Abstract
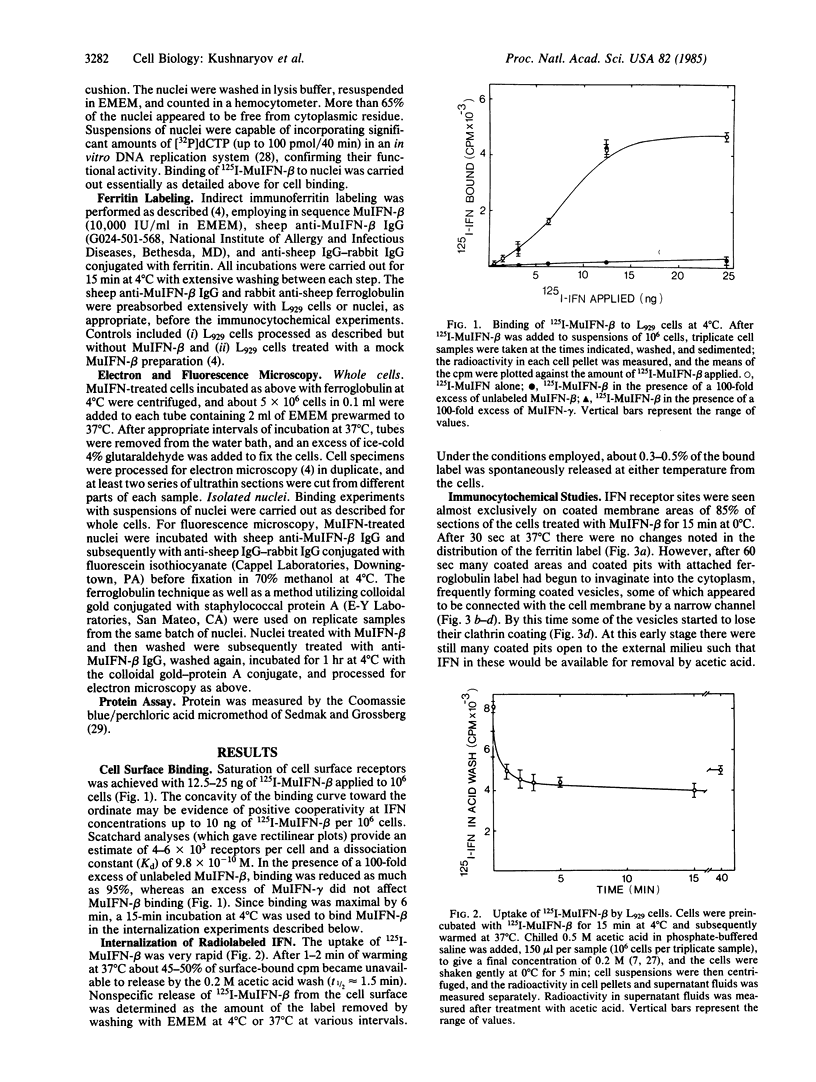
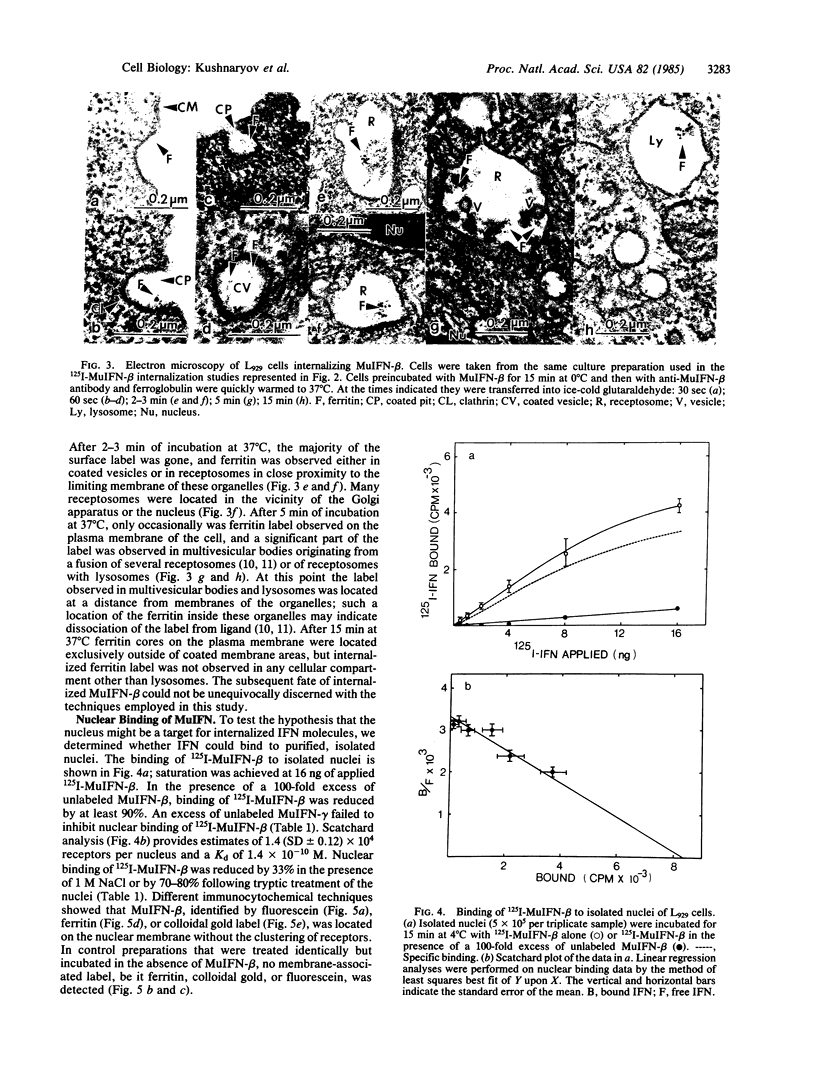
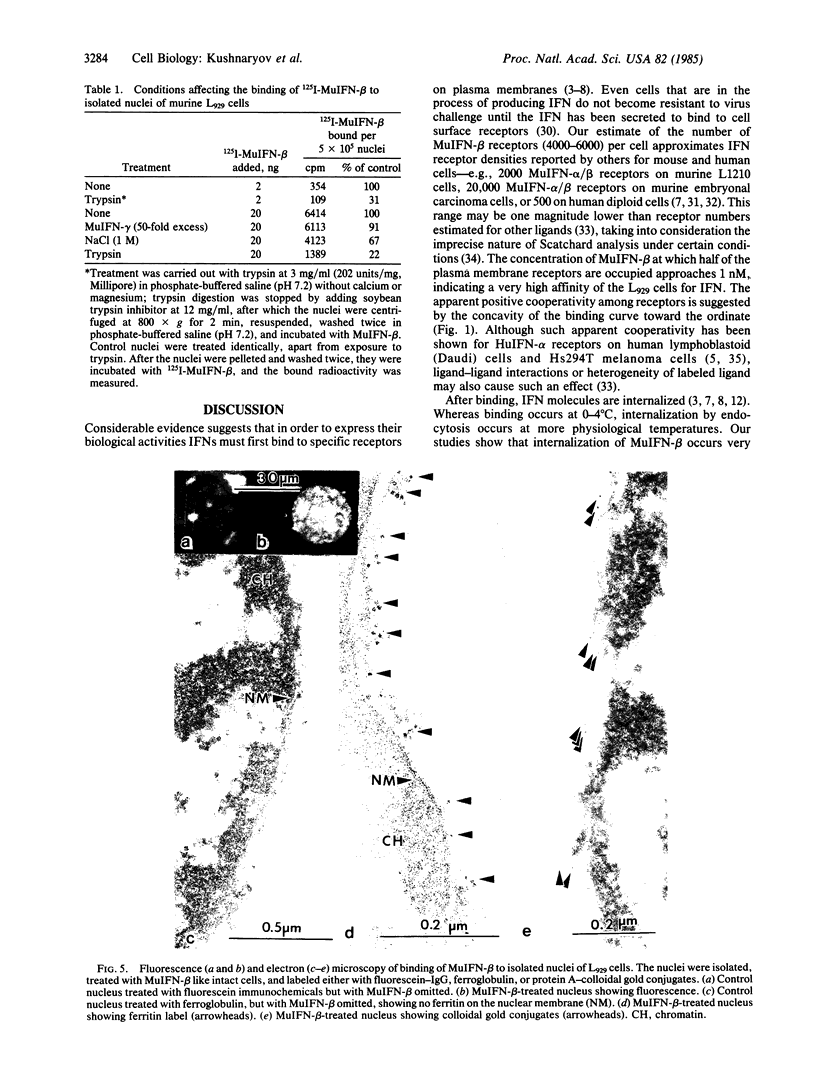
Radioiodinated mouse interferon-beta (125I-MuIFN-beta) bound with high affinity (Kd = 9.8 X 10(-10) M) to plasma membrane of L929 murine fibroblasts (4-6 X 10(3) receptor sites per cell). The binding was saturable and inhibited by a 100-fold excess of unlabeled MuIFN-beta but not by excess mouse IFN-gamma (MuIFN-gamma). MuIFN-beta bound at 4 degrees C was very rapidly internalized upon warming of the cells to 37 degrees C (t 1/2 = 1.5 min). Indirect immunoferritin labeling indicated that MuIFN-beta was initially located in coated pits and subsequently internalized by receptor-mediated endocytosis. Isolated L929 cell nuclei bound 125I-MuIFN-beta with a 7-fold higher affinity (Kd = 1.4 X 10(-10) M) and higher receptor density (about 10(4) per nucleus) than that for the plasma membrane. Binding to the nuclear membrane was inhibited by a 100-fold excess of unlabeled MuIFN-beta but not by excess MuIFN-gamma. Trypsin treatment of nuclei decreased IFN binding by 80%, suggesting that the putative nuclear receptors are protein. Specific binding of MuIFN-beta to nuclei was also shown by fluorescence and electron microscopy. We propose that the very rapid internalization of MuIFN-beta by receptor-mediated endocytosis is important in the cellular processing of IFN and that its high-affinity binding to the nuclear membrane suggests the nucleus as an intracellular site of IFN action.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Gresser I., Hovanessian A. G., Bandu M. T., Blanchard B., Blangy D. Specific high-affinity binding of 125I-labeled mouse interferon to interfevon resistant embryonal carcinoma cells in vitro. Virology. 1981 Oct 30;114(2):585–588. doi: 10.1016/0042-6822(81)90241-5. [DOI] [PubMed] [Google Scholar]
- Anderson P., Vilcek J. Synthesis and biological characterization of a covalent conjugate between interferon and ricin toxin B chain. Virology. 1982 Dec;123(2):457–460. doi: 10.1016/0042-6822(82)90279-3. [DOI] [PubMed] [Google Scholar]
- Anderson P., Yip Y. K., Vilcek J. Human interferon-gamma is internalized and degraded by cultured fibroblasts. J Biol Chem. 1983 May 25;258(10):6497–6502. [PubMed] [Google Scholar]
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Branca A. A., Faltynek C. R., D'Alessandro S. B., Baglioni C. Interaction of interferon with cellular receptors. Internalization and degradation of cell-bound interferon. J Biol Chem. 1982 Nov 25;257(22):13291–13296. [PubMed] [Google Scholar]
- Chany C. Membrane-bound interferon specific cell receptor system: role in the establishment and amplification of the antiviral state. Biomedicine. 1976 Jun;24(3):148–157. [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Smith S. A., Epstein L. B., Epstein C. J. Mouse trisomy 16 as an animal model of human trisomy 21 (Down syndrome): production of viable trisomy 16 diploid mouse chimeras. Dev Biol. 1984 Feb;101(2):416–424. doi: 10.1016/0012-1606(84)90156-8. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Czarniecki C. W., Fennie C. W., Powers D. B., Estell D. A. Synergistic antiviral and antiproliferative activities of Escherichia coli-derived human alpha, beta, and gamma interferons. J Virol. 1984 Feb;49(2):490–496. doi: 10.1128/jvi.49.2.490-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. J., McManus N. H., Epstein L. B., Branca A. A., D'Alessandro S. B., Baglioni C. Direct evidence that the gene product of the human chromosome 21 locus, IFRC, is the interferon-alpha receptor. Biochem Biophys Res Commun. 1982 Aug;107(3):1060–1066. doi: 10.1016/0006-291x(82)90629-5. [DOI] [PubMed] [Google Scholar]
- Faltynek C. R., Branca A. A., McCandless S., Baglioni C. Characterization of an interferon receptor on human lymphoblastoid cells. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3269–3273. doi: 10.1073/pnas.80.11.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Clawson G. A., Smuckler E. A., Purrello F., Vigneri Action of insulin at the nuclear envelope. Mol Cell Biochem. 1982 Oct 1;48(1):3–14. doi: 10.1007/BF00214816. [DOI] [PubMed] [Google Scholar]
- Jameson P., Grossberg S. E. Virus yield-reduction assays for interferon: picornavirus hemagglutination measurements. Methods Enzymol. 1981;78(Pt A):357–368. doi: 10.1016/0076-6879(81)78142-4. [DOI] [PubMed] [Google Scholar]
- Johnson L. K., Vlodavsky I., Baxter J. D., Gospodarowicz D. Nuclear accumulation of epidermal growth factor in cultured rat pituitary cells. Nature. 1980 Sep 25;287(5780):340–343. doi: 10.1038/287340a0. [DOI] [PubMed] [Google Scholar]
- Joshi A. R., Sarkar F. H., Gupta S. L. Interferon receptors. Cross-linking of human leukocyte interferon alpha-2 to its receptor on human cells. J Biol Chem. 1982 Dec 10;257(23):13884–13887. [PubMed] [Google Scholar]
- Klotz I. M. Number of receptor sites from scatchard and klotz graphs: a constructive critique. Science. 1983 May 27;220(4600):981–981. doi: 10.1126/science.220.4600.981. [DOI] [PubMed] [Google Scholar]
- Kushnaryov V. M., MacDonald H. S., Sedmak J. J., Grossberg S. E. Ultrastructural distribution of interferon receptor sites on mouse L fibroblasts grown in suspension: ganglioside blockade of ligand binding. Infect Immun. 1983 Apr;40(1):320–329. doi: 10.1128/iai.40.1.320-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- McDonald J. R., Agutter P. S. The relationship between polyribonucleotide binding and the phosphorylation and dephosphorylation of nuclear envelope protein. FEBS Lett. 1980 Jul 28;116(2):145–148. doi: 10.1016/0014-5793(80)80629-6. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Rosen O. M., Makman M. H., Bloom B. R. Biochemical analysis of mutants of a macrophage cell line resistant to the growth-inhibitory activity of interferon. J Cell Biol. 1984 Apr;98(4):1342–1347. doi: 10.1083/jcb.98.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale R. L. Chromatin replication in vitro. Properties of a HeLa nuclear system. Biochemistry. 1977 Jun 28;16(13):2847–2853. doi: 10.1021/bi00632a007. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Stanley P., Carver J. P. Lectin receptors and lectin resistance in chinese hamster ovary cells. Adv Exp Med Biol. 1977;84:265–284. doi: 10.1007/978-1-4684-3279-4_13. [DOI] [PubMed] [Google Scholar]
- Taylor J. L., Sedmak J. J., Jameson P., Lin Y. G., Grossberg S. E. Markedly enhanced production of gamma interferon in murine T lymphocytes treated with lentil lectin and the diterpene ester, mezerein. J Interferon Res. 1984 Summer;4(3):315–327. doi: 10.1089/jir.1984.4.315. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Rochette-Egly C. Rapid increase in guanosine 3',5'-cyclic-monophosphate interferon treated mouse leukemia L1210 cells: relationship to the development of the antiviral state and inhibition of cell multiplication. Virology. 1981 Dec;115(2):272–281. doi: 10.1016/0042-6822(81)90110-0. [DOI] [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara-Takahashi M. Cell surface receptor-mediated internalization of interferon: its relation to the antiviral activity of interferon. J Gen Virol. 1983 Nov;64(Pt 11):2409–2418. doi: 10.1099/0022-1317-64-11-2409. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Arnheiter H., Zur Nedden D., Fitzgerald D. J., Willingham M. C. Human interferon alpha enters cells by receptor-mediated endocytosis. Virology. 1983 Oct 15;130(1):195–203. doi: 10.1016/0042-6822(83)90127-7. [DOI] [PubMed] [Google Scholar]