Abstract
Cyanide causes toxic effects by inhibiting cytochrome c oxidase, resulting in cellular hypoxia and cytotoxic anoxia, and can eventually lead to death. Cyanide exposure can be verified by direct analysis of cyanide concentrations or analyzing its metabolites, including thiocyanate (SCN−) and 2-amino-2-thiazoline-4-carboxylic acid (ATCA) in blood. To determine the behavior of these markers following cyanide exposure, a toxicokinetics study was performed in three animal models: (i) rats (250–300 g), (ii) rabbits (3.5–4.2 kg) and (iii) swine (47–54 kg). Cyanide reached a maximum in blood and declined rapidly in each animal model as it was absorbed, distributed, metabolized and eliminated. Thiocyanate concentrations rose more slowly as cyanide was enzymatically converted to SCN−. Concentrations of ATCA did not rise significantly above the baseline in the rat model, but rose quickly in rabbits (up to a 40-fold increase) and swine (up to a 3-fold increase) and then fell rapidly, generally following the relative behavior of cyanide. Rats were administered cyanide subcutaneously and the apparent half-life (t1/2) was determined to be 1,510 min. Rabbits were administered cyanide intravenously and the t1/2 was determined to be 177 min. Swine were administered cyanide intravenously and the t1/2 was determined to be 26.9 min. The SCN− t1/2 in rats was 3,010 min, but was not calculated in rabbits and swine because SCN− concentrations did not reach a maximum. The t1/2 of ATCA was 40.7 and 13.9 min in rabbits and swine, respectively, while it could not be determined in rats with confidence. The current study suggests that cyanide exposure may be verified shortly after exposure by determining significantly elevated cyanide and SCN− in each animal model and ATCA may be used when the ATCA detoxification pathway is significant.
Introduction
Cyanide (as HCN or CN−, represented inclusively as CN) is a rapidly acting, toxic chemical that can be readily absorbed by inhalation, ingestion or dermally. After CN is absorbed, it is rapidly distributed throughout the body, causing toxic effects by mechanisms that include inhibiting cytochrome c oxidase, resulting in cellular hypoxia and cytotoxic anoxia, and can eventually result in death (1). Cyanide is volatile and reactive leading to a short half-life (t1/2). It is difficult to determine cyanide exposure by direct CN analysis if significant time has elapsed (2–5). Thus, indirect biomarkers, including thiocyanate (SCN−) and 2-amino-2-thiazoline-4-carboxylic acid (ATCA), are necessary in certain situations for the verification of cyanide poisoning. In the presence of a sulfur donor (e.g. thiosulfate) and a sulfur transferase enzyme (e.g. rhodanese), ∼80% of a dose of CN is metabolized to SCN− (2–4, 6). Although SCN− has been used as the main indirect cyanide exposure marker, it can be difficult to establish definitive CN exposure due to large endogenous SCN− concentrations in biological fluids (7–9). Cyanide can also react with l-cystine through a proposed intermediate, β-thiocyanoalanine, where it is subsequently transformed into ATCA. ATCA is a minor metabolite of CN, and it has been suggested that it accounts for ∼15–20% of cyanide metabolism (6, 10). ATCA may be useful as an alternative for determination of CN exposure because it does not metabolize further (6, 11, 12), and it is a chemically stable metabolite under most storage conditions (13, 14). Although it is a promising marker of CN exposure, there are relatively few studies on the behavior of ATCA following cyanide exposure, and the direct relationship between CN exposure and elevated ATCA concentrations has only tenuously been established (4, 14–17).
The objective of the current study was to simultaneously determine the toxicokinetic behavior of CN, SCN− and ATCA, providing a direct evaluation of the relationship between these biomarkers. In addition, the ability of CN and its detoxification products to serve as cyanide exposure biomarkers was evaluated and a comparison between multiple mammalian species was performed.
Materials and Methods
Chemicals and samples
All chemicals used were at least HPLC grade or higher. Potassium thiocyanate (KSCN), sodium cyanide (NaCN), sodium tetraborate decahydrate and sodium hydroxide (NaOH) were all purchased from Fisher Scientific (Fair Lawn, NJ, USA). Tetrabutylammonium sulfate (TBAS; 50%, w/w, solution in water), used as a phase transfer catalyst, was purchased from Sigma-Aldrich (St. Louis, MO, USA). Pentafluorobenzyl bromide (PFB-Br) was obtained from Thermo Scientific (Hanover Park, IL, USA). Isotopically labeled internal standards (NaS13C15N and Na13C15N) were acquired from Isotech (Miamisburg, OH, USA). Solid-phase extraction (SPE) MCX (mixed-mode cation exchange) columns were acquired from Waters® Corporation (Milford, MA, USA). Deuterated ATCA (ATCA-d2) was prepared by reaction of deuterated l-cysteine (3,3-d2) with cyanamide (18) and provided by the Department of Veterans Affairs Medical Center, Minneapolis, MN, USA. N-Methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) was purchased from Pierce Chemical Company (Rockford, IL, USA).
Animals
Three different animal models were used in this study: (i) Sprague-Dawley rats (Rattus norvegicus), (ii) New Zealand White rabbits (Oryctologus cuniculus) and (iii) Yorkshire swine (Sus scrofa). Male Sprague-Dawley rats weighing 250–300 g (Charles River Breeding Laboratories, Inc., Wilmington, MA, USA) with catheters implanted were kept in temperature and light-controlled rooms (22 ± 2°C, 12 h light/dark cycle), fed Teklad Rodent Diet (W) 8604 (Teklad HSD, Inc., WI, USA) and provided with water at Sam Houston State University (Huntsville, TX, USA). Rabbits weighing 3.5–4.5 kg (Western Oregon Rabbit Supply, Philomath, OR, USA) were housed individually in the Animal Resource Facility (ARF) of the College of Medicine at the University of California, Irvine, CA, USA, fed Purina Pro-lab (St. Louis, MO, USA) and provided with water. Swine (47–54 kg) were purchased from the local USDA licensed breeder (John Albert, Cibolo, TX, USA, USDA #74-A-1246) and were housed in paddocks (outdoor fenced pastures) and moved into indoor pens before the experiments. They were furnished with Purina Pro-Lab's (St. Louis, MO, USA) mini-pig-breeder diet (5082) and provided with water.
All animals were cared for in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication #86–23, revised 1978). All studies involving rats, rabbits or swine were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the appropriate institutions.
Experimental design
Rats (N = 9) were injected with sub-lethal doses (N = 3) of potassium cyanide (KCN) solution subcutaneously [2 mg/kg (25% LD50), 4 mg/kg (50% LD50) or 6 mg/kg (75% LD50)]. In order to establish a baseline, blood was drawn prior to injection for a “zero” time point. Blood samples (320 µL) were also drawn at 5, 15, 30, 60 min, and 2, 4, 6, 12, 15 and 50.5 h post-injection. These blood samples were placed in heparinized tubes to prevent coagulation. The tubes were then centrifuged to separate the plasma from the red blood cells (RBCs). A portion of plasma was removed for ATCA analysis (50 µL) and the rest was hemolyzed to produce whole blood for simultaneous CN and SCN− analysis. The baseline concentration for CN, SCN− and ATCA in saline-treated rats showed no significant change in the concentration over the duration of the experiment.
New Zealand White rabbits (N = 8) were anesthetized with an intramuscular injection of a 2:1 ratio of ketamine HCl (100 mg/mL, Ketaject, Phoenix Pharmaceutical Inc., St. Joseph, MI, USA): xylazine (20 mg/ml, Anased, Lloyd Laboratories, Shenandoah, IA, USA) at a dose of 0.75 cc/kg. After the intramuscular injection, a catheter was placed in the animals' marginal ear vein to administer continuous IV ketamine/xylazine anesthesia. The animals were intubated and were mechanically ventilated (dual phase control respirator, model 32A4BEPM-5R, Harvard Apparatus, Chicago, IL, USA) at a respiratory rate of 32 min−1, a tidal volume of 50 cc, and FiO2 of 100%. Blunt dissection was performed to isolate the femoral artery and vein on the left thigh for cyanide infusion and blood sampling. Sodium cyanide (10 mg) dissolved in 60 mL of 0.9% NaCl was administered intravenously through the femoral line over 60 min. Blood samples (300 µL) were drawn at 11 different time points, including a baseline (time “zero”), 20, 40 and 55 min during CN infusion. After the cyanide infusion was completed, seven additional time points over the next 90 min at 60, 65, 75, 90, 105, 120 and 150 min from the start of the experiment were drawn. Arterial blood samples were collected in heparinized tubes kept on ice and centrifuged to separate the plasma. The plasma samples (150 µL) were then immediately frozen and shipped on ice to South Dakota State University (SDSU) for analysis of CN, SCN− and ATCA. The baseline concentration for CN, SCN− and ATCA in control saline-treated rabbits showed no significant change over the duration of the experiment. At the completion of the experiment, the animals were euthanized with an intravenous injection of 1.0 cc Euthasol (390 mg pentobarbital sodium, 50 mg phenytoin sodium; Vibrac AH, Inc, Fort Worth, TX, USA) administered through the marginal ear vein.
Swine (N = 11) were infused intravenously with approximately (or an average of) 1.7 mg/kg potassium cyanide until apnea occurred. The animals were then observed for an additional 60 min. Arterial blood (20 mL) was sampled prior to cyanide exposure (considered baseline or time ‘zero’), 5 min after the start of the cyanide infusion, 5 min into cyanide administration, at apnea and every 2 min for the first 10 min after apnea, and then every 10 min until 60 min postapnea. Blood (4 mL) was placed in an EDTA tube and centrifuged to separate the plasma. The plasma samples (500 µL) were then frozen and shipped on ice to SDSU for analysis of CN, SCN− and ATCA.
CN and SCN− analysis
The whole blood samples from rats and plasma samples from rabbits and swine were simultaneously analyzed for CN and SCN− by chemical-ionization (CI) GC–MS after chemical modification based on a method previously reported (19). Briefly, blood samples (100 µL) were added to 2 mL microcentrifuge vials. Internal standards (100 µL) of Na13C15N (200 µM) and NaS13C15N (100 µM) were added to the sample vials along with TBAS (800 µL of 10 mM TBAS in a saturated solution of sodium tetraborate decahydrate, pH 9.5) and PFB–Br (500 µL of a 20-mM solution in ethyl acetate) and vortexed for 2 min. Samples were then heated at 70°C for 1 h, and centrifuged for 4 min at 10,000 rpm (9,300 × g) to separate the organic and aqueous layers. The organic layer (200 µL) was collected and analyzed using CI–GC–MS. The concentrations for both CN and SCN− were well above the detection limit of the analytical method (∼1 µM for CN and 50 nM for SCN−) for all samples analyzed.
ATCA analysis
Rat, rabbit and swine plasma samples (50 µL) were analyzed for ATCA according to a slightly modified procedure previously reported (20). Briefly, plasma samples (80 µL), internal standard (ATCA-d2; 120 µL of 100 ng/mL) and 300 µL of 1% HCl in acetone were added to a 2-mL microcentrifuge vial, vortexed for 2 min and centrifuged for 4 min (room temperature) at 10,000 rpm (9,300 × g). The supernatant was transferred to a clean microcentrifuge tube, 1.0 mL of 0.1 M HCl was added, and the sample was aspirated through a prepared mixed-mode cation-exchange solid-phase extraction column (1 mL). The ATCA was eluted from the column into a 2-mL microcentrifuge tube using 1 mL of a water:methanol:ammonium hydroxide solution (25:50:25, by volume). Hydrochloric acid (200 µL of 0.1 M) was added to the microcentrifuge tube to decrease the pH of the sample (pH < 11) and the sample was dried. The dried samples were reacted with 200 µL of 30% MSTFA in hexane for 60 min at 50 °C in capped centrifuge tubes. The samples were then analyzed using electron-ionization GC–MS. The concentrations for ATCA were well above the detection limit of the analytical method (∼170 nM) for each plasma sample tested.
Toxicokinetic analysis
The blood and plasma concentration–time data after subcutaneous or intravenous administration were analyzed using a one-compartment toxicokinetic model which was determined according to the methods previously described (21, 22). Concentration–time curves were used to obtain the maximum concentration (Cmax) of CN, SCN− and ATCA in blood and plasma, elimination constants (Kel) and terminal elimination half-life (t1/2), with interpolation. A linear trapezoidal method was used to calculate the area under the curve (AUC) for cyanide and ATCA. The parameters such as Cmax, Kel, t1/2 and AUC were not calculated for thiocyanate in rabbit and swine models because there was no elimination observed from these experimental subjects throughout the duration of the experiments. The data presented is normalized to the baseline concentration of each compound to allow variation in concentrations of the analytes evaluated (CN, SCN− and ATCA) to be observed on the same figure, such that direct comparison of the relative behavior can more easily be observed.
Results
Behavior of CN, SCN− and ATCA following cyanide exposure in Rats
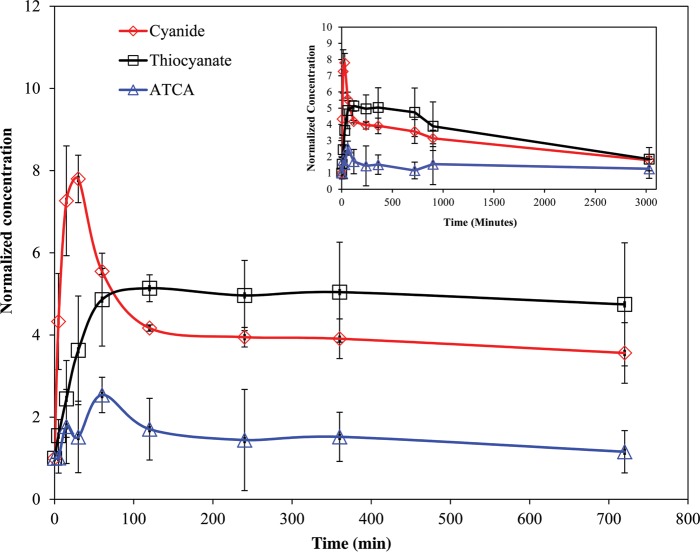
Figure 1 shows the normalized CN, SCN− and ATCA concentrations (i.e., normalized to the baseline concentration of the each compound, listed in Table II) from rats dosed at 6 mg/kg KCN. The baseline concentrations were determined to be 9.95, 38.6 and 0.282 µM for CN, SCN− and ATCA, respectively. As seen in Figure 1, the blood CN concentrations increased rapidly upon subcutaneous injection of KCN to a maximum at ∼30 min and then declined rapidly as cyanide was distributed and metabolized. The CN concentrations then leveled off at ∼100 min post-exposure and slowly declined. Thiocyanate concentrations rose at a slower rate compared with CN and then declined slowly at a rate similar to that of cyanide after reaching a maximum at ∼120 min postexposure. Thiocyanate concentrations remained well above baseline for the duration of the experiment. Considering the variability of the data, ATCA concentrations changed only slightly when compared with the baseline concentration. Similar trends for all markers were observed for each dose of cyanide.
Figure 1.
Whole blood CN, SCN− and plasma ATCA normalized concentrations after cyanide exposure (6 mg/kg body weight KCN injection subcutaneously to rats). Error bars are plotted as standard error of mean (SEM) (N = 3). Inset: Full time course up to 50.5 h post-injection of KCN.
Table II.
Endogenous blood CN or plasma SCN− and ATCA concentrations from humans, rats, rabbits and swine models
| Analyte | Humans (µM) | Rats (µM) |
Rabbits (µM) |
Swine (µM) |
|||
|---|---|---|---|---|---|---|---|
| Current study | Previous studies | Current study | Previous studies | Current study | Previous studies | ||
| CNa | 0.02–2.9 (4), 0.03–10 (5) | 10.78 | 0.19 (28, 40), 3.27 (29, 41) | 5.61 | 3.84 (39) | 8.82 | N/A |
| SCN−b | 4.83–87.5 (4) | 42.91 | 53.0 (23), 20.0 (3) | 8.64 | N/A | 8.17 | 17.1 (3), 41.1 (38) |
| ATCAb | 0.08–0.122 (4) | 0.64 | 0.96 (16), 1.29 (17) | 0.23 | N/A | 1.51 | N/A |
aCN analyzed from whole blood.
bSCN− and ATCA were commonly analyzed from plasma.
Dose–concentration behavior
The dose–blood concentration relationship for CN and SCN− in rats with the Cmax plotted for each dose is shown in Figure 2. As the dose of KCN increased, there was an expected increase in the concentrations of CN and SCN−. Cyanide concentrations showed a linear response to increasing CN dose, whereas the dose–response of thiocyanate was non-linear, leveling off at ∼160 µM, with the 4 mg/kg dose and remaining at that level for the 6 mg/kg dose. The dose response of ATCA was not meaningful because of the large interanimal variability of plasma ATCA concentrations combined with the minor increase in Cmax compared with baseline concentrations.
Figure 2.
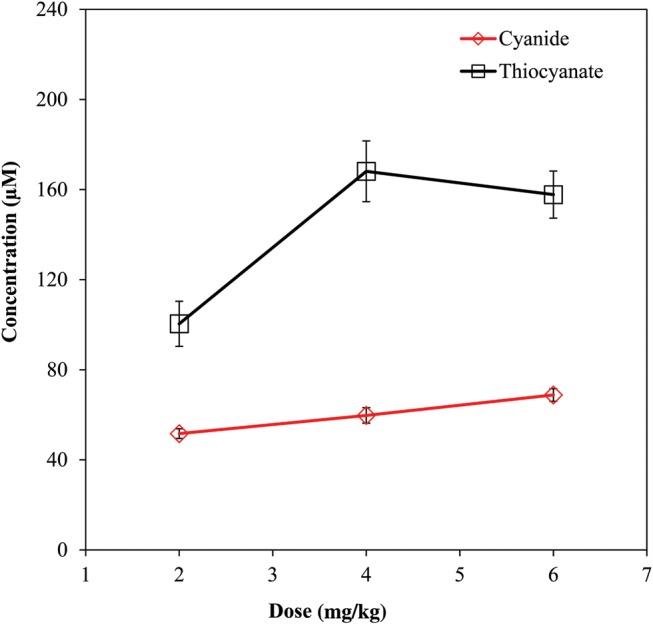
Dose–concentration curve for three different doses of KCN (2, 4 and 6 mg/kg) injected subcutaneously to rats. Error bars are plotted as standard error of mean (SEM) (N = 3).
Behavior of CN, SCN− and ATCA in rabbits after cyanide exposure
Figure 3 shows the normalized plasma concentrations of CN, SCN− and ATCA from rabbits (N = 8) over 150 min. The baseline concentrations were determined to be 5.66, 9.99 and 0.227 µM for CN, SCN− and ATCA, respectively. Plasma CN and ATCA concentrations reached a maximum at 55 min (14.7 and 9.1 µM for CN and ATCA, respectively) and then declined after CN administration was stopped. Plasma SCN− rose slowly throughout the duration of the experiment (150 min). CN, SCN− and ATCA were measurable throughout the study. ATCA concentrations clearly rose above the baseline by a larger ratio than the CN or SCN−.
Figure 3.
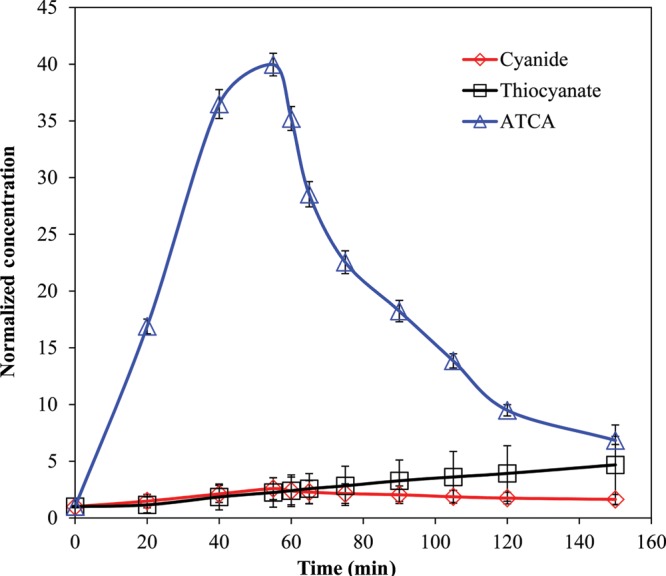
Plasma CN, SCN− and ATCA normalized concentrations after 10 mg NaCN infusion to rabbits. Error bars are plotted as standard error of mean (SEM) (N = 8).
Behavior of CN, SCN− and ATCA after cyanide exposure in swine
Figure 4 shows the normalized CN, SCN− and ATCA concentrations from swine (N = 11) over 40 min. The baseline concentrations were determined to be 3.87, 5.45 and 0.175 µM for CN, SCN− and ATCA, respectively. Plasma CN and ATCA concentrations reached a maximum at apnea (i.e., the 10-min time point) and then declined. The concentrations of each metabolite normalized to the baseline are very similar until 4 min postapnea (i.e., the 14 min time point in Figure 4) where the CN and ATCA continue to decrease, but the SCN− rises slowly through the completion of the experiment (40 min).
Figure 4.
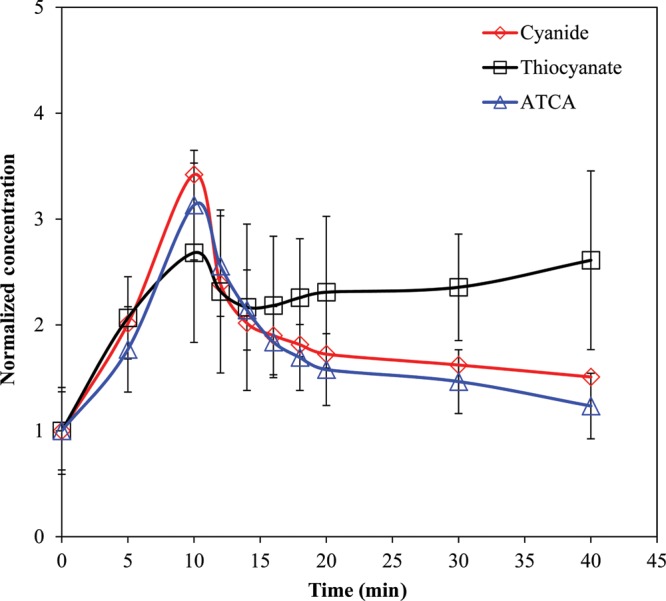
Swine plasma CN, SCN− and ATCA normalized concentrations during and after intravenous dose (0.17 mg/kg/min until apnea; ∼10 min). Error bars are plotted as standard error of mean (SEM) (N = 11).
Toxicokinetics of CN, SCN− and ATCA
A summary of the toxicokinetic parameters for rats, rabbits and swine is presented in Table I. The AUC, Cmax and t1/2 were calculated for all three markers of cyanide exposure with the exception of SCN− in rabbits and swine because it did not reach a maximum, and ATCA in rats because of considerable variability at each time point and the very low concentrations of ATCA measured. In rats, the AUC ranged from 6.53 × 104 to 7.74 × 104 and 2.27 × 104 to 3.07 × 104µM min for cyanide and thiocyanate, respectively. For rabbits, the AUC was found to be 0.33 × 104 and 548 µM min for cyanide and ATCA, respectively. The AUC for swine was determined to be 0.05 × 104 and 75.4 µM min for cyanide and ATCA, respectively.
Table I.
The Toxicokinetic Parameters of Cyanide, Thiocyanate and ATCA in Different Animal Models
| Animals | Analyte | Matrix | CN dose (mg/kg) |
Cmax (µM) |
t1/2 (min) |
|||
|---|---|---|---|---|---|---|---|---|
| Current study | Previous study | Current study | Previous study | Current study | Previous study | |||
| Rats | Cyanide | Blood | 2a, 4b, 6c | 1.0 (23), 3.0 (3) | 51.7a, 59.8b, 68.8c | 25 (23), 89.0 (3) | 1200a, 1370b,1510c | 14.1(23),38.4(3) |
| Thiocyanate | Plasma | 1.0 (23), 3.0 (3) | 100a,168b,158c | 877 (23), 58.1 (3) | 2530a,2860b,3010c | 348 (3) | ||
| ATCA | Plasma | N/A (16), 10.0 (17) | N/Ad | 4.91 (16), 2.82 (17) | N/Ad | 150 (16) | ||
| Pigs | Cyanide | Blood/plasmae | 1.7 | 3.0 (3) | 30.2 | 57.5 (3) | 26.9 | 32.4 (3) |
| Thiocyanate | Plasma | 3.0 (3) | N/Af | 42.8 (3) | N/Af | 297 (3) | ||
| ATCA | Plasma | – | 4.70 | – | 13.9 | – | ||
| Rabbits | Cyanide | Plasma | 2.5 | – | 14.7 | – | 177 | – |
| Thiocyanate | Plasma | – | N/Af | – | N/Af | – | ||
| ATCA | Plasma | – | 9.10 | – | 40.7 | – | ||
| Goats | Cyanide | Blood | – | 3.0 (3) | – | 93.5 (3) | – | 76.8 (3) |
| Thiocyanate | Plasma | – | 3.0 (3) | – | 55.2 (3) | – | 834 (3) | |
| Humans | Cyanide | Blood | – | – | – | – | – | 20–60 (2, 27) |
| Thiocyanate | Plasma | – | – | – | – | – | 1440–8640 (25, 26, 28, 29) | |
| ATCA | Plasma | – | – | – | – | – | – | |
aResults obtained from 2 mg/kg CN dose (11).
bResults obtained from 4 mg/kg CN dose.
cResults obtained from 6 mg/kg CN dose.
dATCA could not be evaluated due to interanimal variability.
ePlasma was used for cyanide analysis in our study.
fSCN− did not reach maximum value in our study.
Discussion
The toxicokinetic parameters found for CN, SCN− and ATCA in this study are presented in Table I, alongside similar studies for comparison. Previously, cyanide has been found to be rapidly absorbed, distributed and subsequently quickly eliminated (2, 3, 23, 24), with a t1/2 ranging from 14 to 60 min. Rapid distribution of CN was also seen in our study for each model tested (Figures 1, 3 and 4). For the rabbits and swine, CN was also rapidly eliminated, with t1/2 values of ∼27 and 178 min, which is in general agreement with similar studies (Table I). Conversely, rats produced a much longer mean elimination half-life. The difference may be due to the duration of our study, 50.5 h post cyanide-exposure versus 1 and 24 h in the Leuschner et al. (23) and Sousa et al. (3) studies, respectively. If the three distribution/elimination time points in our study (i.e., 30, 60 and 120 min) are used, the half-life obtained (t1/2 = 103 min) is comparable with that seen by Sousa et al. (3), and perhaps is more reflective of cyanide distribution than elimination. In addition, the other studies used oral dosing versus subcutaneous injection in the rat model for our study. Subcutaneous injection could potentially cause a rapid absorption and distribution of CN, versus a slower uptake of CN through the digestive tract. If a large dose of cyanide was rapidly absorbed into the erythrocytes, as suggested by Lundquist et al. (25), our data would suggest that the sequestered CN is only slowly released back into rat plasma for transport to tissues to be metabolized. Whole blood concentrations were also measured in multiple samples from each individual animal for the duration of the current study versus multiple animals at each time point in previous studies (3, 23). The fluid volume removed from an individual rat was ∼10% of the total body fluid volume. Thus, by the end of the experiment, despite being provided with food and water ad lib, our experimental subjects could have become dehydrated, causing their hematocrit to be elevated, potentially resulting in a higher level of CN to be measured at later time points. Further investigations would need to be conducted in the rat model to verify the prolonged half-life seen in this study. If verified, the long half-life may indicate the ability of rats to tolerate elevated levels of CN over long periods of time, perhaps due to a relatively large pool of sequestrating agents (e.g., methemoglobin). A relatively large pool of sequestrating agents may reduce the free CN, thereby not overwhelming normal detoxification pathways leading to increased tolerance of CN.
Thiocyanate in the swine and rabbits increased at about the same rate as CN, while the formation of SCN− in rats was markedly slower, which could be due to the method of CN administration, subcutaneous versus intravenous. At each dose, the maximum concentration of thiocyanate in rats occurred at around 1 h after cyanide exposure and stayed relatively consistent for several hours before it began to decrease. The extended consistent SCN− concentrations could be interpreted as having reached a steady-state between formation and elimination of thiocyanate. The mean elimination half-life of SCN− in rats ranged from 2530 to 3010 min, and was much longer than that was found in the Sousa et al. (2003) study, possibly due to the reasons previously presented. That being said, the half-life of SCN− in rats did fall within the range of human SCN− t1/2 (Table I).
Thiocyanate concentrations were still increasing at the end of the rabbit and swine studies, and would be expected to peak and decline as observed in rats if these studies were lengthened. It is interesting to note that SCN− declined immediately after apnea in swine (i.e., after KCN administration was stopped), and then started to rise slowly after the 6-min postapnea time point and continued to rise for the duration of the study (Figure 3). This behavior, which is atypical compared with other similar studies, is likely influenced by several factors. Initially, plasma SCN− levels rose at about the same rate as the infused cyanide levels, reaching a maximum at apnea, suggesting a direct correlation between cyanide and thiocyanate levels. Then the plasma SCN− declined abruptly, possibly due to reduced cardiac output leading to lower cyanide delivery to the detoxification sites and reduced export of thiocyanate due to lack of blood flow in tissues. After several minutes of reduced SCN− production and transport, the levels of SCN− increased, until the end of the experiment, albeit at a slower rate than initially. The secondary rise in thiocyanate production would suggest that once the initial shock of apnea passes, the SCN− detoxification pathway is reinitiated, although the slower rate of SCN− production might suggest some impairment in this process. If any portion of the CN transport or bio-transformation is energy-dependent, the pathway might become partially disabled by undergoing apnea. If the swine study were to be lengthened in time, the SCN− concentrations should reach a maximum and decline, as observed in the rat study.
Compared with cyanide and thiocyanate, ATCA had the lowest mean elimination half-life in rabbit and swine models, resulting in shorter residence time in the plasma (Table I). In the rabbit and swine models, ATCA generally mimicked the behavior of CN, but appears to be a minor metabolic pathway for cyanide detoxification, especially in rats. Although the general behavior of CN and ATCA in each model is similar, the difference in the behavior of normalized ATCA concentrations in rabbits (i.e., the 40-fold increase above baseline), compared with swine and rats (i.e., only a 4-fold increase), is striking.
To investigate the likelihood of a minimized sulfur-donor cyanide detoxification pathway causing increased production of ATCA in rabbits, species-dependent rhodanese concentrations in multiple animal tissues (e.g., liver, kidney, lung, brain, stomach and muscle) were evaluated from literature sources. Among all the tissues, kidney contains relatively large amounts of rhodanese: 6.69, 24.9, 10.44–110.8 and 6.20–7.69 mg/g tissue in humans, swine, rats and rabbits, respectively (6, 26, 30, 31). The lower rhodanese concentrations in humans and rabbits suggest that the sulfur donor pathway for cyanide detoxification may be less active when compared with swine and rats, potentially leading to increased ATCA formation. l-Cystine concentrations were also evaluated with only a few studies reporting liver concentrations ranging from 22 to 77 and 20 to 300 nmol/g tissue in humans and rats, respectively (32, 33). Because the ranges of l-cystine from these studies are similar, it is unclear as to if l-cystine concentrations play a role in the formation of ATCA as opposed to thiocyanate. Further characterization of l-cystine levels may shed some light on this hypothesized explanation of elevated ATCA in rabbits.
As seen in Figure 2, cyanide exhibited a linear relationship between the cyanide dose and blood concentrations under the conditions tested, which fundamentally implies that cyanide has rapid and complete distribution, with first-order kinetic behavior. For SCN−, the dose–concentration behavior was non-linear and as the dose of cyanide was increased, the plasma concentration of SCN− initially increased to 4 mg/kg where it remained essentially constant. This is likely due to saturation of the sulfur-donor pathway for cyanide detoxification. As mentioned here previously, the ATCA concentration–dose behavior was not evaluated due to the large interanimal variability of plasma ATCA concentrations in rats. This is an area of potential future study likely best investigated in rabbits.
The percentage of cyanide converted to ATCA in this study was estimated for rats, rabbits and swine. Cyanide conversion to ATCA was calculated as a percentage by dividing the maximum concentration of ATCA in each model over the total maximum concentration of cyanide, thiocyanate and ATCA. Considering that cyanide is distributed in the range of 70–96% in the red blood cells, with the remainder in the plasma (25, 34), it was estimated that ∼0.10–0.78%, 2.46–9.19% and 0.60–3.7% of the cyanide dose was converted to ATCA in rats, rabbits and swine, respectively. Although the calculation of the percentage of cyanide conversion to ATCA from the current study is meant to be a rough estimate and further studies should be undertaken to accurately determine the amount of cyanide converted to ATCA, our calculations are significantly lower than previously reported estimates of 15–20% (6) and are likely even overestimated for swine and rabbits, due to SCN− failing to reach a maximum. This difference is likely due to differences in experimental protocols, where Wood and Cooley (6) initially administered 20 mg of l-cystine-S35 via tail vein, and after 15 and 25 min, 1 mg of NaCN was subcutaneously administered. The added l-cystine potentially artificially increased the amount of ATCA generated.
It is well known that all biological samples contain endogenous concentrations of cyanide and its metabolites (4, 35–38). Therefore, these concentrations were measured in each animal prior to cyanide exposure and are reported in Table II, alongside previous applicable work. In comparing the endogenous concentrations of CN, SCN− and ATCA, the concentrations found in rabbits are closer to those found in humans than the rats and swine.
Conclusion
If kidney rhodanese and endogenous cyanide concentrations are indicators of similarity between human and animal cyanide metabolism, the rabbits would be more similar to humans than rat and swine. If the rabbit model approximates human behavior, ATCA appears to be a promising candidate for early diagnosis of cyanide poisoning. Specifically, ATCA shows similar behavior relative to cyanide, it increases 40-fold above baseline concentrations, does not metabolize further (11, 14, 39) and it is exceedingly stable during storage of plasma samples (14, 20), something that is a serious issue for CN and SCN− (4, 19). Although the rabbit model appears to be the closest to humans in a number of indirect measurements, future work should address the absorption, distribution and elimination of ATCA in humans (e.g., from nitroprusside patients) in parallel with rabbits in order to confirm applicability of the rabbit to investigate human cyanide metabolism.
Funding
The research was supported by the CounterACT Program, National Institutes of Health Office of the Director and the National Institute of Allergy and Infectious Diseases, Interagency Agreement Numbers Y1-OD-0690-01/A-120-B.P2010-01, Y1-OD-1561-01/A120-B.P2011-01 and the USAMRICD under the auspices of the US Army Research Office of Scientific Services Program Contract No. W911NF-11-D-0001 administered by Battelle (Delivery order 0079, Contract No TCN 11077) and AMRMC W81XWH-12-2-0098. We also gratefully acknowledge funding from the Oak Ridge Institute for Science and Education (ORISE).
Acknowledgments
The authors acknowledge Susan M. Boudreau, RN, BSN, Maria G. Castaneda, MS, Toni E. Vargas, PA-C, MHS and Patricia Dixon, MHS, from the Clinical Research Division, Wilford Hall Medical Center, Lackland AFB, TX, for providing potassium cyanide exposed swine plasma samples for these studies. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army, the National Institutes of Health or the Department of Defense.
References
- 1.Conn EE. Cyanogenesis, the Production of Cyanide, by Plants. Effects of Poisons Plants on Livestock. San Diego: Academic Press; 1978. pp. 301–310. [Google Scholar]
- 2.Ansell M., Lewis FA. A review of cyanide concentrations found in human organs. A survey of literature concerning cyanide metabolism, ‘normal’, non-fatal, and fatal body cyanide levels. Journal of Forensic Medicine. 1970;17:148–155. [PubMed] [Google Scholar]
- 3.Sousa A.B., Manzano H., Soto-Blanco B., Gorniak SL. Toxicokinetics of cyanide in rats, pigs and goats after oral dosing with potassium cyanide. Archives of Toxicology. 2003;77:330–334. doi: 10.1007/s00204-003-0446-y. doi:10.1007/s00204–003–0446-y. [DOI] [PubMed] [Google Scholar]
- 4.Logue BA., Hinkens DM., Baskin SI., Rockwood GA. The analysis of cyanide and its breakdown products in biological samples. Critical Reviews in Analytical Chemistry. 2010;40:122–147. [Google Scholar]
- 5.Minakata K., Nozawa H., Gonmori K., Yamagishi I., Suzuki M., Hasegawa K., et al. Determination of cyanide in blood by electrospray ionization tandem mass spectrometry after direct injection of dicyanogold. Analytical and Bioanalytical Chemistry. 2011;400:1945–1951. doi: 10.1007/s00216-011-4824-7. doi:10.1007/s00216-011-4824-7. [DOI] [PubMed] [Google Scholar]
- 6.Wood JL., Cooley SL. Detoxication of cyanide by cystine. The Journal of Biological Chemistry. 1956;218:449–457. [PubMed] [Google Scholar]
- 7.Hasuike Y., Nakanishi T., Moriguchi R., Otaki Y., Nanami M., Hama Y., et al. Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrology, Dialysis, Transplantation : Official Publication of the European Dialysis and Transplant Association – European Renal Association. 2004;19:1474–1479. doi: 10.1093/ndt/gfh076. doi:10.1093/ndt/gfh076. [DOI] [PubMed] [Google Scholar]
- 8.Pettigrew A.R., Fell GS. Microdiffusion method for estimation of cyanide in whole blood and its application to the study of conversion of cyanide to thiocyanate. Clinical Chemistry. 1973;19:466–471. [PubMed] [Google Scholar]
- 9.Murray S., Lake B.G., Gray S., Edwards A.J., Springall C., Bowey E.A., et al. Effect of cruciferous vegetable consumption on heterocyclic aromatic amine metabolism in man. Carcinogenesis. 2001;22:1413–1420. doi: 10.1093/carcin/22.9.1413. [DOI] [PubMed] [Google Scholar]
- 10.Baskin SI., Brewer T.G. Textbook of Military Medicine. In: Zajtchuk R., Bellamy R.F., editors. TMM Publications; 1997. pp. 271–286. Office of the Surgeion General. [Google Scholar]
- 11.Baskin S.I., Petrikovics I., Kurche J.S., Nicholson J.D., Logue B.A., Maliner B.J., et al. Insights on cyanide toxicity and methods of treatment. In: Flora S.J.S., Romano J.A. Jr, Baskin S.I., Shekhar K., editors. Pharmacological Perspectives of Toxic Chemicals and Their Antidotes. New Delhi, India: Narosa Publishing House; 2004. pp. 105–146. Ch. 9. [Google Scholar]
- 12.Weuffen W., Jess G., Julich W.D., Bernhardt D. Studies on the relationship between 2-iminothiazolidine-4-carboxylic acid and the thiocyanate metabolism in the guinea-pig (author's transl) Pharmazie. 1980;35:221–223. [PubMed] [Google Scholar]
- 13.Bradham L.S., Catsimpoolas N., Wood J.L. Determination of 2-iminothiazolidine-4-carboxylic acid. Analytical Biochemistry. 1965;11:230–237. doi: 10.1016/0003-2697(65)90010-2. [DOI] [PubMed] [Google Scholar]
- 14.Lundquist P., Kagedal B., Nilsson L., Rosling H. Analysis of the cyanide metabolite 2-aminothiazoline-4-carboxylic acid in urine by high-performance liquid chromatography. Analytical Biochemistry. 1995;228:27–34. doi: 10.1006/abio.1995.1310. doi:10.1006/abio.1995.1310. [DOI] [PubMed] [Google Scholar]
- 15.Logue B.A., Maserek W.K., Rockwood G.A., Keebaugh M.W., Baskin S.I. The analysis of 2-amino-2-thiazoline-4-carboxylic acid in the plasma of smokers and non-smokers. Toxicology Mechanisms and Methods. 2009;19:202–208. doi: 10.1080/15376510802488165. doi:10.1080/15376510802488165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrikovics I., Yu J.C., Thompson D.E., Jayanna P., Logue B.A., Nasr J., et al. Plasma persistence of 2-aminothiazoline-4-carboxylic acid in rat system determined by liquid chromatography tandem mass spectrometry. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2012;891–892:81–84. doi: 10.1016/j.jchromb.2012.01.024. doi:10.1016/j.jchromb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Yu J.C., Martin S., Nasr J., Stafford K., Thompson D.E., Petrikovics I. LC-MS/MS analysis of 2-aminothiazoline-4-carboxylic acid as a forensic biomarker for cyanide poisoning. The World Journal of Methodology. 2012;2:33–41. doi: 10.5662/wjm.v2.i5.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagasawa H.T., Cummings S.E., Baskin S.I. The structure of "ITCA", a urinary metabolite of cyanide. Organic Preparations and Procedures International. 2004;36:178–182. doi:10.1080/00304940409355393. [Google Scholar]
- 19.Bhandari R.K., Oda R.P., Youso S.L., Petrikovics I., Bebarta V.S., Rockwood G.A., et al. Simultaneous determination of cyanide and thiocyanate in plasma by chemical ionization gas chromatography mass-spectrometry (CI-GC-MS) Analytical and Bioanalytical Chemistry. 2012;404:2287–2294. doi: 10.1007/s00216-012-6360-5. doi:10.1007/s00216-012-6360-5. [DOI] [PubMed] [Google Scholar]
- 20.Logue B.A., Kirschten N.P., Petrikovics I., Moser M.A., Rockwood G.A., Baskin S.I. Determination of the cyanide metabolite 2-aminothiazoline-4-carboxylic acid in urine and plasma by gas chromatography-mass spectrometry. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2005;819:237–244. doi: 10.1016/j.jchromb.2005.01.045. doi:10.1016/j.jchromb.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 21.Shargel L., Wu-Pong S., Yu A.B.C. Applied Biopharmaceuticals and Pharmacokinetics. New York: McGraw Hill; 2005. One-compartment open model: intravenous bolus administration. 6th edition. [Google Scholar]
- 22.Welling P.G. Pharmacokinetic principles. In: Welling P G., De La Iglesia F.A., editors. Drug Toxicokinetics. New York: Marcel Dekker, Inc; 1993. [Google Scholar]
- 23.Leuschner J., Winkler A., Leuschner F. Toxicokinetic aspects of chronic cyanide exposure in the rat. Toxicology Letters. 1991;57:195–201. doi: 10.1016/0378-4274(91)90146-w. doi:10.1016/0378-4274(91)90146-W. [DOI] [PubMed] [Google Scholar]
- 24.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Cyanide. Atlanta, USA: US Department of Health and Human Services; 2006. 2006. [PubMed] [Google Scholar]
- 25.Lundquist P., Rosling H., Sorbo B. Determination of cyanide in whole blood, erythrocytes, and plasma. Clinical Chemistry. 1985;31:591–595. [PubMed] [Google Scholar]
- 26.Himwich W.A., Saunders J.P. Enzymatic conversion of cyanide to thiocyanate. American Journal of Physiology. 1948;153:348–354. doi: 10.1152/ajplegacy.1948.153.2.348. [DOI] [PubMed] [Google Scholar]
- 27.Hartung R. Cyanides and nitriles. In: Clayton G.D., Clayton F.E., editors. Patty's Industrial Hygiene. 3rd edition. New York: John Wiley & Sons, Inc.; 1982. pp. 4845–4900. [Google Scholar]
- 28.Schulz V., Bonn R., Kindler J. Kinetics of elimination of thiocyanate in 7 healthy subjects and in 8 subjects with renal failure. Klinische Wochenschrift. 1979;57:243–247. doi: 10.1007/BF01477493. [DOI] [PubMed] [Google Scholar]
- 29.Junge B. Changes in serum thiocyanate concentration on stopping smoking. British Medical Journal. 1985;291:22. doi: 10.1136/bmj.291.6487.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westley J. Thiosulfate: cyanide sulfurtransferase (rhodanese) Methods in Enzymology. 1981;77:285–291. doi: 10.1016/s0076-6879(81)77039-3. [DOI] [PubMed] [Google Scholar]
- 31.Aminlari M., Malekhusseini A., Akrami F., Ebrahimnejad H. Cyanide-metabolizing enzyme rhodanese in human tissues: comparison with domestic animals. Comparative Clinical Pathology. 2007;16:47–51. [Google Scholar]
- 32.Lee J.I., Londono M., Hirschberger L.L., Stipanuk M.H. Regulation of cysteine dioxygenase and gamma-glutamylcysteine synthetase is associated with hepatic cysteine level. The Journal of Nutritional Biochemistry. 2004;15:112–122. doi: 10.1016/j.jnutbio.2003.10.005. doi:10.1016/j.jnutbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Stipanuk M.H., Dominy J.E., Jr, Lee J.I., Coloso R.M. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. The Journal of Nutrition. 2006;136(6 Suppl.):1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 34.Baar S. The micro determination of cyanide: its application to the analysis of whole blood. The Analyst. 1966;91:268–272. doi: 10.1039/an9669100268. [DOI] [PubMed] [Google Scholar]
- 35.Manzano H., de Sousa A.B., Soto-Blanco B., Guerra J.L., Maiorka P.C., Gorniak S.L. Effects of long-term cyanide ingestion by pigs. Veterinary Research Communications. 2007;31:93–104. doi: 10.1007/s11259-006-3361-x. doi:10.1007/s11259-006-3361-x. [DOI] [PubMed] [Google Scholar]
- 36.Shen X., Wang P., Hu S., Yang Z., Ma H., Gao W., et al. Simultaneous determination of oxygen, nitrogen and hydrogen in metals by pulse heating and time of flight mass spectrometric method. Talanta. 2011;84:1057–1062. doi: 10.1016/j.talanta.2011.03.007. doi:10.1016/j.talanta.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Takeda S., Inada Y., Tomaru T., Ikeda T., Tashiro N., Morimoto F., et al. Plasma and red blood cell cyanide concentrations during hypotension induced by sodium nitroprusside or by a nitroprusside-trimetaphan mixture in rabbits. Masui The Japanese Journal of Anesthesiology. 1990;39:701–707. [PubMed] [Google Scholar]
- 38.Toida T., Togawa T., Tanabe S., Imanari T. Determination of cyanide and thiocyanate in blood plasma and red cells by high-performance liquid chromatography with fluorometric detection. Journal of Chromatography. 1984;308:133–141. doi: 10.1016/s0021-9673(01)87540-3. [DOI] [PubMed] [Google Scholar]
- 39.Weuffen W., Jess G., Jülich W.D., Bernhardt D. Studies on the relationship between 2-iminothiazolidine-4-carboxylic acid and the thiocyanate metabolism in the guinea-pig (author's transl) Die Pharmazie. 1980;35:221–223. [PubMed] [Google Scholar]
- 40.Shibata M., Inoue K., Yoshimura Y., Nakazawa H., Seto Y. Simultaneous determination of hydrogen cyanide and volatile aliphatic nitriles by headspace gas chromatography, and its application to an in vivo study of the metabolism of acrylonitrile in the rat. Archives of Toxicology. 2004;78:301–305. doi: 10.1007/s00204-004-0545-4. doi:10.1007/s00204-004-0545-4. [DOI] [PubMed] [Google Scholar]
- 41.Tor-Agbidye J., Palmer V.S., Sabri M.I., Craig A.M., Blythe L.L., Spencer P.S. Dietary deficiency of cystine and methionine in rats alters thiol homeostasis required for cyanide detoxification. Journal of Toxicology and Environmental Health Part A. 1998;55:583–595. doi: 10.1080/009841098158269. doi:10.1080/009841098158269. [DOI] [PubMed] [Google Scholar]