Abstract
Maternally transmitted antigen (Mta) is a murine cell surface class I-like antigen that is defined by specific cytotoxic lymphocyte reactivity. Mta is unique in that its expression requires cooperation between genetic elements both in the Qa/Tla region of chromosome 17 and in the cytoplasm. In view of the known cytoplasmic, and thus maternal, inheritance of mitochondria, we have directly assessed their potential involvement in Mta expression. The mitochondria-specific lethal dye rhodamine 6G (R6G) was used to control the input of mitochondria into cell hybrids. The parental lines, one of BALB/c and one of NZB origin, were known to differ in Mta and mtDNA phenotype. Our data show that most control BALB/c-NZB hybrids expressed the BALB/c Mta phenotype and likewise contained only BALB/c-type mtDNA. The NZB Mta phenotype was not coexpressed in the control hybrids. However, when the mitochondrial contribution from BALB/c was prevented by R6G treatment, the majority of the resultant hybrids expressed only the NZB Mta type and likewise contained only NZB mtDNA. The exceptional R6G-treated hybrids that continued to express the BALB/c Mta phenotype likewise contained only BALB/c mtDNA. Thus, in every case the mtDNA phenotype correlated with the Mta phenotype of the cells. Together, the data support the remarkable conclusion that mitochondria modulate the phenotypic expression of a cell surface molecule.
Full text
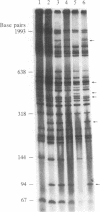
PDF

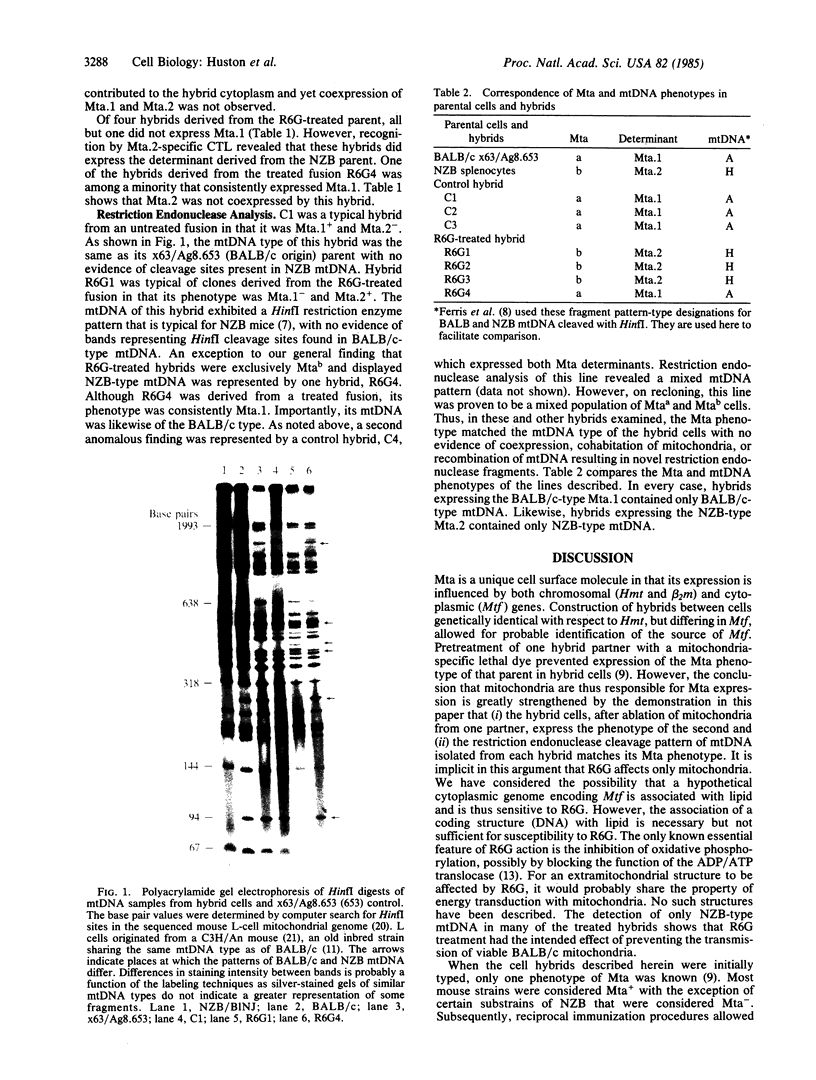


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Blackler A. W. Maternal and cytoplasmic inheritance of mitochondrial DNA in Xenopus. Dev Biol. 1972 Oct;29(2):152–161. doi: 10.1016/0012-1606(72)90052-8. [DOI] [PubMed] [Google Scholar]
- De Francesco L., Attardi G., Croce C. M. Uniparental propagation of mitochondrial DNA in mouse-human cell hybrids. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4079–4083. doi: 10.1073/pnas.77.7.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Ritte U., Lindahl K. F., Prager E. M., Wilson A. C. Unusual type of mitochondrial DNA in mice lacking a maternally transmitted antigen. Nucleic Acids Res. 1983 May 11;11(9):2917–2926. doi: 10.1093/nar/11.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Sage R. D., Wilson A. C. Evidence from mtDNA sequences that common laboratory strains of inbred mice are descended from a single female. Nature. 1982 Jan 14;295(5845):163–165. doi: 10.1038/295163a0. [DOI] [PubMed] [Google Scholar]
- Fischer Lindahl K., Bocchieri M., Riblet R. Maternally transmitted target antigen for unrestricted killing by NZB T lymphocytes. J Exp Med. 1980 Dec 1;152(6):1583–1595. doi: 10.1084/jem.152.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear A. R. Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3628–3637. [PubMed] [Google Scholar]
- Huston D. P., Jenkins R. N., Gresens S. E., Smith R., 3rd, Rich R. R. Cellular requirements for the generation of primary cell-mediated lympholysis responses to Qa-1 antigens. J Immunol. 1985 Apr;134(4):2198–2204. [PubMed] [Google Scholar]
- Huston M. M., Smith R., 3rd, Huston D. P., Rich R. R. Differences in maternal lineages of New Zealand Black mice defined by restriction endonuclease analysis of mitochondrial DNA and by expression of maternally transmitted antigen. J Exp Med. 1983 Jun 1;157(6):2154–2159. doi: 10.1084/jem.157.6.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. N., Rich R. R. Characterization of determinants encoded by four Qa-1 genotypes and their recognition by cloned cytotoxic T lymphocytes. J Immunol. 1983 Nov;131(5):2147–2153. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Lindahl K. F., Bürki K. Mta, a maternally inherited cell surface antigen of the mouse, is transmitted in the egg. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5362–5366. doi: 10.1073/pnas.79.17.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl K. F., Hausmann B., Chapman V. M. A new H-2-linked class I gene whose expression depends on a maternally inherited factor. Nature. 1983 Nov 24;306(5941):383–385. doi: 10.1038/306383a0. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Hausmann B. Cytoplasmic inheritance of a cell surface antigen in the mouse. Genetics. 1983 Mar;103(3):483–494. doi: 10.1093/genetics/103.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl K. F., Langhorne J. Medial histocompatibility antigens. Scand J Immunol. 1981 Dec;14(6):643–654. doi: 10.1111/j.1365-3083.1981.tb00607.x. [DOI] [PubMed] [Google Scholar]
- McGrath J., Solter D. Maternal Thp lethality in the mouse is a nuclear, not cytoplasmic, defect. Nature. 1984 Apr 5;308(5959):550–551. doi: 10.1038/308550a0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., 3rd, Huston D. P., Rich R. R. Primary cell-mediated lympholysis response to a maternally transmitted antigen. J Exp Med. 1982 Dec 1;156(6):1866–1871. doi: 10.1084/jem.156.6.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., 3rd, Huston M. M., Jenkins R. N., Huston D. P., Rich R. R. Mitochondria control expression of a murine cell surface antigen. Nature. 1983 Dec 8;306(5943):599–601. doi: 10.1038/306599a0. [DOI] [PubMed] [Google Scholar]
- Smith R., 3rd, Rich R. R. Polymorphism and tissue distribution of maternally transmitted antigen defined by cytotoxic T lymphocyte lines. J Immunol. 1985 Apr;134(4):2191–2197. [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Mitotic segregation of cytoplasmic determinants for chloramphenicol resistance in mammalian cells II: Fusions with human cell lines. Somatic Cell Genet. 1977 Jan;3(1):93–119. doi: 10.1007/BF01550989. [DOI] [PubMed] [Google Scholar]
- Weide L. G., Clark M. A., Rupert C. S., Shay J. W. Detrimental effect of mitochondria on hybrid cell survival. Somatic Cell Genet. 1982 Jan;8(1):15–21. doi: 10.1007/BF01538647. [DOI] [PubMed] [Google Scholar]
- Yonekawa H., Moriwaki K., Gotoh O., Miyashita N., Migita S., Bonhomme F., Hjorth J. P., Petras M. L., Tagashira Y. Origins of laboratory mice deduced from restriction patterns of mitochondrial DNA. Differentiation. 1982;22(3):222–226. doi: 10.1111/j.1432-0436.1982.tb01255.x. [DOI] [PubMed] [Google Scholar]
- Ziegler M. L., Davidson R. L. Elimination of mitochondrial elements and improved viability in hybrid cells. Somatic Cell Genet. 1981 Jan;7(1):73–88. doi: 10.1007/BF01544749. [DOI] [PubMed] [Google Scholar]