Abstract
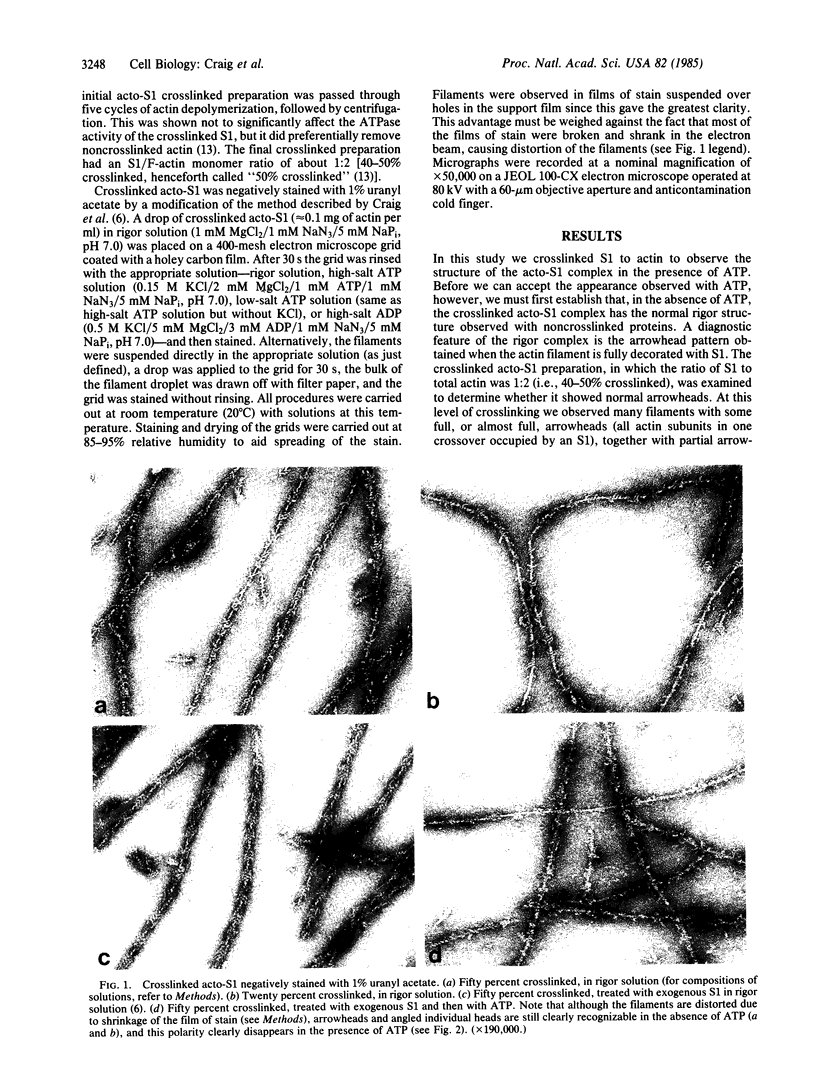
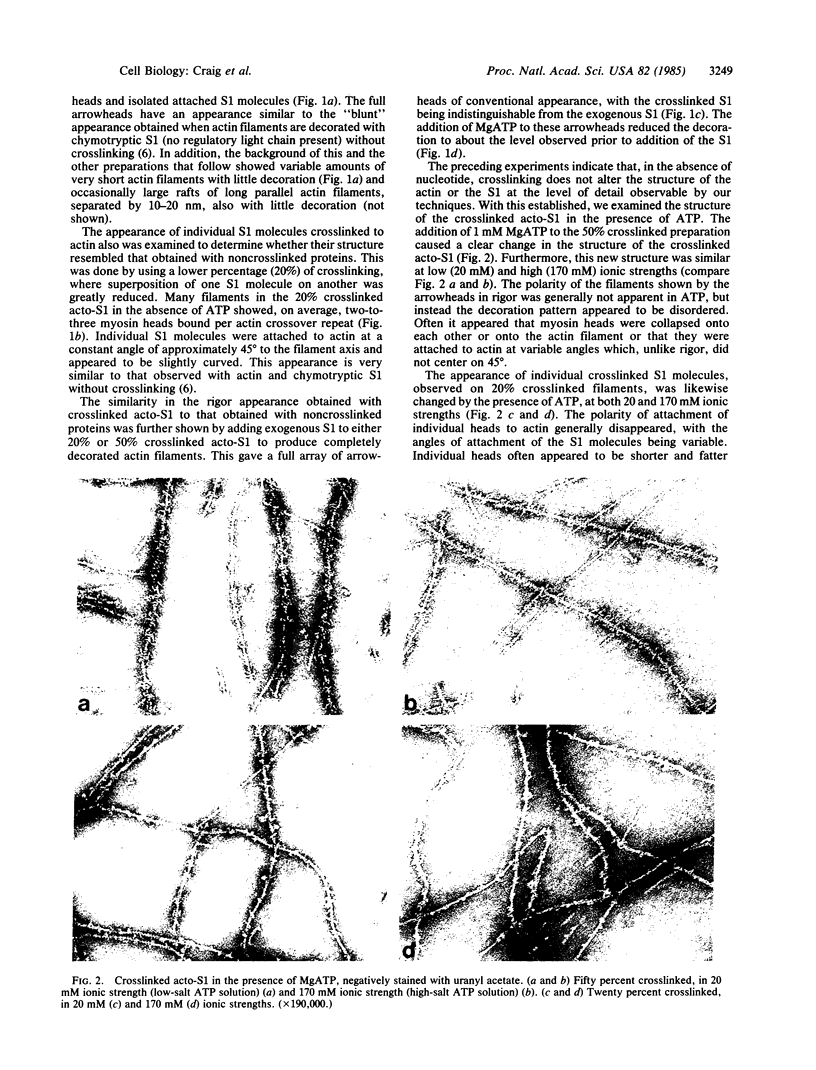
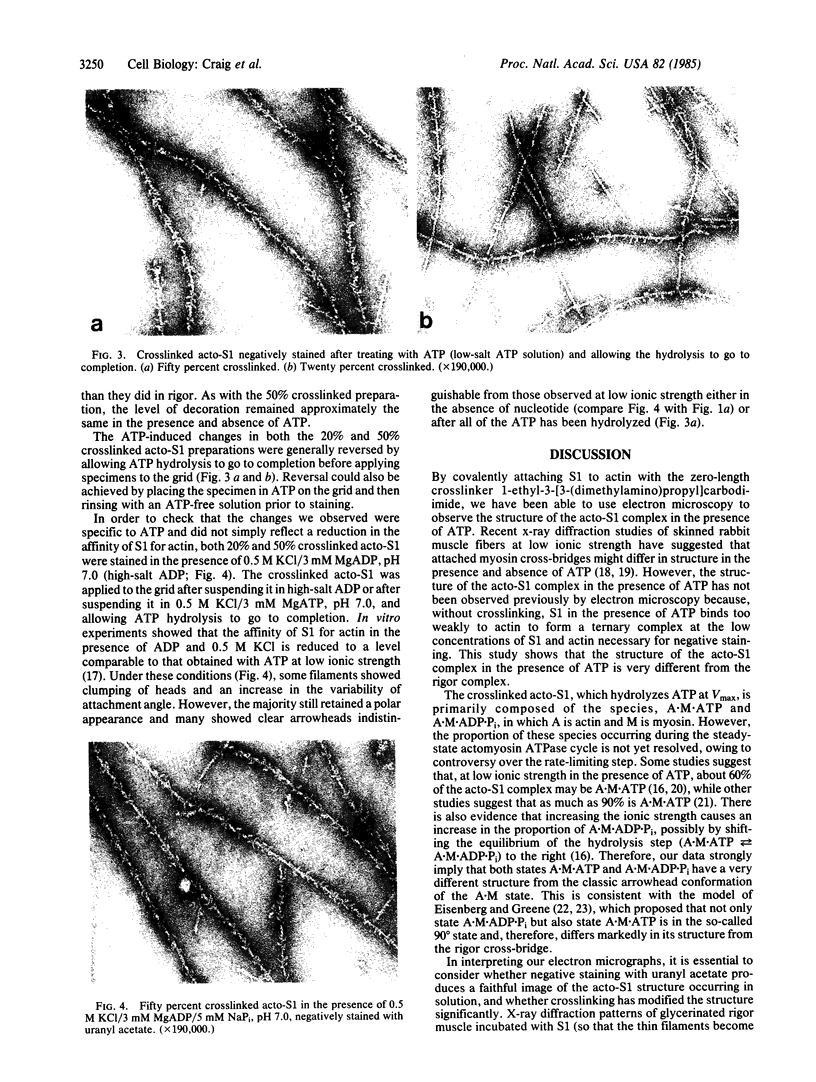
The structure of the complex between actin and myosin subfragment 1 (S1), designated the acto-S1 complex, in the presence of ATP was examined by electron microscopy. This was accomplished by using negative staining to study a complex of S1 covalently crosslinked to actin by the zero-length crosslinker, 1-ethyl-3-[3-(dimethylamino)-propyl]carbodiimide. Two levels of S1 binding were studied, with a molar ratio of crosslinked S1 to total actin of either 20% or 50%. The lower percentage was used to observe individual S1 molecules attached to actin, while the higher percentage was used to look at the overall pattern of S1 decoration of the actin filament. In the absence of ATP, the appearances of both the 20% and 50% crosslinked filaments closely resembled the rigor appearances obtained with noncrosslinked proteins. The arrowheads observed had the conventional structure, and individual S1 molecules were elongated and curved and appeared to make an angle of 45 degrees with the thin filament. Addition of ATP to the crosslinked acto-S1 complex caused a radical change in the structure of the cross-bridges. At both 20 and 170 mM ionic strengths, individual S1 molecules appeared to be attached at variable angles which, in contrast to rigor, did not center on 45 degrees. In addition, the S1 molecules often appeared shorter and fatter than in rigor. The 50% crosslinked acto-S1 preparation no longer showed the arrowhead pattern of S1 decoration but instead appeared to be disordered with little obvious polarity. Control experiments with ADP suggest that these effects were not due simply to a weakening of the binding of S1 to actin in the presence of nucleotide but most likely were ATP-specific. The crosslinked acto-S1 complex, which hydrolyzes ATP at about the same rate as the maximal actin-activated ATPase of S1 (Vmax), is composed of a mixture of states A X M X ATP and A X M X ADP X Pi (in which A = actin and M = myosin), with more than 50% of the crosslinked S-1 occurring in state A X M X ATP. Therefore, it appears that both states A X M X ATP and A X M X ADP X Pi have a very different conformation from the classic arrowhead conformation of the A X M state.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A., Huxley H. E., Holmes K. C., Goody R. S., Taylor K. A. Structural evidence that myosin heads may interact with two sites on F-actin. Nature. 1982 Sep 30;299(5882):467–469. doi: 10.1038/299467a0. [DOI] [PubMed] [Google Scholar]
- Begg D. A., Rodewald R., Rebhun L. I. The visualization of actin filament polarity in thin sections. Evidence for the uniform polarity of membrane-associated filaments. J Cell Biol. 1978 Dec;79(3):846–852. doi: 10.1083/jcb.79.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Podolsky R. J. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J. 1984 Sep;46(3):299–306. doi: 10.1016/S0006-3495(84)84026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R., Szent-Györgyi A. G., Beese L., Flicker P., Vibert P., Cohen C. Electron microscopy of thin filaments decorated with a Ca2+-regulated myosin. J Mol Biol. 1980 Jun 15;140(1):35–55. doi: 10.1016/0022-2836(80)90355-1. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Zobel C. R., Moos C. Subfragment 1 of myosin: adenosine triphophatase activation by actin. Biochemistry. 1968 Sep;7(9):3186–3194. doi: 10.1021/bi00849a022. [DOI] [PubMed] [Google Scholar]
- Greene L. E. Comparison of the equilibrium binding of heavy meromyosin and myosin to F-actin in the presence and absence of the troponin-tropomyosin complex. FEBS Lett. 1982 Mar 22;139(2):233–236. doi: 10.1016/0014-5793(82)80859-4. [DOI] [PubMed] [Google Scholar]
- Greene L. E. Stoichiometry of actin X S-1 cross-linked complex. J Biol Chem. 1984 Jun 25;259(12):7363–7366. [PubMed] [Google Scholar]
- Heaphy S., Tregear R. Stoichiometry of covalent actin-subfragment 1 complexes formed on reaction with a zero-length cross-linking compound. Biochemistry. 1984 May 8;23(10):2211–2214. doi: 10.1021/bi00305a017. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Cooke R. Actin-myosin interactions visualized by the quick-freeze, deep-etch replica technique. J Mol Biol. 1983 Sep 5;169(1):97–122. doi: 10.1016/s0022-2836(83)80177-6. [DOI] [PubMed] [Google Scholar]
- Heuser J. E. Procedure for freeze-drying molecules adsorbed to mica flakes. J Mol Biol. 1983 Sep 5;169(1):155–195. doi: 10.1016/s0022-2836(83)80179-x. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Podolsky R. J. X-ray evidence for two structural states of the actomyosin cross-bridge in muscle fibers. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2364–2368. doi: 10.1073/pnas.81.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet D., Bertrand R., Pantel P., Audemard E., Kassab R. Structure of the actin-myosin interface. Nature. 1981 Jul 23;292(5821):301–306. doi: 10.1038/292301a0. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol. 1967;17:1–60. doi: 10.1016/0079-6107(67)90003-x. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. The ATPase mechanism of skeletal and smooth muscle acto-subfragment 1. J Biol Chem. 1984 Oct 10;259(19):11908–11919. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stein L. A., Chock P. B., Eisenberg E. The rate-limiting step in the actomyosin adenosinetriphosphatase cycle. Biochemistry. 1984 Mar 27;23(7):1555–1563. doi: 10.1021/bi00302a033. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Greene L. E., Chock P. B., Eisenberg E. Rate-limiting step in the actomyosin adenosinetriphosphatase cycle: studies with myosin subfragment 1 cross-linked to actin. Biochemistry. 1985 Mar 12;24(6):1357–1363. doi: 10.1021/bi00327a013. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Sutoh K. An actin-binding site on the 20K fragment of myosin subfragment 1. Biochemistry. 1982 Sep 14;21(19):4800–4804. doi: 10.1021/bi00262a043. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Mapping of actin-binding sites on the heavy chain of myosin subfragment 1. Biochemistry. 1983 Mar 29;22(7):1579–1585. doi: 10.1021/bi00276a009. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Reedy M. C., Córdova L., Reedy M. K. Three-dimensional reconstruction of rigor insect flight muscle from tilted thin sections. 1984 Jul 26-Aug 1Nature. 310(5975):285–291. doi: 10.1038/310285a0. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P., Craig R. Electron microscopy and image analysis of myosin filaments from scallop striated muscle. J Mol Biol. 1983 Apr 5;165(2):303–320. doi: 10.1016/s0022-2836(83)80259-9. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]