Abstract
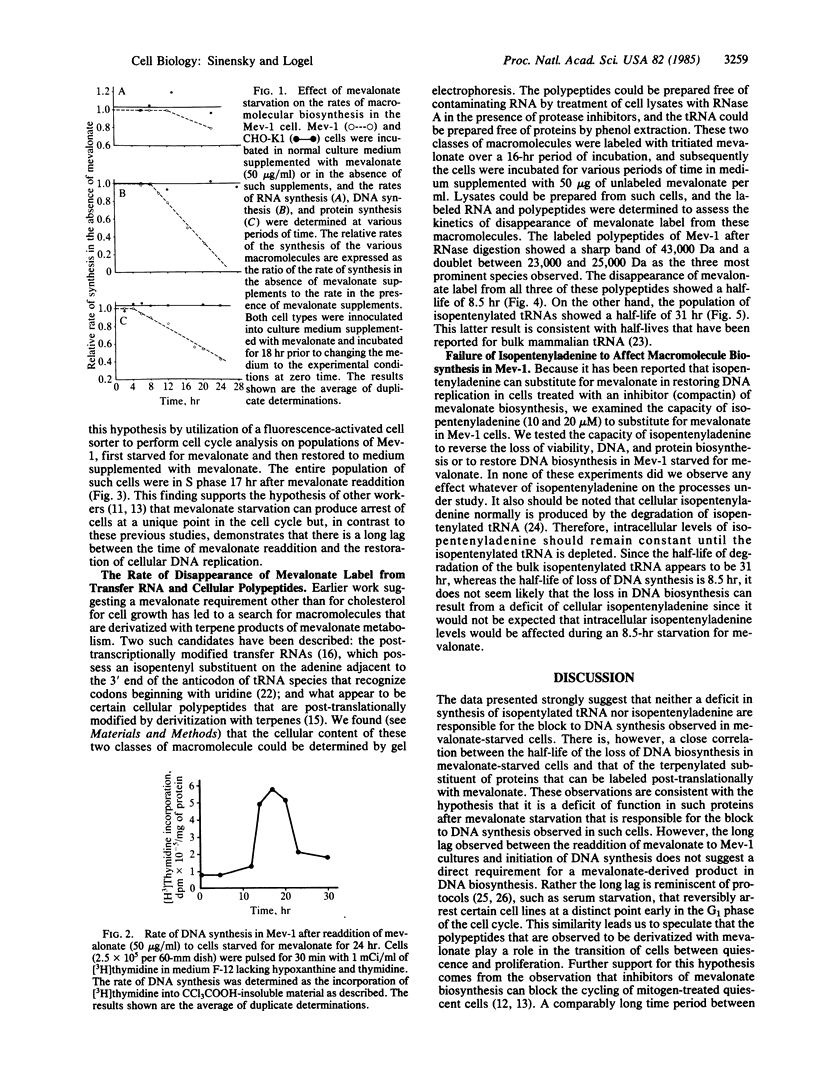
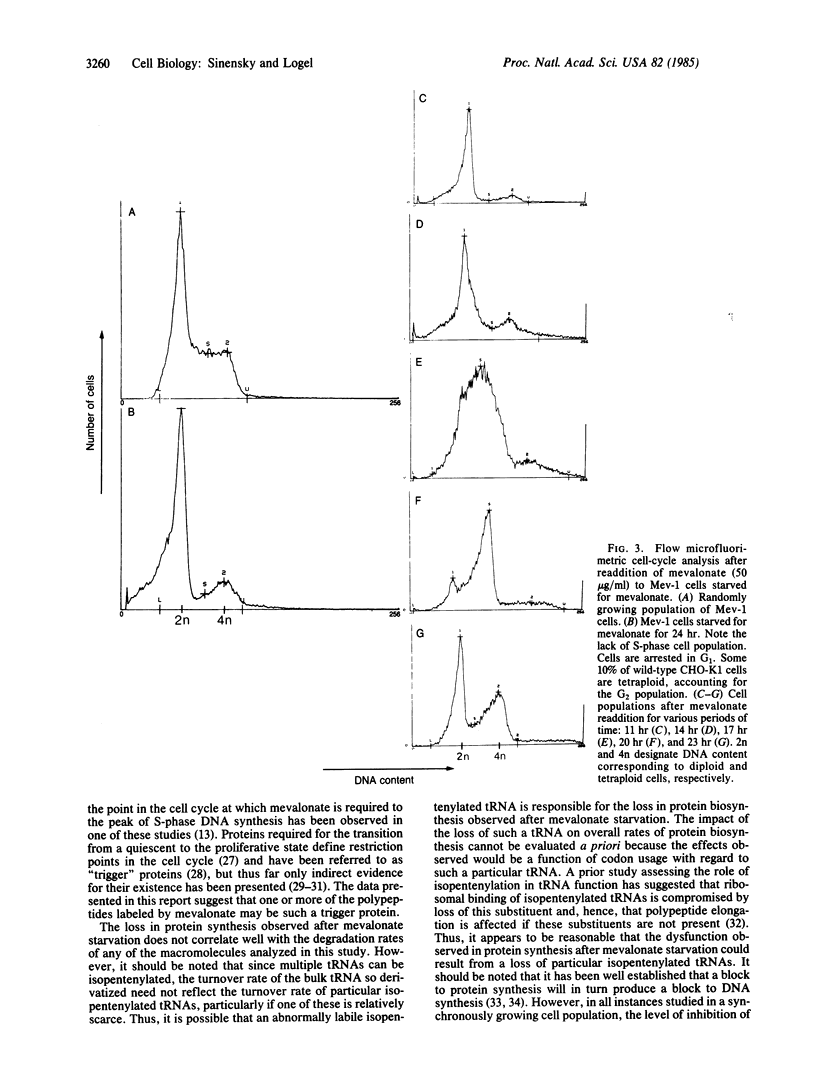
The isolation of a somatic cell mutant (Mev-1) with a block in one of the mevalonate-biosynthesizing enzymes (3-hydroxy-3-methylglutaryl-coenzyme A synthase, EC 4.1.3.5) has afforded us the opportunity to test and to extend the hypothesis that a product of mevalonate biosynthesis other than cholesterol is required for cellular proliferation. We present evidence here that both DNA synthesis and protein synthesis are inhibited in this mutant by mevalonate starvation, although RNA synthesis appears to be unaffected. The loss of DNA synthesis and the loss of protein synthesis in this mutant appear to be due to independent processes. DNA synthesis is reversibly inhibited by mevalonate starvation at a unique point in the cell cycle. Resumption of DNA synthesis after readdition of mevalonate exhibits a long lag; the peak of S-phase DNA synthesis occurs approximately 17 hr after mevalonate readdition, suggesting that mevalonate starvation puts cells into a quiescent (G0) state owing to their failure to transit a restriction point. The loss of DNA biosynthesis in the Mev-1 cell is well correlated with the rate of turnover of mevalonate label of certain terpenylated polypeptides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977 Sep;12(1):311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Chang T. Y., Limanek J. S. Regulation of cytosolic acetoacetyl coenzyme A thiolase, 3-hydroxy-3-methylglutaryl coenzyme A synthase, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and mevalonate kinase by low density lipoprotein and by 25-hydroxycholesterol in Chinese hamster ovary cells. J Biol Chem. 1980 Aug 25;255(16):7787–7795. [PubMed] [Google Scholar]
- Croy R. G., Pardee A. B. Enhanced synthesis and stabilization of Mr 68,000 protein in transformed BALB/c-3T3 cells: candidate for restriction point control of cell growth. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4699–4703. doi: 10.1073/pnas.80.15.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks K. P., Witte L. D., Goodman D. S. Relationship between mevalonate and mitogenesis in human fibroblasts stimulated with platelet-derived growth factor. J Biol Chem. 1984 Feb 10;259(3):1546–1551. [PubMed] [Google Scholar]
- Faust J. R., Brown M. S., Goldstein J. L. Synthesis of delta 2-isopentenyl tRNA from mevalonate in cultured human fibroblasts. J Biol Chem. 1980 Jul 25;255(14):6546–6548. [PubMed] [Google Scholar]
- Faust J. R., Luskey K. L., Chin D. J., Goldstein J. L., Brown M. S. Regulation of synthesis and degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by low density lipoprotein and 25-hydroxycholesterol in UT-1 cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5205–5209. doi: 10.1073/pnas.79.17.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., Ross R. Relation of cholesterol and mevalonic acid to the cell cycle in smooth muscle and swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1980 Jun 10;255(11):5134–5140. [PubMed] [Google Scholar]
- Huneeus V. Q., Wiley M. H., Siperstein M. D. Isopentenyladenine as a mediator of mevalonate-regulated DNA replication. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5842–5846. doi: 10.1073/pnas.77.10.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IX. Quantitation of mutagenesis by physical and chemical agents. J Cell Physiol. 1969 Dec;74(3):245–258. doi: 10.1002/jcp.1040740305. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Quesney-Huneeus V., Wiley M. H., Siperstein M. D. Essential role for mevalonate synthesis in DNA replication. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5056–5060. doi: 10.1073/pnas.76.10.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R. A., Iversen P., Rechsteiner M. The turnover of tRNAs microinjected into animal cells. Nucleic Acids Res. 1978 Oct;5(10):3715–3729. doi: 10.1093/nar/5.10.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. A., Schneider C. J., Glomset J. A. Evidence for post-translational incorporation of a product of mevalonic acid into Swiss 3T3 cell proteins. J Biol Chem. 1984 Aug 25;259(16):10175–10180. [PubMed] [Google Scholar]
- Schnitzer-Polokoff R., Torget R., Logel J., Sinensky M. Analysis of the coordinate expression of 3-hydroxy-3-methylglutaryl coenzyme A synthase and reductase activities in Chinese hamster ovary fibroblasts. Arch Biochem Biophys. 1983 Nov;227(1):71–80. doi: 10.1016/0003-9861(83)90348-x. [DOI] [PubMed] [Google Scholar]
- Schnitzer-Polokoff R., von Gunten C., Logel J., Torget R., Sinensky M. Isolation and characterization of a mammalian cell mutant defective in 3-hydroxy-3-methylglutaryl coenzyme A synthase. J Biol Chem. 1982 Jan 10;257(1):472–476. [PubMed] [Google Scholar]
- Scott R. E., Florine D. L., Wille J. J., Jr, Yun K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc Natl Acad Sci U S A. 1982 Feb;79(3):845–849. doi: 10.1073/pnas.79.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M., Torget R., Edwards P. A. Radioimmune precipitation of 3-hydroxy-3-methylglutaryl coenzyme A reductase from Chinese hamster fibroblasts. Effect of 25-hydroxycholesterol. J Biol Chem. 1981 Nov 25;256(22):11774–11779. [PubMed] [Google Scholar]
- Sinensky M., Torget R., Schnitzer-Polokoff R., Edwards P. A. Analysis of regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in a somatic cell mutant auxotrophic for mevalonate. J Biol Chem. 1982 Jul 10;257(13):7284–7286. [PubMed] [Google Scholar]
- Stimac E., Housman D., Huberman J. A. Effects of inhibition of protein synthesis on DNA replication in cultured mammalian cells. J Mol Biol. 1977 Sep 25;115(3):485–511. doi: 10.1016/0022-2836(77)90167-x. [DOI] [PubMed] [Google Scholar]
- Tyson J., Garcia-Herdugo G., Sachsenmaier W. Control of nuclear division in Physarum polycephalum: Comparison of cycloheximide pulse treatment, uv irradiation, and heat shock. Exp Cell Res. 1979 Mar 1;119(1):87–98. doi: 10.1016/0014-4827(79)90338-0. [DOI] [PubMed] [Google Scholar]