Abstract
The present studies evaluated the role of α1 and α5 subunit-containing GABAA receptors (α1GABAA and α5GABAA receptors, respectively) in the ability of benzodiazepine (BZ)-type drugs to alter performance in the cognitive domain of executive function. Five adult female rhesus monkeys (ages 9–17 years old) were trained on the object retrieval with detours (ORD) task of executive function. For the ORD task, monkeys were required to retrieve food items from a clear box with one open end that was rotated to different positions along with varying placements of food. When the non-selective BZ triazolam and the α1GABAA-preferring agonists zolpidem and zaleplon were evaluated in the ORD task, deficits in performance occurred at doses that did not increase the latency of monkeys to initiate responding and/or increase the percentage of reaches that were incorrect (i.e., reaches in which food was not obtained). Cognition-impairing effects of triazolam and zolpidem in ORD were blocked by the α1GABAA-preferring antagonist, βCCT, whereas the α5GABAA-preferring antagonist XLi-093 blocked the effects of triazolam but not zolpidem. While these findings suggest a role for both α1GABAA and α5GABAA receptor mechanisms, α1GABAA receptor mechanisms appear to be sufficient for impairments in executive function induced by BZ-type drugs.
1. Introduction
Drugs that act at the γ-aminobutyric acid type-A (GABAA) receptor, such as the benzodiazepines (BZs) have been shown to alter learning and memory processes (Arolfo and Brioni, 1991, Buffett-Jerrott and Stewart, 2002). Cognitive deficits are a major impediment to the clinical use of BZ-type drugs as anxiolytics and hypnotics, especially in populations already suffering from neurocognitive disorders. At present, relatively little is known about the mechanisms underlying cognitive deficits induced by BZ-type drugs, or if there are any differences among the clinically available compounds.
GABAA receptors are heteropentameric chloride ion channels that are assembled in a typical stoichiometry of 2 α, 2 β, and 1 γ subunits, and conventional BZs bind specifically to GABAA receptors containing α1, α2, α3 or α5 subunits (α1GABAA, α2GABAA, α3GABAA or α5GABAA receptors, respectively; for review, see Rudolph and Knoflach, 2011). The α1GABAA receptor has been shown to account for approximately 60% of all GABAA receptors and is particularly dense on hippocampal and cortical interneurons (Fritschy et al., 1992, Gao and Fritschy, 1994, McKernan and Whiting, 1996, Rudolph and Knoflach, 2011). The α2GABAA and α3GABAA receptors represent a smaller percentage of the GABAA receptor population, with the α2GABAA receptor particularly dense in hippocampus, cortex, and basal ganglia, whereas the α3GABAA receptor is expressed in cortex and thalamus. In contrast, the α5GABAA receptor is found almost exclusively in hippocampus and deep layers of cortex (for review, see Rudolph and Knoflach, 2011).
BZs and related drugs have long been documented to disrupt memory in human and non-human subjects (e.g., Duka et al., 1996, Ghoneim and Mewaldt, 1975, McNaughton and Morris, 1987; for review, see Stewart, 2005). Broadly stated, in human subjects, administration of BZ-type drugs can result in a loss of the ability to form new memories (i.e., anterograde amnesia). Early reports in human subjects concluded that BZs blocked the acquisition of new information (e.g., Ghoneim et al., 1984a, Ghoneim et al., 1984b), and a number of clinically available BZ-type drugs have been shown to impair memories for facts that are explicitly stored and retrieved (explicit, or declarative, memory; cf. Mintzer et al., 1997, Mintzer and Griffiths, 1999). Although the exact cognitive processes underlying these types of memory deficits are unknown, it has been suggested that BZ-type drugs specifically impair memory for contextual information, i.e., information peripheral to an event, such as time and place in which an event is experienced (Brown and Brown, 1990, Duka et al., 1996, Mintzer and Griffiths, 1999).
Relatively little information is available on the role that GABAA receptor subtypes play in the ability of BZ-type drugs to impair memory processes. One finding from human laboratory studies is that the hypnotic BZ agonist zolpidem did not disrupt memory for spatial contextual information, in contrast to the non-selective BZ agonist triazolam (Mintzer and Griffiths, 1999). Zolpidem displays highest affinity for the α1GABAA receptor subtype and does not bind appreciably to the α5GABAA receptor (Hadingham et al., 1993); the latter of which are densely expressed in hippocampus (for reviews, see Rudolph and Knoflach, 2011, Sieghart and Sperk, 2002). Based on these observations, Mintzer and Griffiths (1999) suggested that zolpidem’s apparent lack of impairment of a visual-cues based task (see also Balkin et al., 1992) is consistent with hippocampal regulation of spatial memory. Interestingly, studies in non-human animals generally suggest α1GABAA-selective agonists are less effective than non-selective BZs in engendering memory deficits (Noguchi et al., 2002, Sanger et al., 1986). Moreover, some memory-impairing effects of BZs are not blocked by the α1GABAA-selective BZ antagonist, β-carboline-3-carboxylate-3-butyl-ester (βCCT; Belzung et al., 2000, Savic et al., 2008).
Although research in recent years has focused on the role of hippocampal α5GABAA receptors in the memory-impairing effects of BZ-type drugs, as noted, BZ-sensitive GABAA receptors exist throughout the CNS (for reviews, see Rudolph and Knoflach, 2011, Sieghart and Sperk, 2002). Consistent with the relatively ubiquitous distribution of GABAA receptor subtypes throughout the brain, BZ-type drugs impair performance on tasks that likely involve regions other than hippocampal/temporal areas (e.g., attentional set-shifting, paired-associates learning, (Coull et al., 1999, Coull et al., 1995).
A cognitive domain often associated with prefrontal cortical areas and not the hippocampus is executive function. This domain characteristically includes processes such as goal formation, planning, initiation, preservation and alteration of goal-directed behavior, problem solving, response inhibition, and cognitive flexibility (Kehagia et al., 2010). To our knowledge, no previous studies have evaluated systematically the effects of BZ-type drugs on executive function. However, Ballard et al. (2009) did demonstrate enhancement of performance in an executive function task in monkeys by administration of an α5GABAA-selective inverse agonist. To the extent that agonists act in an opposite fashion to inverse agonists, these data would suggest that “positive” intrinsic efficacy at the α5GABAA receptor would result in impairments of executive function
The object retrieval with detours (ORD) task in monkeys is often described as providing a measure of executive functioning (Ballard et al., 2009, Jentsch et al., 1999, Taylor et al., 1990). Lesion studies in monkeys have indicated the involvement of prefrontal cortex-striatal circuitry in mediating behavior in the ORD task, with no involvement of the α5GABAA receptor-enriched hippocampus (e.g., Diamond et al., 1989, Dias et al., 1996). It is important to note, however, that α5GABAA receptors are found in deep cortical layers, albeit at relatively low expression levels (Rudolph and Knoflach, 2011) in rodents, and importantly, the expression of α5GABAA receptors in primate prefrontal cortex is unknown.
This experiment is part of a series of studies designed to evaluate the extent to which BZ-type drugs alter cognitive function via α1GABAA and/or α5GABAA receptor subtypes. Our overall hypotheses, based on the relative distributions of the two subtypes, are that (1) the α1GABAA receptor is involved in cognitive impairments in tasks involving cortical regions; and (2) the α5GABAA receptor plays a primary role in cognitive tasks that engage the hippocampus. For this report, we evaluated the role of α1GABAA and α5GABAA receptors in the ORD task of executive function described by Ballard et al. (2009), with the prediction that α1GABAA, but not α5GABAA receptors, would mediate impairments in ORD performance. In contrast to this prediction, evidence was obtained for a role of both α1GABAA and α5GABAA receptors in the ORD task.
2. Methods
2.1. Animals
Subjects were five adult female rhesus monkeys (Macaca mulatta), age 9 to 17 years (9, 11, 13, 17, and 17 years old) with no history of exposure to drug (except for occasional analgesics, anesthetics and/or sedatives for clinical exams/surgeries) or experimental compounds. The monkeys weighed between 6 and 9 kg during the course of the study. Monkeys were individually housed and maintained on a 12 hour lights on/12 hour lights off cycle, with water available ad libitum. All animals were maintained on 20–30 biscuits per day of commercially-available macaque food (LabDiet 5038), which allowed maximum allotment of food availability without a decrease in daily performance during experimental sessions, with the monkeys maintaining steady weights and body conditions as assessed by the clinical veterinary staff. Three times per week, the monkeys were given a small allotment of fruits and/or vegetables as part of the New England Primate Research Center’s environmental enrichment program. Animals in this study were maintained in accordance with the guidelines of the Committee on Animals, Office of Research Subject Protection, of the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals (8th edition, 2011). Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
2.2. Surgery
Monkeys were prepared with chronic indwelling venous catheters (polyvinyl chloride, i.d.: 0.64 mm; o.d.: 1.35 mm) following the general surgical procedures described by Platt et al. (2011). Monkeys initially were anesthetized with 10–20 mg/kg i.m. injection of ketamine for transport to the surgical suite and preparation for the procedure. Throughout surgery, anesthesia was maintained by an isofluorane/oxygen mixture. Under aseptic conditions, a catheter was implanted in the femoral, brachial, or jugular vein and passed to the level of the right atrium. The distal end of the catheter was passed subcutaneously and exited in the mid-scapular region. The external end of the catheter was fed through a fitted jacket and tether system (Lomir Biomedical, Toronto, Canada) and attached to a fluid swivel mounted to the animal’s cage. The exit site of the catheter was inspected routinely and the catheters were flushed two-three times per week with heparinized saline (150–200 U/ml). Physical exams were conducted that occasionally included contrast-dye infused into the catheter, followed by radiography to verify catheter patency and proper placement.
2.3. Overall design
The general design consisted of two phases: (1) determination of dose-response functions for the non-selective BZ triazolam and the α1GABAA receptor-preferring drugs zolpidem and/or zaleplon, and if effects were evident, then (2) evaluation of blockade of a maximally-effective dose of triazolam and zolpidem (supplies of zaleplon were too limited for the antagonism studies) by the α1GABAA receptor-preferring antagonist βCCT and the α5GABAA receptor-preferring antagonist, XLi-093 (1,3-bis(8-ethynyl-5,6-dihydro-5-methyl-6-oxo-4H-imidazo-[1,5a][1,4]benzodiazepine-3-carboxy)propyl diester; Li et al., 2003). All drugs/compounds were administered by the i.v. route, in order to make direct comparisons with other procedures in our laboratory that use this route (e.g., i.v. self-administration). In general, animals were trained in the ORD task until performance reached a priori criteria (see below), and tests of individual drugs/compounds, their respective vehicles, or selected doses of agonists plus antagonists were conducted up to 3 times per week, with training days interspersed. A test session consisted of a 5-min pretreatment with one of a range of doses of agonist or vehicle (phase 1) or a dose of agonist (determined in phase 1) with one of a range of doses of antagonist or vehicle (phase 2). For the phase 2 studies, the two injections were given in separate syringes, with the antagonist administered first and the agonist second. Within a phase, the doses were tested in irregular order, except that an agonist or agonist + antagonist combination was completed before studying the next agonist or agonist + antagonist combination.
2.4. Object Retrieval with Detours (ORD)
Five female rhesus monkeys were trained in the ORD task. Monkeys were individually housed in stainless-steel primate cages (Lab Products Inc, Seaford, DE) which also served as the experimental chamber, allowing the object retrieval apparatus (Metal Smiths, Boston, MA) to be attached to the home cage. A transparent barrier was affixed to the front of an open cage during test sessions. Three arm holes in the transparent barrier allowed for the subject to reach the testing apparatus and to discourage hand preference when obtaining food rewards (either a single marshmallow or dried cranberry, depending on each monkey’s preference). An additional screen was placed in front of the barrier when a trial was not in session and lifted off of the barrier at the beginning of each trial. A transparent test box (6″ × 6″) with only one open face was situated on a tray attached to the barrier. When the screen was lifted from the barrier, the monkeys were expected to reach through one of the three holes to obtain the reward. The reward was located in one of three positions relative to the open face of the box: (1) directly outside of the open face, considered “easy” trials; (2) directly on the inside near the open face, considered “easy” trials; (2) deep within the box, considered a “difficult” trial. Additionally, orientation of the open face varied from line of sight (LOS), left and right from the monkey’s perspective. Complexity of the trial was also dependent on the location of the open face. When the open face was in the LOS, this was considered “easy” and when positioned to the left and right, the complexity of the trial was dependent on the depth of the reward inside the box. The position of the box and the position of the reward in the box were randomly predetermined prior to a daily session so that the animal was given approximately 50% easy and difficult trials.
A session consisted of 15 trials and monkeys were given up to 2 minutes to complete each trial. Correct trials were indicated by the animal reaching for the correct hole and successfully obtaining the treat. An “incorrect reach” occurred if the animal reached for the correct hole, but did not successfully obtain the treat, usually due to the monkey dropping the treat or not completing the act of retrieving the treat. A “barrier reach” occurred when the animal touched a closed side of the test apparatus. An incorrect or barrier reach resulted in termination of the trial. Monkeys were trained until they reached ≥ 75% percent successful responses, for 3 consecutive sessions. Data were calculated as percentage of successful trials in a session. The latency for the animal to initiate the trial was also recorded. Barrier reaches were analyzed as the percentage of total reaches.
2.5. Data analysis
The measures analyzed were the percentage of correct trials, the latencies to initiate a response, and the percentage of touches that consisted of barrier reaches. These data were analyzed for each drug with one-way repeated measures ANOVA, with dose as the factor. Multiple comparisons were made using Bonferroni t-tests, comparing each dose to the vehicle condition. For all comparisons, the α level was set at p ≤ 0.05.
2.6. Drug/compound preparation
Triazolam and zolpidem (zolpidem hemitartate) were purchased from Sigma-Aldrich. Zaleplon was a gift from Wyeth Pharmaceuticals. βCCT and XLi-093 were synthesized by the laboratory of Dr. James M. Cook at the University of Wisconsin-Milwaukee. Triazolam, zolpidem and zaleplon were dissolved in propylene glycol and diluted in 0.9 % sterile saline (final concentration of propylene glycol of 50%) and sterilized by filtration. βCCT was dissolved in 5% 1N HCl, 80% propylene glycol and 15% sterile distilled water and XLi-093 was dissolved in 80% propylene glycol and 20% sterile distilled water. Doses were expressed as the base forms of the drugs. All experimental compounds were administered in injections volumes ranging from 0.03 to 0.15 ml/kg, via the i.v. route, approximately 5 min prior to the initiation of a task. Injections were administered by hand over an approximately 10 second period.
3. Results
3.1. Triazolam, zolpidem, and zaleplon alone
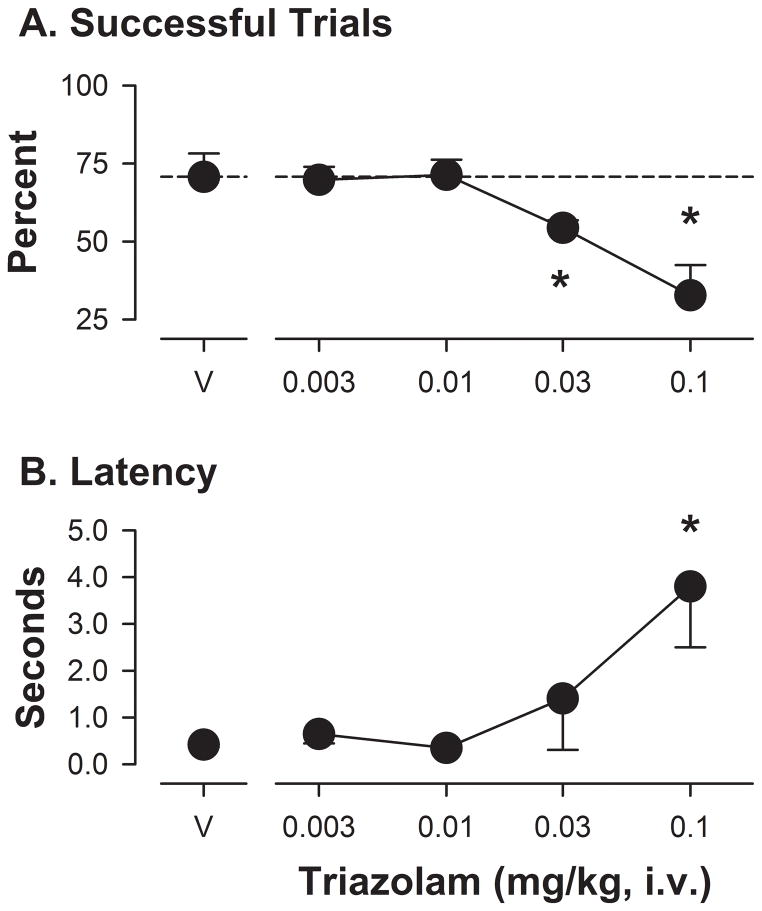
In the ORD task, monkeys consistently achieved ~75% successful trials once trained to criterion levels (number of training sessions ranged from 6 to 30) with very short latencies to initiate the task (often less than 1 second). Most errors during the latter stages of training, as well as during vehicle tests, were barrier reaches (84 – 100% of total errors). Administration of i.v. doses of triazolam prior to an ORD session resulted in a significant effect of dose (F[4,14]= 20.39, p<0.05). Bonferroni t-tests showed that both the 0.03 and 0.1 mg/kg doses of triazolam significantly reduced the percentage of successful trials (Figure 1A). In contrast, no significant effect of triazolam was found with ANOVA performed on the latency to initiate a trial; however, Bonferroni t-tests demonstrated that latencies were significantly increased at the highest triazolam dose tested (Figure 1B, 0.1 mg/kg). For barrier reaches, no significant effect of triazolam was found with ANOVA, but with Bonferroni t-tests, the highest dose of triazolam reduced the percentage of errors that consisted of barrier reaches (i.e., reaches in which a closed side of the box was touched, expressed as means ± SEM: vehicle, 98% ± 2.0; triazolam 0.1 mg/kg, 68% ± 7.3), due to an increase in incorrect reaches (i.e., reaches in which the treat was dropped or otherwise not placed in the mouth).
Figure 1.
Performance on the object retrieval with detours (ORD) task following triazolam administration in rhesus monkeys (N=5). Panel A: Mean (± SEM) percentage of successful trials, defined as a single reach into the apparatus, resulting in food retrieval into the mouth with no errors (i.e., dropping food, placing hand on closed end of box), following vehicle (V) or increasing mg/kg (i.v.) doses of drug. Panel B: Mean (± SEM) latency (in sec) to initiate a trial, defined as hand entering the platform area on which the box was mounted (see Methods for details of the apparatus). Note that *p<0.05 compared with vehicle, Bonferroni t-tests.
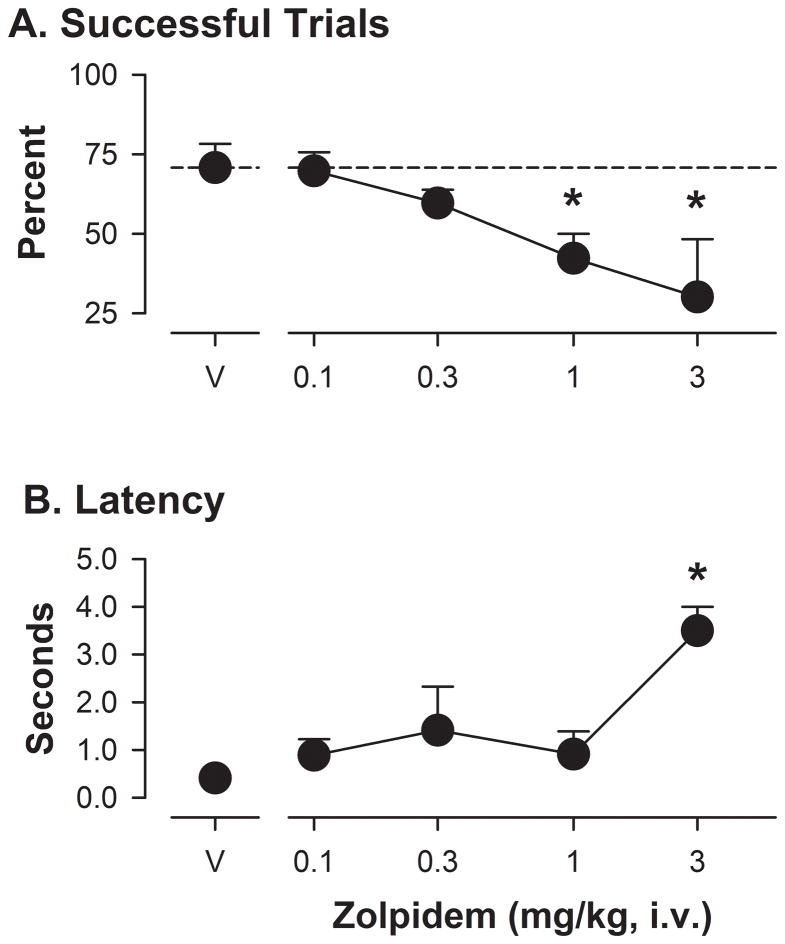
As with triazolam, zolpidem reduced the percentage of successful trials (F[4,15]= 5.05, p<0.05) in a dose-dependent manner, with the averages for the two highest doses (1.0 and 3.0 mg/kg) significantly lower than those obtained after vehicle treatment (Figure 2A). For latency to initiate a trial, the ANOVA approached, but did not achieve significance (F[4,15]= 2.82, p= 0.078) and Bonferroni t-tests revealed that only the highest dose of zolpidem (3.0 mg/kg) significantly reduced average latencies compared with the vehicle control condition (Figure 2B). For barrier reaches, no significant effect of zolpidem was found with ANOVA, but with Bonferroni t-tests, the highest dose of zolpidem reduced the percentage of errors that consisted of barrier reaches (means ± SEM: vehicle, 94% ± 2.9; zolpidem 3.0 mg/kg, 51% ± 9.3).
Figure 2.
Performance on the object retrieval with detours (ORD) task following zolpidem administration in rhesus monkeys (N=5). Other details as in Figure 3.
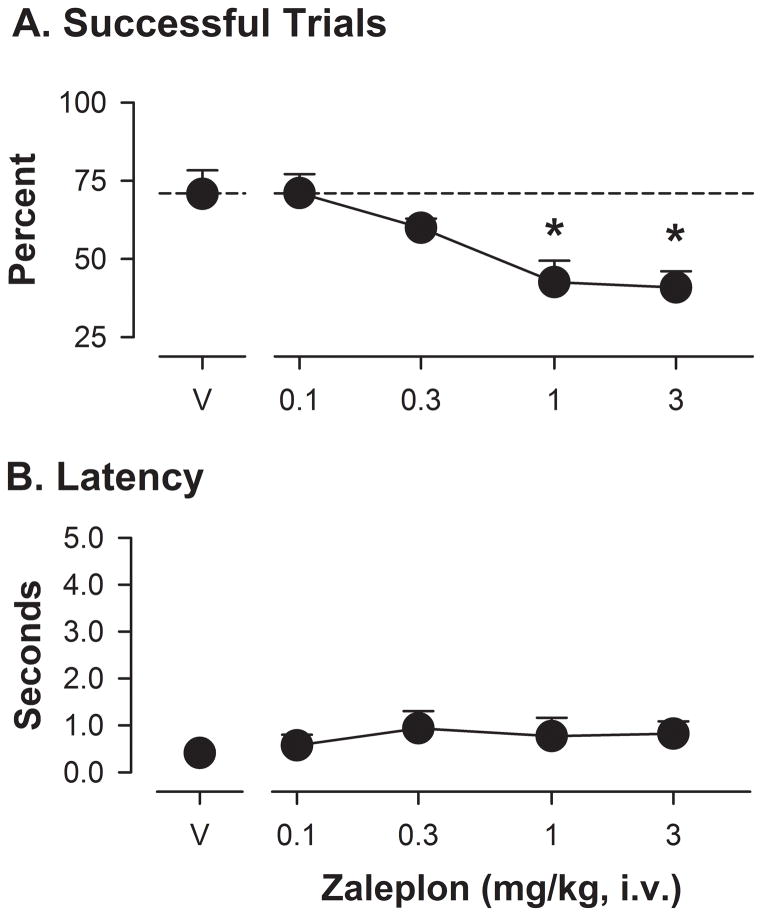
Another α1GABAA-preferring drug, zaleplon, was evaluated in the ORD task (Figure 3). This drug, similar to triazolam and zolpidem, significantly altered the percentage of successful trials (F[4,15]= 15.25, p<0.05). Bonferroni t-tests showed that the 1.0 and 3.0 mg/kg doses of zaleplon resulted in average percentage of successful trials that were lower than these values following vehicle administration (Figure 3A). Interestingly, the ANOVA and Bonferroni tests revealed no significant differences for average latencies to initiate the trial (Figure 3B). For barrier reaches, no significant effect of zaleplon was found with ANOVA, but with Bonferroni t-tests, the highest dose of zaleplon reduced the percentage of errors that were barrier reaches (means ± SEM: vehicle, 98% ± 2.0; zaleplon 3.0 mg/kg, 61% ± 7.5).
Figure 3.
Performance on the object retrieval with detours (ORD) task following zaleplon administration in rhesus monkeys (N=5). Other details as in Figure 3.
3.2. Antagonism studies
In initial studies, the highest doses of the two antagonists βCCT and XLi-093 (1.0 and 3.0 mg/kg for both) had no effects on performance in the ORD task (ANOVA, F’s<1.0; data not shown). For the α1GABAA-preferring antagonist βCCT, a dose range of 0.1 to 3.0 mg/kg, i.v., was evaluated, except when it became clear that this antagonist was more potent at blocking the effects of zolpidem than triazolam and a lower dose (0.03 mg/kg) was added. For the α5GABAA-preferring antagonist XLi-093, a dose range of 0.1 to 3.0 mg/kg, i.v., was evaluated. In both sets of studies, the doses of triazolam and zolpidem chosen were those that reduced the percentage of successful trials without altering the latency to initiate a trial or the percentage of errors that were barrier reaches (0.03 mg/kg and 1.0 mg/kg, respectively). Note that zaleplon was not evaluated in the antagonism studies due to limited supplies of this drug.
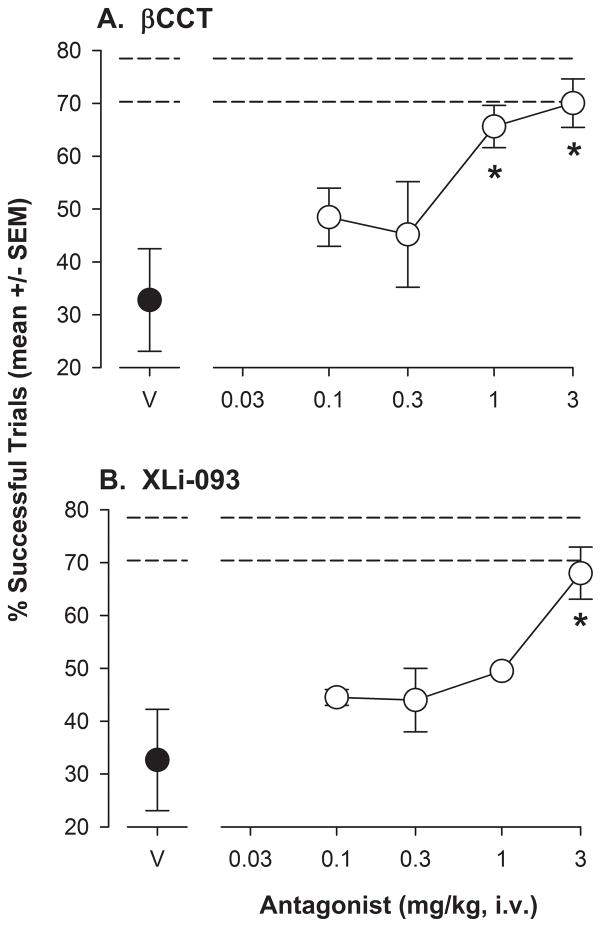
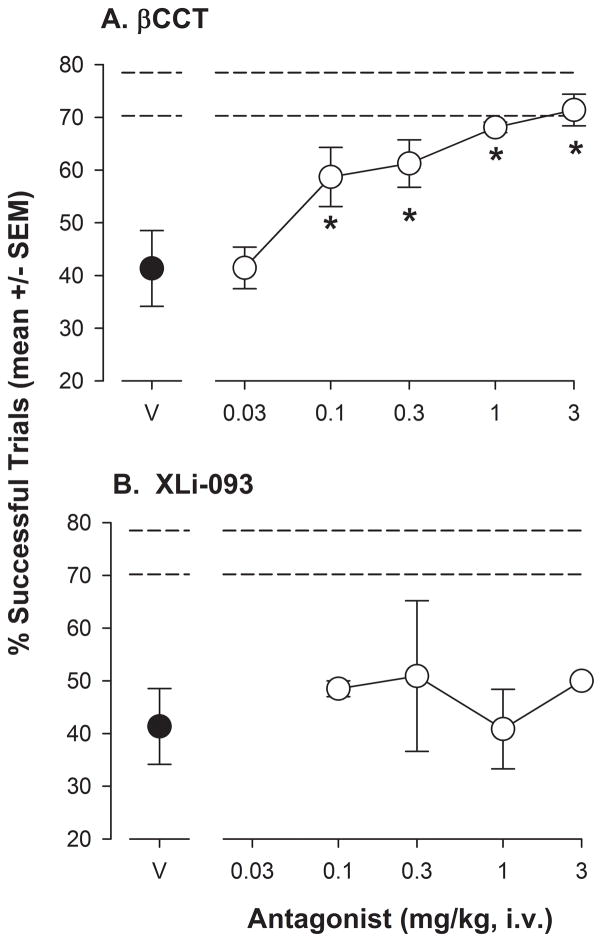
When administered prior to ORD test sessions with 0.03 mg/kg of triazolam, βCCT treatments resulted in a significant alteration in the percentage of successful trials (F[4,16]= 6.57, p<0.05). Bonferroni t-tests comparing βCCT doses to vehicle revealed a significant increase in the percentage of successful trials at doses of 1.0 and 3.0 mg/kg, such that performance essentially returned to untreated levels (represented by the horizontal dashed lines, upper and lower SEMS; Figure 4A). Similarly, XLi-093 altered the percentage of successful trials (F[4,16]= 8.11, p<0.05), with the 3.0 mg/kg dose significantly enhanced compared to vehicle levels (Bonferroni t-tests, Figure 4B). As above, the increase in percentage of successful trials essentially returned to untreated levels.
Figure 4.
Performance on the object retrieval with detours (ORD) task following 0.03 mg/kg triazolam and a range of doses of the antagonists βCCT or XLi-093 in rhesus monkeys (N=5). Both triazolam and the antagonists were administered i.v., 5 min before the session. Horizontal dashed lines represent upper and lower SEMs for performance on the ORD task without drug treatments. Panel A: Percentage of successful trials (see Figure 3 for details) following triazolam with βCCT vehicle (V) and increasing doses of βCCT. Panel B: Percentage of successful trials (see Figure 3 for details) following triazolam with XLi-093 vehicle (V) and increasing doses of XLi-093. Note that *p<0.05 compared with vehicle, Bonferroni t-tests.
Increasing doses of βCCT (0.1–3.0 mg/kg) resulted in an increase in the percentage of successful trials that occurred after 1.0 mg/kg of zolpidem (F[5,20]= 12.36, p<0.05 and Bonferroni t-tests; Figure 5A). In contrast to triazolam, βCCT was approximately 10-fold more potent at blocking zolpidem in the ORD task, based on the lowest significant doses (0.1 mg/kg for zolpidem, 1.0 mg/kg for triazolam). The percentage of successful trials returned to untreated levels, represented by the horizontal dashed lines (upper and lower SEM values) in the Figure. Interestingly, over the dose range tested, XLi-093 had no effects on the percentage of successful trials after treatment with 1.0 mg/kg of zolpidem (Figure 5B).
Figure 5.
Performance on the object retrieval with detours (ORD) task following 1.0 mg/kg zolpidem and a range of doses of the antagonists βCCT or XLi-093 in rhesus monkeys (N=5). Other details as in Figure 6.
4. Discussion
Drugs that act at the GABAA receptor have been shown to alter learning and memory processes (Arolfo and Brioni, 1991, Buffett-Jerrott and Stewart, 2002, Coull et al., 1999, Mintzer and Griffiths, 2005). Cognitive deficits induced by BZ-type drugs in particular are a major impediment to their clinical use as anxiolytics and hypnotics; however, little is known about the mechanisms underlying cognitive deficits induced by BZ-type drugs. Previous studies have illustrated that the diverse behavioral effects of BZ-like drugs may reflect action at different subtypes of the GABAA receptor (Rudolph and Knoflach, 2011). Based on findings in human and non-human animals, we hypothesized that tasks involving primarily frontal circuitry would involve mostly α1GABAA receptors, whereas the α5GABAA receptor is involved in hippocampal-dependent tasks.
The frontostriatal-based ORD task is often described as providing a measure of executive functioning (Ballard et al., 2009, Jentsch et al., 1999, Taylor et al., 1990). In the present study, triazolam, zolpidem, and zaleplon all reduced accuracy in terms of the percentage of successful trials. These deficits occurred for at least one dose in which there were no changes in either latency to initiate a trial or percentage of errors as barrier reaches. Based on these findings, we predict that BZ-type drugs may impair at least some aspect of executive functioning, irrespective of selectivity for α1GABAA receptor subtypes.
The primary type of error that contributed to the percentage of successful trials measure was “barrier” reaches, i.e., reaches that resulted in the monkey touching a closed side of the box. In untreated monkeys, the other type of error—incorrect reaches, in which the reward was dropped—occurred relatively infrequently. The latter type of error increased significantly only at the highest doses tested for all three drugs, which also corresponded to doses that increased latency to respond. Based on research in our laboratories with these drugs and other procedures in rhesus monkeys (e.g., Rowlett et al., 2005), the most parsimonious explanation for increased incorrect reaches and latencies likely is the emergence of motor coordination deficits and/or sedation. The parsing out of barrier and incorrect reaches was of interest due to the fact that changes in barrier reaches often have been attributed to alterations in “impulsivity” (Jentsch et al., 1999), and some drugs do alter barrier reaches selectively (e.g., phencyclidine). Because the proportion of barrier reaches to incorrect reaches did not change at doses of BZ-type drugs that reduced the percentage of successful trials, we do not have evidence for BZ-induced changes in impulsive behavior, at least within the context of the ORD task.
To our knowledge, no previous studies have evaluated systematically the effects of BZ-type drugs in the ORD task. However, Ballard et al. (2009) did demonstrate enhancement of performance in ORD by administration of an α5GABAA-selective inverse agonist. To the extent that agonists act in an opposite fashion to inverse agonists, these data would suggest that “positive” intrinsic efficacy at the α5GABAA receptor would result in a decline in performance. Lesion studies in monkeys, however, have indicated the involvement of prefrontal cortex-striatal circuitry in mediating the ORD task, with no involvement of the α5GABAA receptor-enriched hippocampus (e.g., Dias et al., 1996). It is important to note, however, that α5GABAA receptors are found in deep cortical layers, albeit at relatively low expression levels (Rudolph and Knoflach, 2011) in rodents, and importantly, the expression of α5GABAA receptors in primate prefrontal cortex is unknown.
In order to explore further the role of α1GABAA and α5GABAA receptors in mediating the cognitive-impairing effects of BZ-type drugs in the ORD task, we evaluated performance of these drugs when co-administered with βCCT and XLi-093, antagonists with selectivity for α1GABAA and α5GABAA receptors, respectively. We found that both βCCT and XLi-093 dose-dependently reversed the impairments induced by the non-selective BZ triazolam. In contrast, while βCCT blocked the effects of the α1GABAA receptor-preferring agonist zolpidem, XLi-093 did not block the deficits induced by zolpidem over the same dose range that was effective at blocking the effects of triazolam. Moreover, our results indicated that βCCT was approximately 10-fold more potent in blocking the effects of zolpidem than it was in blocking the effects of triazolam. Collectively, these findings suggest that BZ-type drugs may impair performance on the ORD task by different mechanisms, with both α1GABAA and α5GABAA receptors playing a role in the effects of a non-selective BZ, whereas only α1GABAA receptors were important for the impairments induced by zolpidem. The latter results further suggest that stimulation of α1GABAA receptors may be sufficient to induce deficits in this executive function task.
As discussed above, Ballard et al. (2009) demonstrated that an inverse agonist at α5GABAA receptors enhanced performance on an ORD task similar to the one used in the present study. Our data with the α5GABAA receptor-preferring antagonist, XLi-093, provide additional support to an unexpected role for this subtype in a prefrontal cortex-striatal task. Interestingly, a recent report by (Koh et al., 2013)) demonstrated that compounds that act as functionally-selective agonists at α5GABAA receptors can improve various indices of memory in cognitively-impaired aged rats, whereas a functionally-selective α5GABAA inverse agonist had no effect, yet improved memory performance in young rats. In the present study, the monkeys generally fell into the category of “middle age” for rhesus macaques, according to analyses of survival rates, age of sexual maturity, and other environmental factors (Peters et al., 1996). Moreover, the aged rats (~2 years old) in the Koh et al. (2013) study were pre-selected quantitatively for relatively impaired performance on spatial learning tasks. It is difficult to make direct comparisons regarding the relative ages of monkeys and rats across the studies; nevertheless, the extent to which α5GABAA agonists vs. inverse agonists improve cognition in senescent (i.e., 20+ year old) rhesus monkeys is of considerable interest.
5. Conclusions
The BZ-like drugs characteristically are some of the most frequently used medications for the treatment of anxiety and insomnia. The use of these drugs in treating anxiety and sleep disorders is limited by unwanted side effects, and a major side effect of BZ-type drugs is the detrimental effects on memory processing (Coenen, 1989, Curran and Birch, 1991, Lister, 1985, Mintzer and Griffiths, 2005). At present, the mechanisms of action responsible for cognitive deficits associated with the use of BZ-type drugs are understood poorly. In the present study, we evaluated the ability of a BZ, triazolam, which binds non-selectively to all BZ-sensitive GABAA receptor subtypes, as well as the α1GABAA receptor-preferring drugs zolpidem and zaleplon, on a task associated with executive function (ORD task). Contrary to expectations, all drugs impaired cognitive performance on the ORD task at doses that did not alter responding directly (as assessed by latency to initiate a response and percentage of barrier reaches). Studies with selective antagonists suggested that the α1GABAA receptor subtype is sufficient to induce impairment of ORD performance by BZ-type drugs; however, the α5GABAA receptor subtype can contribute to the deficits in an as-yet specified manner. These findings raise the possibility that BZ-type drugs can induce a deficit in at least certain aspect(s) of executive functioning via an α1GABAA receptor mechanism.
Highlights.
Triazolam (non-selective benzodiazepine), zolpidem, and zaleplon (both preferential agonists at α1 subunit-containing GABAA receptors) impaired performance on the object retrieval with detours (ORD) task of executive function.
The effects of triazolam and zolpidem in the ORD task were reversed by an antagonist that binds preferentially to α1 subunit-containing GABAA receptors.
The effects of triazolam, but not zolpidem, were reversed by an antagonist that binds preferentially to α5 subunit-containing GABAA receptors.
Positive modulation of the α1 subunit-containing GABAA receptor was sufficient to induce impairments in a task of executive function, although evidence for a role of α5 subunit-containing GABAA receptors was obtained.
Acknowledgments
The authors wish to thank Dr. Jeffrey A. Vivian for assistance with development of the ORD task in our laboratories, and Drs. D. Platt and L. Wachtman for comments on earlier versions of this manuscript. This research was supported through an investigator-initiated research grant program from Sepracor, Inc., The Lynde and Harry Bradley Foundation, and USPHS grants AG035361, DA011792, MH046851 and OD011103 (formerly RR000168).
References
- Arolfo MP, Brioni JD. Diazepam impairs place learning in the Morris water maze. Behav Neural Biol. 1991;55:131–6. doi: 10.1016/0163-1047(91)80133-y. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, O’Donnell VM, Wesensten N, McCann U, Belenky G. Comparison of the daytime sleep and performance effects of zolpidem versus triazolam. Psychopharmacology (Berl) 1992;107:83–8. doi: 10.1007/BF02244970. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, et al. RO4938581, a novel cognitive enhancer acting at GABAA alpha5 subunit-containing receptors. Psychopharmacology (Berl) 2009;202:207–23. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Griebel G. Beta-CCT, a selective BZ-omega1 receptor antagonist, blocks the anti-anxiety but not the amnesic action of chlordiazepoxide in mice. Behav Pharmacol. 2000;11:125–31. doi: 10.1097/00008877-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Brown J, Brown MW. The effects of repeating a recognition test in lorazepam-induced amnesia: evidence for impaired contextual memory as a cause of amnesia. Q J Exp Psychol A. 1990;42:279–90. doi: 10.1080/14640749008401222. [DOI] [PubMed] [Google Scholar]
- Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8:45–58. doi: 10.2174/1381612023396654. [DOI] [PubMed] [Google Scholar]
- Coenen AM, van Poppel HC, Gribnau FW, Vossen JM, van Luijtelaar EL. Benzodiazepines and cognition: effects on intentional and incidental learning. In: Horne J, editor. Sleep ‘88. Stuttgart, New York: Gustav Fischer Verlag; 1989. pp. 201–4. [Google Scholar]
- Coull JT, Frith CD, Dolan RJ. Dissociating neuromodulatory effects of diazepam on episodic memory encoding and executive function. Psychopharmacology (Berl) 1999;145:213–22. doi: 10.1007/s002130051051. [DOI] [PubMed] [Google Scholar]
- Coull JT, Middleton HC, Robbins TW, Sahakian BJ. Clonidine and diazepam have differential effects on tests of attention and learning. Psychopharmacology (Berl) 1995;120:322–32. doi: 10.1007/BF02311180. [DOI] [PubMed] [Google Scholar]
- Curran HV, Birch B. Differentiating the sedative, psychomotor and amnesic effects of benzodiazepines: a study with midazolam and the benzodiazepine antagonist, flumazenil. Psychopharmacology (Berl) 1991;103:519–23. doi: 10.1007/BF02244252. [DOI] [PubMed] [Google Scholar]
- Diamond A, Zola-Morgan S, Squire LR. Successful performance by monkeys with lesions of the hippocampal formation on AB and object retrieval, two tasks that mark developmental changes in human infants. Behav Neurosci. 1989;103:526–37. doi: 10.1037//0735-7044.103.3.526. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–86. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Duka T, Curran HV, Rusted JM, Weingartner HJ. Perspectives on cognitive psychopharmacology research. Behav Pharmacol. 1996;7:401–10. [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci U S A. 1992;89:6726–30. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the alpha 1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci. 1994;6:837–53. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Hinrichs JV, Mewaldt SP. Dose-response analysis of the behavioral effects of diazepam: I. Learning and memory. Psychopharmacology (Berl) 1984a;82:291–5. doi: 10.1007/BF00427672. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP. Effects of diazepam and scopolamine on storage, retrieval and organizational processes in memory. Psychopharmacologia. 1975;44:257–62. doi: 10.1007/BF00428903. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP, Hinrichs JV. Dose-response analysis of the behavioral effects of diazepam: II. Psychomotor performance, cognition and mood. Psychopharmacology (Berl) 1984b;82:296–300. doi: 10.1007/BF00427673. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993;43:970–5. [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Elsworth JD, Redmond DE, Jr, Roth RH. Altered frontal cortical dopaminergic transmission in monkeys after subchronic phencyclidine exposure: involvement in frontostriatal cognitive deficits. Neuroscience. 1999;90:823–32. doi: 10.1016/s0306-4522(98)00481-3. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–13. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABA(A) alpha5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013;64:145–52. doi: 10.1016/j.neuropharm.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cao H, Zhang C, Furtmueller R, Fuchs K, Huck S, et al. Synthesis, in vitro affinity, and efficacy of a bis 8-ethynyl-4H-imidazo[1,5a]- [1,4]benzodiazepine analogue, the first bivalent alpha5 subtype selective BzR/GABA(A) antagonist. J Med Chem. 2003;46:5567–70. doi: 10.1021/jm034164c. [DOI] [PubMed] [Google Scholar]
- Lister RG. The amnesic action of benzodiazepines in man. Neurosci Biobehav Rev. 1985;9:87–94. doi: 10.1016/0149-7634(85)90034-x. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–43. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Morris RG. Chlordiazepoxide, an anxiolytic benzodiazepine, impairs place navigation in rats. Behav Brain Res. 1987;24:39–46. doi: 10.1016/0166-4328(87)90034-9. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997;8:561–74. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Triazolam and zolpidem: effects on human memory and attentional processes. Psychopharmacology (Berl) 1999;144:8–19. doi: 10.1007/s002130050971. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Drugs, memory, and metamemory: a dose-effect study with lorazepam and scopolamine. Exp Clin Psychopharmacol. 2005;13:336–47. doi: 10.1037/1064-1297.13.4.336. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Kitazumi K, Mori M, Shiba T. Effect of zaleplon on learning and memory in rats. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:183–8. doi: 10.1007/s00210-002-0576-4. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, et al. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861–74. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD. Models of neurological disease (substance abuse): self-administration in monkeys. Curr Protoc Pharmacol. 2011;Chapter 10(Unit10):5. doi: 10.1002/0471141755.ph1005s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A. 2005;102:915–20. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–97. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ, Joly D, Zivkovic B. Effects of zolpidem, a new imidazopyridine hypnotic, on the acquisition of conditioned fear in mice. Comparison with triazolam and CL 218,872. Psychopharmacology (Berl) 1986;90:207–10. doi: 10.1007/BF00181243. [DOI] [PubMed] [Google Scholar]
- Savic MM, Clayton T, Furtmuller R, Gavrilovic I, Samardzic J, Savic S, et al. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res. 2008;1208:150–9. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. 2005;66 (Suppl 2):9–13. [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Redmond DE., Jr Cognitive and motor deficits in the acquisition of an object retrieval/detour task in MPTP-treated monkeys. Brain. 1990;113 ( Pt 3):617–37. doi: 10.1093/brain/113.3.617. [DOI] [PubMed] [Google Scholar]