNA of bamboo mosaic potexvirus. J Gen Virol. 1994 Sep;75(Pt 9):2513–2518. doi: 10.1099/0022-1317-75-9-2513. [DOI] [PubMed] [Google Scholar]
- Liu J. S., Lin N. S. Satellite RNA associated with bamboo mosaic potexvirus shares similarity with satellites associated with sobemoviruses. Arch Virol. 1995;140(8):1511–1514. doi: 10.1007/BF01322678. [DOI] [PubMed] [Google Scholar]
- Liu Y. Y., Cooper J. I. The multiplication in plants of arabis mosaic virus satellite RNA requires the encoded protein. J Gen Virol. 1993 Jul;74(Pt 7):1471–1474. doi: 10.1099/0022-1317-74-7-1471. [DOI] [PubMed] [Google Scholar]
- Roossinck M. J., Sleat D., Palukaitis P. Satellite RNAs of plant viruses: structures and biological effects. Microbiol Rev. 1992 Jun;56(2):265–279. doi: 10.1128/mr.56.2.265-279.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh G., Dodds J. A., Fitzmaurice L., Mirkov T. E. Characterization of deletion and frameshift mutants of satellite tobacco mosaic virus. Virology. 1995 Sep 10;212(1):121–127. doi: 10.1006/viro.1995.1460. [DOI] [PubMed] [Google Scholar]
- Takamatsu N., Ishikawa M., Meshi T., Okada Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 1987 Feb;6(2):307–311. doi: 10.1002/j.1460-2075.1987.tb04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Bancroft J. B., Mackie G. A. Coding capacity determines in vivo accumulation of a defective RNA of clover yellow mosaic virus. J Virol. 1992 May;66(5):3069–3076. doi: 10.1128/jvi.66.5.3069-3076.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Bancroft J. B., Mackie G. A. Mutagenesis of a hexanucleotide sequence conserved in potexvirus RNAs. Virology. 1992 Aug;189(2):817–820. doi: 10.1016/0042-6822(92)90614-u. [DOI] [PubMed] [Google Scholar]
- Yeh S. J., Lin F. C., Hung J. S., Wu D. Significance of bundle branch or fascicular block complicating acute myocardial infarction. Taiwan Yi Xue Hui Za Zhi. 1982 Dec;81(12):1543–1551. [PubMed] [Google Scholar]
- Zhang C., Simon A. E. Effect of template size on accumulation of defective interfering RNAs in protoplasts. J Virol. 1994 Dec;68(12):8466–8469. doi: 10.1128/jvi.68.12.8466-8469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]