Abstract
Introduction
Additional oral antidiabetic agents to metformin, sulfonylureas (SU) and thiazolidinediones (TZD) are approved for the treatment of type 2 diabetes.
Areas covered
The efficacy and safety of metformin, SUs, TZDs, dipeptidyl peptidase-IV (DPP-4) inhibitors, meglitinide analogs, α-glucosidase inhibitors (AGIs), bile-acid sequestrants (BAS) and bromocriptine will be reviewed.
Expert opinion
Several new oral agents have been approved for type 2 diabetes management in recent years. It is important to understand the efficacy and safety of these medications in addition to the older agents to best maximize oral drug therapy for diabetes. Of the recently introduced oral hypoglycemic/antihyperglycemic agents, the DPP-4 inhibitors are moderately efficacious compared with mainstay treatment with metformin with a low side-effect profile and have good efficacy in combination with other oral agents and insulin. They are a recommended alternative when metformin use is limited by gastrointestinal (GI) side effects or when SU treatment results in significant hypoglycemia or weight gain. Meglitinide analogs are limited by their frequent dosing, expense and hypoglycemia (repaglinide > nateglinide), while AGIs are also limited by their dosing schedule and GI side-effect profile. BAS and bromocriptine have the lowest efficacy with regard to HbA1c reduction, also are plagued by GI adverse reactions, but have a low risk of hypoglycemia.
Keywords: α-glucosidase inhibitor, bile-acid sequestrant, bromocriptine, DPP-4 inhibitor, meglitinide, metformin, sulfonylurea, thiazolidinedione, type 2 diabetes
1. Introduction
Treatment of type 2 diabetes is based on an interplay of patient characteristics, severity of hyperglycemia and available therapeutic options. Metformin, sulfonylureas (SU) and thiazolidinediones (TZD) are the most studied of the oral medications used worldwide. They play a prominent initial role in the type 2 diabetes treatment algorithm recommended by the American Diabetes Association (ADA) and the European Diabetes Association for the Study of Diabetes (EASD) [1]. Metformin is considered first-line therapy unless not tolerated or contraindicated. Second-line therapy then includes SUs, TZDs, dipeptidyl peptidase-IV (DPP-4) inhibitors, glucagon-like polypeptide-1 (GLP-1) agonists or insulin. DPP-4 inhibitors are relatively newer and are the only oral agent in the incretin family of therapeutic targets. In addition, multiple oral medications have been approved for the treatment of type 2 diabetes that are less widely used. Meglitinides are suggested as an alternative to SU therapy for patients with irregular meal times or late post-prandial hypoglycemia with traditional SU therapy. α-Glucosidase inhibitors (AGIs), bile-acid sequestrants (BAS) and bromocriptine have no current place on the formal treatment algorithm, suggested briefly as potential therapy in selected patients. This review will predominantly focus on the efficacy and safety of DPP-4 inhibitors, meglitinide analogs, AGIs, BAS and bromocriptine but will also address metformin, SUs and TZDs that have been used for a longer duration in clinical practice.
The expected improvement in HbA1c with the use of metformin, SUs and TZDs is approximately 1 -- 1.5% [2]. In pooled analysis, metformin therapy is associated with a greater reduction in weight and low-density lipoprotein (LDL) cholesterol compared with SUs and TZDs and less risk of hypoglycemia compared with SU therapy. Efficacy for the SU class has clearly been shown but increased risk of major cardiovascular events compared with metformin use was recently demonstrated in a retrospective cohort study of Veterans who initiated either metformin or SU monotherapy [3]. TZDs have proven essentially equally efficacious as metformin and SU therapy. Subjects treated with pioglitazone also have the added benefit of significant decreases in triglycerides (TG) and of the dense, more atherogenic LDL particle concentration combined with increases in high-density lipoprotein (HDL) cholesterol levels [4,5]. Supporting the above, the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROACTIVE) trial did find vascular benefits in patients with type 2 diabetes when treated with pioglitazone [6]. Rosiglitazone on the other hand, increases TG levels and LDL particle concentrations and increases HDL levels to a lesser extent than pioglitazone. Both pioglitazone and rosiglitazone increase LDL cholesterol levels but pioglitazone does so to a lesser extent than rosiglitazone [4]. Peroxisome proliferator-activated receptor-gamma (PPAR-γ), the receptor for TZDs, modulates CD36, a macrophage cell surface protein which binds oxidized LDL and contributes to foam cell accumulation in arteries during atherosclerosis [7]. Targeted disruption of the CD36 gene has been shown to protect against atherosclerotic lesion development [8]. Unfortunately, TZDs have been plagued by safety concerns. Rosiglitazone was recently withdrawn over concerns of increased risk for myocardial infarction and in 2011 pioglitazone was associated with an increased risk for bladder cancer [9,10]. Further risks associated with TZDs include edema and bone fracture [11,12].
2. DPP-4 inhibitors
The DPP-4 inhibitors increase concentrations of both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) by blocking their degradation by the DPP-4 enzyme. Demonstration of the incretin effect occurred in the 1960s when an intrajejunal glucose infusion was found to produce a larger insulin response compared with an equivalent intravenous glucose infusion [13]. GLP-1 and GIP are responsible for the majority of nutrient-stimulated insulin secretion. GLP-1 action also results in suppression of glucagon secretion [14], slowing of gastric emptying [15] and in mice/rats an increase in β-cell mass [16,17] and maintenance of β-cell function [18]. Since diabetes is characterized by a loss of the incretin effect, treatments that mimic and enhance the incretin axis were sought. DPP-4 inhibitor use results in increased levels of GLP-1, GIP and insulin, but decreased levels of glucagon [19].
Sitagliptin was the first DPP-4 inhibitor approved in the USA in 2006, followed by saxagliptin in 2009 and linagliptin in 2011. Each are administered once daily, with maximum dosing being sitagliptin 100 mg, saxagliptin 5 mg and linagliptin 5 mg. Vildagliptin is approved for use in Europe and several countries outside of the USA while alogliptin is currently approved for clinical use in Japan but not yet in the USA or Europe. The maximum recommended dose for vildagliptin is 100 mg/day while alogliptin is recommended at 25 mg/day.
The pharmacokinetics across the DPP-4 class of oral diabetes medications is, in general, similar with a few exceptions [20,21]. The agents in this class have good oral bioavailability which remains essentially unchanged with food intake. The relatively long half-lives of sitagliptin, linagliptin and alogliptin allow for once-daily dosing. Saxagliptin and vildagliptin both have shorter half-lives but saxagliptin is still dosed once daily because of its active metabolite while vildagliptin is dosed twice daily (b.i.d.). DPP-4 inhibitors are mainly excreted via the kidney, linagliptin and vildagliptin (eliminated both by the liver and kidney) being the exceptions [22]. Because of renal excretion, dose adjustment of all agents except linagliptin is recommended in renal impairment. Vildagliptin is not recommended for use in hepatic impairment, but otherwise there are no recommendations for dose adjustment with hepatic impairment which may be partly due to a lack of experience in patients with severe liver dysfunction. Drug--drug interactions are of most concern with saxagliptin for which a dose reduction is recommended when co-administered with a CYP3A4 inhibitor, such as ketoconazole, clarithromycin and atazanavir. In pharmacodynamic testing, DPP-4 inhibition is achieved and an increase in GLP-1 levels by each agent is most evident after a meal challenge [23–27]. DPP-4 inhibitors have been studied extensively in several large trials comparing efficacy with placebo, other oral agents and as combination therapy (Table 1).
Table 1.
Clinical efficacy of DPP-4 inhibitors.
| Study | Duration (months) | Patients (n) | Background therapy | Intervention | Baseline HbA1C, mean (%) | Δ from placebo, HbA1c (%) | Δ from placebo, FPG (mg/dl) | Δ from placebo, PPG (mg/dl) |
|---|---|---|---|---|---|---|---|---|
| Aschner et al. [28] | 6 | 741 | Washout | Sitagliptin 200 mg vs placebo | 8.1 | −0.9* | −21* | −54* |
| Sitagliptin 100 mg vs placebo | 8.0 | −0.8* | −17* | −47* | ||||
| Raz et al. [29] | 4.5 | 521 | Washout | Sitagliptin 200 mg vs placebo | 8.1 | −0.5* | −16* | −52* |
| Sitagliptin 100 mg vs placebo | 8.0 | −0.6* | −20* | −47* | ||||
| Rosenstock et al. [30] | 6 | 401 | Diet | Saxagliptin 10 mg vs placebo | 7.8 | −0.7* | −23* | −48* |
| Saxagliptin 5 mg vs placebo | 8.0 | −0.7* | −15* | −37* | ||||
| Saxagliptin 2.5 mg vs placebo | 7.9 | −0.6* | −21* | −39* | ||||
| Del Prato et al. [31] | 6 | 503 | Washout | Linagliptin 5 mg vs placebo | 8.0 | −0.7* | −23* | −58* |
| Pi-Sunyer et al. [32] | 6 | 354 | Diet | Vildagliptin 100 mg/day vs placebo | 8.3 | −0.9* | −23* | Not recorded |
| Vildagliptin 50 mg b.i.d. vs placebo | 8.4 | −0.7* | −23* | |||||
| Vildagliptin 50 mg/day vs placebo | 8.4 | −0.5* | −11 | |||||
| DeFronzo et al. [33] | 6.5 | 329 | Diet | Alogliptin 25 mg vs placebo | 7.9 | −0.6* | −28* | Not recorded |
| Alogliptin 12.5 mg vs placebo | −0.5* | −22* | ||||||
| Aschner et al. [34] | 6 | 1050 | Diet | Sitagliptin 100 mg | 7.2 | +0.1‡ | +8‡ | Not recorded |
| Metformin, maximum 2 g | 7.2 | (vs metformin) | ||||||
| Schweizer et al. [35] | 12 | 780 | Diet | Vildagliptin 100 mg | 8.7 | +0.4 | +18 | Not recorded |
| Metformin, maximum 2 g | 8.7 | (vs metformin) | ||||||
| Nauck et al. [37] | 12 | 1172 | Metformin | Sitagliptin 100 mg | 7.7 | 0‡ | −2‡ | Not recorded |
| Glipizide, maximum 20 mg | 7.6 | (vs glipizide) | ||||||
| Gallwitz et al. [38] | 24 | 1552 | Metformin | Linagliptin 5 mg | 7.7 | +0.2‡ | +6‡ | −58† |
| Glimepiride, maximum 4 mg | 7.7 | (vs glimepiride) | ||||||
| Goldstein et al. [41] | 6 | 1091 | Washout | Sitagliptin 100 mg/metformin | 8.8 | −2* | −70* | −117* |
| 2000 mg vs placebo | ||||||||
| Sitagliptin 100 mg/metformin | 8.8 | −1.6* | −53* | −93* | ||||
| 1000 mg vs placebo | ||||||||
| Metformin 2000 mg vs placebo | 8.7 | −1.3* | −35* | −78* | ||||
| Metformin 1000 mg vs placebo | 8.9 | −1.0* | −33* | −54* | ||||
| Sitagliptin 100 mg vs placebo | 8.9 | −0.8* | −23* | −52* | ||||
| Defronzo et al. [42] | 6 | 743 | Metformin | Saxagliptin 10 mg vs placebo | 8.0 | −0.7* | −22* | −32* |
| Saxagliptin 5 mg vs placebo | 8.1 | −0.8* | −23* | −40* | ||||
| Saxagliptin 2.5 mg vs placebo | 8.1 | −0.7* | −16* | −44* | ||||
| Hermansen et al. [43] | 6 | 441 | Glimepiride ± metformin | Sitagliptin 100 mg vs placebo | 8.3 | −0.7* | −20* | −36* |
| Rosenstock et al. [44] | 6 | 353 | Pioglitazone | Sitagliptin 100 mg vs placebo | 8.1 | −0.7* | −18* | Not recorded |
| Vilsboll etal. [45] | 6 | 641 | Insulin ± metformin | Sitagliptin 100 mg vs placebo | 8.7 | −0.6* | −15* | −36* |
| Fonseca et al. [46] | 6 | 296 | Insulin | Vildagliptin 50 mg b.i.d. vs placebo | 8.4 | −0.3 | Not recorded | Not recorded |
p ≤ 0.001.
Non-inferior.
DPP-4: Dipeptidyl peptidase-IV; FPG: Fasting plasma glucose; HbA1c: Glycosylated HbA1c; PPG: Post-prandial glucose.
2.1 DPP-4 inhibitor monotherapy
There are many randomized, placebo-controlled trials on each of the available DPP-4 inhibitors highlighting their efficacy. In a 24-week trial of sitagliptin use, subjects on background diet or oral hypoglycemic agents (OHA) underwent a washout period and then received placebo, sitagliptin 100 or 200 mg [28]. Changes in HbA1c from baseline (8.0%) were +0.2, −0.6 and −0.8%, respectively (p < 0.001). Decreases in fasting plasma glucose (FPG) of 13 and 16 mg/dl occurred in the sitagliptin 100 and 200 mg groups, respectively, while an increase of 5 mg/dl occurred in the placebo group (p < 0.001). Post-prandial glucose (PPG) decreased by 2, 49 and 56 mg/dl in the placebo, sitagliptin 100 and 200 mg groups, respectively (p < 0.001). In a similar 18-week study, significant reductions in HbA1c, FPG and PPG were again seen with sitagliptin treatment compared with placebo [29]. From a mean baseline HbA1c of 8.1%, decreases of 0.5 and 0.4% were seen in the sitagliptin 100 and 200 mg groups, respectively, while an increase of 0.2% occurred in the placebo group (p < 0.001). Patients with a higher baseline HbA1c experienced greater reductions with treatment. Together these data suggested similarity in efficacy of sitagliptin 100- and 200-mg doses which resulted in ultimate approval of 100 mg as the recommended dose.
Saxagliptin monotherapy was evaluated in a 24-week trial of subjects with inadequate glycemic control on diet and exercise [30]. From a mean baseline HbA1c of 7.9%, subjects randomized to saxagliptin 2.5, 5 or 10 mg/day experienced decreases of 0.4, 0.5 and 0.5%, respectively compared with an increase of 0.2% for the placebo group (p < 0.0001). For those with a baseline HbA1c ≥ 9.0%, the reduction for saxagliptin-treated groups ranged from 0.8 to 1.3% versus an increase of 0.1% for placebo. FPG changes from baseline of −15 (p = 0.0002), −9 (p = 0.0074) and −17 mg/dl (p < 0.001) were seen for saxagliptin 2.5, 5 and 10 mg groups, respectively while a +6 mg/dl change was seen for those receiving placebo. PPG changes from baseline were −45 (p = 0.0007), −43 (p = 0.0009) and −54 mg/dl (p < 0.0001) for saxagliptin 2.5, 5 and 10 mg, respectively versus −6 mg/dl for placebo. Studies of saxagliptin have shown maximal responses in HbA1c, FPG and PPG with the saxagliptin 5 mg dose without evidence of a dose--response relationship at greater than 5 mg. Accordingly, saxagliptin 5 mg is the recommended dose.
Linagliptin proved efficacious in a 24-week trial of participants randomized to linagliptin 5 mg/day or placebo [31]. From a mean baseline HbA1c of 8.0%, linagliptin resulted in a decrease of 0.4% compared with an increase of 0.3% with placebo (p < 0.0001). The subgroup of linagliptin-treated patients with baseline HbA1c ≥ 9% experienced even greater decreases in HbA1c of 0.9%. A mean decrease in FPG of 9 mg/dl was seen in the linagliptin-treated group while an increase of 14 mg/dl was seen in the placebo group (p < 0.0001). Significant decreases in 2-h PPG of 34 mg/dl occurred in the linagliptin-treated group compared with an increase of 25 mg/dl in the placebo group (p < 0.0001).
Vildagliptin proved effective compared with placebo in inadequately controlled drug-naïve patients randomized to vildagliptin 50 mg/day, 50 mg b.i.d., 100 mg/day or placebo [32]. From a mean baseline HbA1c of 8.4%, decreases of 0.4 (p = 0.011), 0.7 (p < 0.001) and 0.8% (p < 0.001) versus an increase of 0.1% were seen in vildagliptin 50 mg, 50 mg b.i.d., 100 mg/day and placebo groups, respectively. Greater decreases in HbA1c were observed in participants with baseline HbA1c > 8.0%. Significant decreases in FPG were seen in the groups receiving a total of 100 mg of vildagliptin daily but not in the 50 mg/day group. The efficacy of vildagliptin was clearly dose-related and therefore the recommended daily dose is 100 mg except when used in combination with a SU when 50 mg/day is preferred.
Alogliptin therapy examined as monotherapy in treatment-naïve subjects over 26 weeks resulted in HbA1c decreases from baseline of 0.6% for both alogliptin 12.5 and 25 mg groups compared with no change for placebo (p < 0.001) [33]. Reductions in FPG of 10 and 14 mg/dl were seen in the alogliptin 12.5 and 25 mg groups, respectively versus an increase of 11 mg/dl in the placebo-treated group (p < 0.001).
2.2 DPP-4 inhibitors versus metformin
Since metformin is recommended as first-line therapy, it has been of interest to compare DPP-4 inhibitors with metformin. Randomization to sitagliptin 100 mg or metformin 2000 mg/day in subjects inadequately controlled on diet and exercise resulted in decreases in HbA1c from baseline (7.2%) of 0.4 and 0.6%, respectively, demonstrating non-inferiority for sitagliptin treatment [34]. Changes in FPG were greater with metformin than sitagliptin treatment (-19 vs -12 mg/dl, respectively). More patients reached a goal HbA1c of < 7% in the metformin than the sitagliptin group (76 vs 69%). This study was limited by the relatively low baseline HbA1c of participants resulting in less significant decreases in HbA1c than would be expected at higher baseline values. Another limitation was the non-inferiority design of the study.
In another study comparing DPP-4 inhibitor therapy with metformin, subjects inadequately controlled on diet were randomized to vildagliptin 100 mg/day or metformin (titrated to 2000 mg/day) and studied over 52 weeks [35]. Decreases in HbA1c from a baseline of 8.4% were 1.0% for vildagliptin (p < 0.001 vs baseline) and 1.4% for metformin (p < 0.001 vs baseline and vildagliptin). Non-inferiority of vildagliptin to metformin was not established. HbA1c level < 7.0% was seen in 35% of subjects treated with vildagliptin and 45% treated with metformin. The mean change in FPG from baseline was −16 mg/dl in the vildagliptin-treated group (p < 0.001 vs baseline) and −34 mg/dl in the metformin-treated group (p < 0.001 vs baseline and vildagliptin).
A recent meta-analysis was conducted of randomized controlled trials comparing DPP-4 inhibitor therapy with metformin as monotherapy or in combination with other agents [36]. DPP-4 inhibitor compared with metformin treatment resulted in a smaller decline in HbA1c (weighted mean difference 0.20, 95% confidence interval (CI) 0.08 – 0.32) and in body weight (weighted mean difference 1.50, 95% CI 0.90–2.11). In addition, patients receiving DPP-4 inhibitors were less likely than those receiving metformin to reach goal HbA1c of < 7.0% (risk ratio in favor of metformin 1.18, 95% CI 1.07 – 1.29).
2.3 DPP-4 inhibitors versus SU
Given the well-established effectiveness of SU therapy, it also has been of interest to compare DPP-4 inhibitors with this class. The efficacy of sitagliptin 100 mg/day versus glipizide 5 mg/day (titrated to a potential maximum of 20 mg/day) in subjects inadequately controlled on metformin alone was studied over 52 weeks [37]. The change in HbA1c for both groups from a mean baseline of 7.7% was −0.7%, confirming non-inferiority of sitagliptin to glipizide. The percentage of patients with HbA1c value < 7% at week 52 was similar between the sitagliptin (63%) and glipizide (59%) groups as was the change in FPG from baseline (−10 mg/dl, sitagliptin; −8 mg/dl, glipizide).
In another study comparing DDP-4 and SU therapy, subjects with inadequate glycemic control on metformin alone or with one additional antidiabetic agent (washed-out during screening) were randomized to linagliptin 5 mg/day or glimepiride 1 mg/day (possibility of titration to 4 mg/day) [38]. Over a 2-year period, decreases in HbA1c from a mean baseline of 7.7% were similar between groups (−0.2%, linagliptin and −0.4%, glimepiride) demonstrating non-inferiority of linagliptin to glimepiride. At week 104, HbA1c value < 7.0% was achieved by 30% of patients receiving linagliptin and 35% receiving glimepiride. Other studies have shown similar results indicating non-inferiority of DPP-4 inhibitors to SUs [38–40].
2.4 DPP-4 inhibitors in combination
DPP-4 inhibitors have also been shown to be efficacious and safe in combination therapy with metformin, SUs, TZDs and insulin. In a 24-week study, subjects were randomized to placebo, sitagliptin 100 mg/day (S100), metformin 500 mg or 1000 mg b.i.d. (M1000, M2000) or 50 mg of sitagliptin b.i.d. in combination with 500 mg or 1000 mg of metformin b.i.d. (S100/M1000, S100/M2000) [41]. Changes in HbA1c from baseline (mean 8.8%) were: +0.2% (placebo), −0.7% (S100), −0.8% (M1000), −1.1% (M2000), −1.4% (S100/M1000) and −1.9% (S100/M2000), each group with a statistically significant change relative to placebo (p ≤ 0.001). The combination therapy provided significant improvements in HbA1c, FPG and PPG compared with placebo, to metformin alone, and to sitagliptin alone (p < 0.001). This study demonstrated the additive effect of the DPP-4 class of agents with metformin in those inadequately controlled on diet and exercise, likely due to their complementary mechanisms of action. It showed that this combination is also a good initial treatment option in patients with elevated HbA1c values unlikely to be controlled on monotherapy.
In another study of DPP-4/metformin combination therapy, subjects with inadequate glycemic control on metformin alone were randomized to the addition of placebo or saxagliptin 2.5, 5 or 10 mg/day [42]. From a mean baseline HbA1c of 8.0%, decreases of 0.6, 0.7 and 0.6% were seen in the saxagliptin 2.5, 5 and 10 mg groups, respectively versus an increase of 0.1% in the placebo/metformin group (p ≤ 0.0001). There were also statistically significant decreases in FPG and PPG for the saxagliptin versus placebo add-on groups. Given the proven efficacy of DPP-4 and metformin combination therapy, each of the agents in the DPP-4 inhibitor class is available in a single pill combination tablet with metformin for ease of administration.
Also studied extensively has been the addition of DPP-4 inhibitor to SU therapy. In subjects inadequately controlled on glimepiride ± metformin, the addition of sitagliptin resulted in a change in HbA1c from baseline (8.3%) of −0.5% while an increase of 0.3% was seen with placebo (p < 0.001) [43]. Decreases in FPG and PPG of 4 and 23 mg/dl, respectively were seen in the sitagliptin add-on group, compared with increases of 16 and 14 mg/dl in the placebo group. The improved glycemic control with sitagliptin added to glimepiride ± metformin was at the expense of a higher incidence of overall (60 vs 47%) and drug-related adverse experiences (15 vs 7%) in the sitagliptin than placebo group. The increased incidence of adverse events was mainly accounted for by the higher incidence of hypoglycemia with sitagliptin added to SU ± metformin therapy.
DPP-4 inhibitor therapy also proved efficacious in combination with TZDs when sitagliptin was added to patients with poor glycemic control on pioglitazone therapy. The addition of sitagliptin resulted in a decrease in HbA1c of 0.9% compared with 0.2% for placebo from a mean baseline of 8.0% after 24 weeks (p < 0.001) [44]. FPG decreased by 17 mg/dl in the sitagliptin group while it increased by 1 mg/dl in the placebo group (p < 0.001). The addition of sitagliptin to pioglitazone was overall well-tolerated with a similar incidence of overall adverse events and hypoglycemia between the sitagliptin and placebo add-on groups.
There has also been success with DPP-4 inhibitor and insulin combination therapy. In subjects with inadequate glycemic control on insulin monotherapy or in combination with metformin (baseline HbA1c 8.7%), the addition of sitagliptin decreased HbA1c by 0.6% compared with no change from baseline in the placebo group (p < 0.001) [45]. Both FPG and PPG decreased significantly when sitagliptin was added to insulin therapy versus placebo (p ≤ 0.001). Despite improved glycemic control, a higher incidence of adverse events was reported with sitagliptin (52%) compared with placebo (43%), due mainly to the increased incidence of hypoglycemia (sitagliptin, 16% vs placebo, 8%). Insulin and metformin doses remained stable throughout the study unless hypoglycemia occurred. In a study of vildagliptin added to insulin therapy, patients in the vildagliptin/insulin group had decreases in HbA1c of 0.5% compared with decreases of 0.2% in the insulin-only group from a mean baseline of 8.4% (p = 0.01) [46]. In a subgroup analysis of patients ≥ 65 years, greater decreases in HbA1c of 0.7% were seen in the vildagliptin add-on group compared with decreases of only 0.1% in the placebo/insulin group (p < 0.001). Insulin adjustments were allowed throughout this study. Interestingly, significantly lower rates of hypoglycemia were seen in patients receiving vildagliptin than those receiving placebo (p < 0.001).
2.5 Comparison between DPP-4 inhibitors
Head-to-head comparison trials between DPP-4 inhibitors are limited as there have not been any randomized controlled trials to date comparing agents in this class. Indirect comparisons suggest that they are similar in terms of efficacy and safety profiles. An 18-week non-inferiority trial was published comparing sitagliptin 100 mg/day to saxagliptin 5 mg/day in those inadequately controlled on metformin [47]. The adjusted mean changes in HbA1c were −0.6% for sitagliptin and −0.5% for saxagliptin, demonstrating non-inferiority for saxagliptin. Adverse events were comparable between groups. A meta-analysis of studies comparing sitagliptin or vildagliptin with placebo showed that both agents promoted significant reduction of HbA1c values, −0.6% with sitagliptin and −0.7% with vildagliptin (p < 0.00001) [48]. Sitagliptin and vildagliptin treatments were compared in a trial examining their effects on daily blood glucose fluctuations [49]. Decreases in HbA1c, FPG and PPG were similar in the two groups after 3 months while the amplitude of glycemic excursions decreased more significantly with vildagliptin treatment.
2.6 Safety and tolerability
There are no significant differences in the incidence of overall, serious or drug-related clinical adverse events, including gastrointestinal (GI) events or hypoglycemia in DPP-4- versus placebo-treated patients [28,29,32,41–43]. Certain adverse events are seen at a higher but not statistically significant rate in DPP-4-treated groups including constipation, nasopharyngitis, urinary tract infection, myalgias, arthralgias, headache and dizziness [28,29,32,37,44]. Compared with metformin, a lower incidence of drug-related adverse events is reported, likely related to the lower incidence of GI side effects [34,35]. Combination of DPP-4 inhibitor and metformin does not produce a statistically significant increase in the incidence of GI adverse experiences compared with metformin alone [41]. DPP-4 is found in multiple tissues including immune-related cells and is identical to CD26, a marker for activated T cells [50]. Concern arose over potential immune-modulating effects of DPP-4 inhibitors. However, immune activation does not seem to be affected by specific DPP-4 inhibition, but rather by DPP-8 and DPP-9 [51].
Several studies have shown a small mean increase from baseline in white blood cell count and uric acid and a decrease in alkaline phosphatase with DPP-4 inhibitor treatment but these differences have not been statistically or clinically meaningful [29,34,43,44]. Case reports of pancreatitis, including 88 post-marketing cases between 2006 and 2009 led to the Food and Drug Administration (FDA) requiring a warning statement on all product labels. A review of preclinical data examining pancreatic histology in multiple animal species did not reveal any evidence of treatment-related pancreatitis [52]. A pooled analysis of controlled clinical trials has also failed to show an increased incidence of pancreatitis in patients treated with the DPP-4 inhibitor class compared with placebo or other agents for diabetes. Rare cases of hepatic dysfunction have been reported with vildagliptin 100 mg once daily and therefore monitoring of liver function tests is recommended before and during treatment [53].
Hypoglycemia is an uncommon complication of DPP-4 inhibitor therapy. A higher incidence of hypoglycemia is more likely to occur with SU than DPP-4 inhibitor therapy [37]. When a DPP-4 inhibitor is added to a SU, but not metformin, there is a higher incidence of drug-related adverse events, mainly accounted for by the higher incidence of hypoglycemia [43,45]. This increased incidence of hypoglycemia can also be seen when a DPP-4 inhibitor is added to insulin if there is not careful monitoring for adjustment of the insulin dose [45,46].
DPP-4 inhibitor therapy has a neutral effect on body weight [28,30]. Metformin produces more significant weight loss [34,35] while SU therapy leads to weight gain compared with gliptins [37].
There does not appear to be any increased risk of cardiovascular or cerebrovascular events with use of various DPP-4 inhibitors [54–56]. These retrospective studies were pooled data from Phase II and III trials. Data also suggest that there may be a cardioprotective effect that necessitates further inquiry [54,55]. Currently, Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 Trial (SAVOR-TIMI 53), Sitagliptin Cardiovascular Outcomes Study (TECOS), Cardiovascular Outcomes Study of Alogliptin in Subjects with Type 2 Diabetes and Acute Coronary Syndrome (EXAMINE) and Cardiovascular Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes (CAROLINA) are investigating the long-term safety and cardiovascular outcomes in patients treated with DPP-4 inhibitors [57–60].
DPP-4 inhibitors are efficacious and safe in the elderly [61]. They are particularly a wise choice in this population due to the low risk of hypoglycemia. In addition, with the exception of saxagliptin which is metabolized by CYP3A4/5, there is a low risk of drug–drug interactions due to lack of interaction with the CYP isoforms [62,63]. The DPP-4 class of medications has proven safe and efficacious in renal impairment [64,65]. In addition, they are safe to be used in hepatic impairment, with exception of vildagliptin, with no clinically significant changes in their pharmacokinetics seen in hepatic impairment [66–68].
3. Meglitinides
The meglitinide analogs `glinides' are short-acting insulin secretagogues that target one of the main defects that characterizes type 2 diabetes: the progressive loss of early phase prandial insulin secretion [69]. They act in a glucose-dependent manner to close adenosine triphosphate (ATP)-dependent potassium channels on the β-cell membrane, depolarize the β-cell, resulting in the opening of calcium channels, increased calcium influx and insulin secretion [70,71]. The currently approved drugs in this class include the benzoic acid derivative repaglinide, approved in 1997, and nateglinide, a d-phenylalanine derivative, approved in 2000. Despite similar mechanisms of action, the molecular binding site of repaglinide is different from that of nateglinide, as well as SUs [72]. In addition, the onset of inhibition of ATP-dependent potassium channels by nateglinide is more rapid than that by repaglinide, as is the time to reversal of channel inhibition. The nature of nateglinide's interaction with its receptor and at the KATP channel results in its shorter duration of action and reduced risk of hypoglycemia compared with repaglinide. The glinides are rapidly absorbed from the GI tract with augmentation of insulin secretion occurring within 30 minutes of commencing a meal [73]. The recommended time for administration is 15 minutes prior to each meal. The maximum dosing is 4 mg per meal with repaglinide and 120 mg per meal with nateglinide. The major glinide efficacy trials are summarized in Table 2.
Table 2.
Clinical efficacy of meglitinides.
| Study | Duration (months) | Patients (n) | Background therapy | Intervention | Baseline HbA1C, mean (%) | Δ from placebo/other, HbA1c (%) | Δ from placebo/other, FPG (mg/dl) |
|---|---|---|---|---|---|---|---|
| Jovanovic et al. [74] | 6 | 361 | Washout | Repaglinide 4 mg AC vs placebo | 8.7 | −1.9* | −66* |
| Repaglinide 1 mg AC vs placebo | 8.9 | −2.1* | −68* | ||||
| Moses et al. [75] | 4 | 408 | Diet and exercise | Repaglinide, maximum 1 mg AC vs placebo | 7.8 | −1* | −26* |
| Derosa et al. [76] | 12 | 112 | Diet and exercise | Repaglinide, maximum 4 mg/day | 7.6 | +0.1 | −3 |
| Metformin, maximum 2500 mg/day | 7.4 | (vs metformin) | |||||
| Madsbad et al. [77] | 12 | 256 | Washout | Repaglinide, maximum 4 mg AC | 7.3 | −0.6* | −16* |
| Glipizide, maximum 15 mg/day | 7.2 | (vs glipizide) | |||||
| Marbury et al. [78] | 12 | 576 | Washout | Repaglinide, maximum 4 mg AC | 8.7 | 0 | +3.1 |
| Glyburide, maximum 15 mg/day | 8.9 | (vs glyburide) | |||||
| Moses et al. [79] | 6 | 83 | Metformin (1 -- 3 g/day) | Metformin + repaglinide, maximum 4 mg AC | 8.3 | −1.1* (combination vs metformin) | −36* |
| Metformin + placebo | 8.6 | −1* (combination vs repaglinide) | −48* | ||||
| Repaglinide, maximum 4 mg AC + placebo (off metformin) | 8.6 | ||||||
| Jovanovic et al. [80] | 6 | 246 | SU ± metformin | Repaglinide, maximum 4 mg AC + pioglitazone 30 mg/day | 9.3 | −2.1* (combination vs pioglitazone) | −64* |
| Pioglitazone 30 mg/day + placebo | 9.1 | −1.6* (combination vs repaglinide) | −48* | ||||
| Repaglinide, maximum 4 mg AC + placebo | 9.0 | ||||||
| Rosenstock et al. [81] | 150 | Diet and exercise | Repaglinide, maximum 4 mg AC | 8.9 | −0.6* | −39* | |
| Nateglinide, maximum 120 mg AC | 8.9 | (vs nateglinide) |
p < 0.05.
AC: With meals; HbA1c: Glycosylated HbA1c; FPG: Fasting plasma glucose.
3.1 Meglitinide monotherapy
Repaglinide has been shown to improve glycemic control over placebo in several randomized, double-blind multicenter trials. Over 24 weeks, subjects on background diet or OHA underwent a washout period and then received repaglinide 1 or 4 mg with meals [74]. From a mean baseline of 8.7%, HbA1c increased by 1.4% in the placebo group, while it decreased by 0.7 and 0.5% in the repaglinide 1 and 4 mg groups, respectively (p < 0.001). Mean FPG changes of +19, −47 and −49 mg/dl were seen in the placebo, repaglinide 1 and 4 mg groups, respectively (p < 0.001). In another study over 16 weeks in participants poorly controlled by diet alone, decreases in HbA1c of 1.1 and 0.2% (from 7.7%) were seen in repaglinide- and placebo-treated patients, respectively (p < 0.001) [75]. Repaglinide treatment improved FPG with respect to baseline and placebo with a mean reduction of 32 mg/dl (p < 0.001).
3.2 Meglitinide versus metformin
Repaglinide monotherapy provides similarly significant improvements in glycemic control as with metformin monotherapy. At 12 months, pharmacotherapy-naïve participants randomized to repaglinide or metformin experienced significant decreases from baseline in HbA1c, FPG and PPG (p < 0.05) [76]. HbA1c decreases of 0.8 and 0.9% from a mean baseline of 7.5% occurred in repaglinide- and metformin-treated subjects, respectively. The between-group difference was not significant for HbA1c or FPG but the fall in PPG was significantly greater in the repaglinide-treated group (p < 0.05).
3.3 Meglitinides versus SU
Studied over 1 year, patients underwent a washout period and then received repaglinide or glipizide (up to 15 mg/day) [77]. Although initial improvement in HbA1c was noted, HbA1c rose from a mean of 7.3% by 0.2 and 0.8% when treated with repaglinide and glipizide, respectively (p < 0.05 between groups). In both treatment groups, FPG initially decreased from baseline, but subsequently increased. The increase in FPG was greater in the glipizide than repaglinide group (23 vs 9 mg/dl, p < 0.05). In a similar study of subjects randomized to either repaglinide or glyburide monotherapy studied over 1 year, HbA1c increased by 0.1% from a mean baseline of 8.8% in both treatment groups [78]. Better efficacy was seen in OHA-naïve participants who experienced similar but significant decreases in HbA1c of 1.3 and 1.1% when treated with repaglinide or glyburide. FPG changes were similar between groups.
3.4 Meglitinides in combination
In those inadequately controlled on metformin alone, repaglinide added to metformin resulted in a mean HbA1c decrease of 1.4% (from 8.3%) (p < 0.05) [79]. Non-significant decreases in HbA1c of 0.4 and 0.3% occurred in the repaglinide and metformin monotherapy groups, respectively (p < 0.05 compared with combination therapy). The mean FPG decreased by 40 mg/dl (p < 0.05) in the combination group while no significant change was seen in either mono-therapy group. Proven in this study was not only the efficacy of combination therapy but also repaglinide as a viable substitution to metformin, with no deterioration in glycemic control seen when switched from metformin to repaglinide monotherapy. Repaglinide was studied in combination and against pioglitazone therapy in a 6-month trial [80]. Patients with inadequate control on metformin or SU therapy were randomized to combination repaglinide/pioglitazone therapy, repaglinide monotherapy or pioglitazone monotherapy. Changes in HbA1c from an overall mean baseline of 9.1% were −1.8, −0.2 and +0.3% for combination therapy, repaglinide monotherapy and pioglitazone monotherapy, respectively (p < 0.001 combination vs monotherapy). The monotherapy groups did not significantly differ from one another. A reduction in FPG of 82 mg/dl was seen with combination therapy, significantly greater than the reduction with either repaglinide or pioglitazone monotherapy (−34 and −19 mg/dl, respectively; p < 0.001).
3.5 Repaglinide versus nateglinide
Repaglinide and nateglinide monotherapies were compared over 16 weeks in subjects uncontrolled on diet and exercise [81]. The reduction in HbA1c from baseline (8.0%) was significantly greater with repaglinide than nateglinide monotherapy (−1.6 vs −1.0%; p = 0.002) as was the reduction in FPG (−57 vs −18 mg/dl; p < 0.001). Similar post-prandial glycemic effects were seen between groups.
3.6 Safety and tolerability
Hypoglycemia is the most commonly reported adverse event with glinide use, especially repaglinide. Hypoglycemic symptoms were experienced in 11, 27 and 35% of placebo-, repaglinide 1 and 4 mg treated subjects, respectively [74]. However, only two subjects had hypoglycemic symptoms with a concomitant blood glucose value < 45 mg/dl, both in the repaglinide 4 mg group. Furthermore, no events were severe, requiring third-party assistance or hospitalization. Hypoglycemic events are more frequent in subjects who are OHA-naïve or have a baseline HbA1c < 8% and are seen less frequently with lower doses of repaglinide with 3, 11 and 18% of subjects reporting symptoms with placebo, repaglinide 0.5 and 1 mg, respectively [75]. Repaglinide and some SUs result in a similar incidence of hypoglycemic symptoms (15% of repaglinide-treated subjects and 19% of glipizide or glyburide-treated patients) [77,78]. Blood glucose levels recorded at the time of hypoglycemic symptoms, however, were lower and more often recorded at < 45 mg/dl with SU treatment. When compared, repaglinide has a higher risk of hypoglycemia compared with nateglinide which has been attributed to the difference in interaction at the KATP channel [81]. Minor hypoglycemic events, defined as a documented blood glucose level of < 50 mg/dl, were seen in 7% of the repaglinide group but 0% of the nateglinide group. No major hypoglycemic events, requiring third-party assistance were needed.
The glinides appear to be weight neutral [75,77–80]. A slight increase in weight of 0.4 kg occurred in repaglinide-treated subjects, not significantly different from placebo-treated patients. No significant difference in weight change was seen when compared with SU therapy. Repaglinide produced less weight gain than pioglitazone but more than metformin.
In a large study (n = 9306), patients with impaired glucose tolerance were randomized to receive nateglinide or placebo and were followed for a median of 5 years to evaluate the risk of diabetes or cardiovascular events [82]. Unfortunately, nateglinide did not diminish the incidence of new diabetes, nor did it improve cardiovascular outcomes, death from any cause or cardiovascular death.
Nateglinide has been studied in treatment-naïve elderly subjects and found to be well tolerated and safe [83,84]. The meglitinides are extensively metabolized by the cytochrome P450 enzymes and should be used with caution in patients also taking inhibitors or inducers of these enzymes such as ketoconazole, gemfibrizol, trimethoprim, cyclosoporin and rifampin [85–89]. Patients switched to repaglinide therapy with varying degrees of renal dysfunction were studied over a 3-month period [90]. With the exception of the runin period, there was no significant increased incidence of hypoglycemia in relation to the degree of renal dysfunction. However, with increasing renal dysfunction, there was a significant tendency toward a lower final dose of the drug (p = 0.03). In pharmacokinetic studies of nateglinide, renal clearance of nateglinide tended to decrease with increasing renal dysfunction but there was no significant increase in plasma nateglinide area under the curve or half-life [91]. Nateglinide was well tolerated in patients with renal dysfunction or undergoing hemodialysis [84,91]. Glinides appear safe in liver dysfunction, but have been associated with rare hepatotoxicity [92–95].
4. α-Glucosidase inhibitors
AGIs were approved for treatment of type 2 diabetes in the 1990s. AGIs competitively inhibit α-glucosidase enzymes at the enterocyte brush border [96]. The absorption of consumed carbohydrates is delayed resulting in a reduction in plasma glucose. There is no direct effect on insulin secretion, but there is evidence that GLP-1 secretion is enhanced [97–100]. Currently, only acarbose and miglitol are approved in the USA. Both are dosed with meals, with maximal dosing of 100 mg three times per day (t.i.d.). Focusing on acarbose as the most widely investigated of the AGIs, Table 3 shows trials studying acarbose as either monotherapy or combination therapy with most other available diabetes treatments.
Table 3.
Clinical efficacy of acarbose.
| Study | Duration (months) | Patients (n) | Background therapy | Intervention | Baseline HbA1C, mean (%) | Δ from placebo, HbA1c (%) | Δ from placebo, FPG (mg/dl) | Δ from placebo, PPG (mg/dl) |
|---|---|---|---|---|---|---|---|---|
| Derosa et al. [101] | 7 | 188 | Diet and exercise | Acarbose 100 mg t.i.d. vs placebo | 6.8 | −0.7* | −14* | −15* |
| Halimi et al. [104] | 6 | 152 | Metformin 850 mg b.i.d. or t.i.d. | Acarbose 100 mg t.i.d. vs placebo | 8.6 | −0.9* | −41* | −45* |
| Costa and Pinol [107] | 6 | 65 | Glibenclamide > 10 mg/day | Acarbose 100 mg t.i.d. vs placebo | 9.0 | −0.6* | −27* | −27* |
| Chiasson et al. [108] | 12 | 354 | Diet | Acarbose, maximum dose | 6.7 | −0.9‡ | −38‡ | −81‡ |
| Diet + metformin | 200 mg/day vs placebo | 7.8 | −0.8‡ | −27 | −63‡ | |||
| Diet + SU | 8.0 | −0.9‡ | −25‡ | −74‡ | ||||
| Diet + insulin | 7.7 | −0.4§ | 0 | −54‡ |
| Δ from baseline HbA1c (%) | Δ from baseline FPG (mg/dl) | Δ from baseline PPG (mg/dl) | ||||||
|---|---|---|---|---|---|---|---|---|
| Hoffmann and Spengler (Essen trial) [102] | 6 | 96 | Diet | Acarbose 100 mg t.i.d. | 8.3 | −1¶ | −25¶ | −38¶ |
| Glibenclamide, mean dose of 4.3 mg/day | 8.3 | −0.8¶ | −29¶ | −31¶ | ||||
| Placebo | 8.3 | +0.1 | 0 | +2 | ||||
| Hoffmann and Spengler (Essen II trial) [103] | 6 | 94 | Diet | Acarbose 100 mg t.i.d. | 9.6 | −1.1¶ | −27¶ | −43¶ |
| Metformin 850 mg b.i.d. | 9.7 | −1¶ | −18¶ | −34¶ | ||||
| Placebo | 9.4 | +0.4 | +9 | 0 |
p-Value not provided.
p < 0.01.
p < 0.011 by ANCOVA.
Statistically significant (p < 0.001) by ANCOVA for acarbose and metformin compared with placebo, but no difference between acarbose and glibenclamide or metformin.
ANCOVA: Analysis of covariance; FPG: Fasting plasma glucose; HbA1c: Glycosylated hemoglobin A1c; PPG: Post-prandial glucose; SU: Sulfonylurea.
4.1 Acarbose monotherapy versus metformin versus SU
Against diet alone, acarbose was more effective in reducing HbA1c, FPG and PPG (p < 0.01, < 0.05 and < 0.05, respectively) [101]. HbA1c was reduced by 0.7% compared with placebo, from a baseline of 6.8%. The Essen and Essen II trials compared the efficacy of acarbose as first-line therapy versus placebo, SU or metformin in treatment-naïve type 2 diabetes patients [102,103]. There was no statistical difference in mean reduction in HbA1c, FPG and PPG from baseline between acarbose and metformin or acarbose and glibenclamide therapy over 24 weeks. However, across all treatment groups, the reduction in HbA1c from baseline was about 1%.
4.2 Acarbose in combination
Added to background of metformin or SU therapy, acarbose demonstrated a HbA1c reduction of 0.6 -- 0.9% from placebo, with an average reduction in FPG and PPG from placebo of about 30 mg/dl [104–107]. In a 1-year study by Chiasson et al., acarbose therapy was added to background diet, metformin, SU and insulin therapy [108]. In all groups, acarbose-treated patients had a decrease in HbA1c from placebo, ranging from 0.9% (diet, p = 0.005) to 0.4% (insulin, p = 0.07). Only add-on to diet and SU therapy had a statistically significant reduction in FPG (p < 0.001 and p = 0.013, respectively), but all groups had a statistically significant reduction in PPG with add-on acarbose therapy (p ≤ 0.01). These data were supported by a similar 3-year study of acarbose added to pre-existing therapy where acarbose-treated patients had a reduction in HbA1c from placebo, ranging from 0.7% (multiple insulin, p = 0.025) to 0.1% (SU plus insulin, p = 0.9) [109]. In all groups combined, there was a statistically significant mean change from baseline in HbA1c of 0.5% (p < 0.001), but no significant change in FPG after 3 years of treatment.
In pooled analysis of 41 trials (n = 8130) of AGIs, 30 of which studied acarbose, there was an average reduction from placebo of HbA1c of 0.8%, FPG 20 mg/dl and PPG of 41 mg/dl [110,111].
4.3 Safety and tolerability
Acarbose has a high non-compliance rate (as high as 49% after 1 year of treatment), owing mostly to its increased incidence of flatulence (30 vs 12%, p < 0.00001) and diarrhea (16 vs 8%, p < 0.0001) compared with placebo [109,112]. There was no significant net difference in urinary albumin, β-cell function or insulin sensitivity from placebo at 3 years. Hypoglycemia is a rare complication and mainly occurs when acarbose is added to SU or insulin therapy [102,108].
Acarbose is weight neutral in placebo-controlled trials, but has a weight beneficial change when compared with SU-controlled studies [111]. In pooled analysis, there was a −1.9 kg effect advantage of acarbose-treated patients compared with SU-treated patients.
In the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) trial, 1429 patients with impaired glucose tolerance were treated with acarbose for 3 years. Results showed a 25% relative risk reduction in new-onset diabetes and a 34% relative risk reduction in new-onset hypertension [113,114]. Myocardial infarction was significantly reduced (hazard ratio (HR) 0.09, p = 0.02) as was any cardiovascular event (HR 0.51, p = 0.03) [114]. Authors suggest that targeting PPG potentially resulted in these cardiovascular benefits [113,114].
Elderly patients tolerate acarbose monotherapy well [115]. When studied over 12 months, elderly patients (average age 68 years) who received acarbose versus placebo, had improved insulin sensitivity by glucose clamp and insulin resistance by homeostasis model assessment (HOMA) [116]. Acarbose may affect the bioavailability of digoxin [117]. There are no long-term data in patients with serum creatinine levels > 2.0 mg/dl, however, with increasing renal dysfunction these is a relative increase in plasma concentrations of acarbose [118]. Liver transaminase elevation can be seen rarely (62 post-marketing cases in > 3 million patient years) and occurred in patients who received 100 mg t.i.d. or greater in dosing [118].
5. Bile-acid sequestrants
Initially developed for treatment of hyperlipidemia, BAS were coincidentally found to reduce plasma glucose in studies investigating the lipid-lowering effects of the drug [119]. The mechanism by which this occurs is unknown [120]. Endogenous glucose production may be reduced by effects on the farsenoid X receptor within the liver and intestine. In addition, BAS may augment the secretion of incretin hormones [121]. Currently, colesevelam is the only BAS approved for use in type 2 diabetes in the USA and Europe. Colesevelam 3.8 g is recommended to be taken once daily or in divided doses with meals.
5.1 BAS in combination
There have been no studies of colesevelam as monotherapy but it has been examined in combination therapy (Table 4). Against background therapy of metformin, SU or insulin, colesevelam reduced HbA1c from placebo by 0.5% and average FPG from placebo by 14 mg/dl [122–124]. When stratified, patients with a baseline HbA1c > 8% showed a slightly greater HbA1c reduction compared with those with HbA1c < 8%. Greater than 47% of colesevelam-treated patients were able to achieve a > 0.7% reduction in HbA1c and > 30 mg/dl reduction in FPG from baseline in all background groups. At completion of the above studies, patients with > 80% compliance (n = 509) were eligible to enroll in a 52-week open-label extension trial of colesevelam [125]. At the completion of the study, the patients who had continued on colesevelam treatment had a 0.1% reduction in HbA1c and a 4 mg/dl reduction in FPG from baseline compared with patients who had received placebo and then initiated colesevelam for the open-label extension trial (−0.3% and −9.5 mg/dl, respectively). When studied on a background of metformin therapy against rosiglitazone and sitagliptin, colesevelam 3.75 mg/day reduced HbA1c from baseline by 0.3% (p = 0.031) compared with rosiglitazone 4 mg/day (−0.6%, p < 0.001) and sitagliptin 100 mg/day (−0.4%, p = 0.009) [126].
Table 4.
Clinical efficacy trials of colesevelam.
| Study | Duration (months) | Patients (n) | Background therapy | Intervention | Baseline HbA1C, mean (%) | Δ from placebo, HbA1c (%) | Δ from placebo, FPG (mg/dl) | Δ from placebo, LDL-C (%) | Δ from placebo, TG (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bays et al. [122] | 6.5 | 316 | Metformin | Colesevelam 3.6 mg/day vs placebo | 8.2 | −0.5* | −14* | −16* | +5 |
| Fonseca et al. [123] | 6.5 | 461 | SU | Colesevelam 3.6 mg/day vs placebo | 8.2 | −0.5* | −14* | −17* | +18* |
| Goldberg et al. [124] | 4 | 287 | Insulin | Colesevelam 3.6 mg/day vs placebo | 8.3 | −0.5* | −15 | −13* | +22* |
p ≤ 0.01, effect reported as least squares mean difference between colesevelam treatment and placebo with the last observation carried forward.
FPG: Fasting plasma glucose; HbA1c: Glycosylated hemoglobin A1c: LDL-C: Low-density lipoprotein cholesterol; SU: Sulfonylurea; TG: Triglycerides.
Beyond glycemic benefits, addition of colesevelam had a favorable effect on lipid parameters. The average reduction in LDL cholesterol from placebo was 15% in colesevelam-treated patients [122–124]. TG levels increased by ~ 16% in colesevelam-treated patients whose baseline average TG levels were < 180 mg/dl. Similar trends in LDL reduction and increase in TG levels were observed in the open-label extension trial [125]. Colesevelam significantly reduced LDL cholesterol by 11% from baseline (p = 0.001) compared with rosiglitazone (8%, p = 0.04) and sitagliptin (8%, p = 0.029). TG levels were significantly elevated in the colesevelam- and rosiglitazone-treated groups (15 and 24%, respectively, p < 0.001) compared with baseline [126].
5.2 Safety and tolerability
In addition to efficacy, the 52-week open-label extension trial evaluated the safety of colesevelam [125]. Of the 509 patients who enrolled, 361 patients completed the extension trial. Seventy-one percent of patients experienced an adverse event with 11% attributed to colesevelam treatment. The most common adverse events were GI, including constipation and flatulence. Seventeen patients experienced hypoglycemia (16/17 considered to be mild-moderate), 1 from the metformin study, 5 from the SU study and 11 from the insulin study. There was a non-significant weight reduction of 0.2 kg in the subjects who completed the extension portion.
There are no long-term data on colesevelam's effects to elevate TG levels with regards to pancreatitis or cardiovascular outcomes in diabetes patients. Additionally, there are no long-term data on the cardiovascular outcomes related to improvement of LDL cholesterol, which authors suggest may be attenuated by the elevation in TG levels [125].
Within the large efficacy trials of colesevelam (n = 1128), 22% of participants were aged > 65 years with no overall difference in efficacy compared with subjects < 65 years [122–124,127]. Colesevelam should be administered 4 hours prior to cyclosporine, levothyroxine, glyburide, ethinyl estradiol and norethindrone [128–130]. There is no recommendation for renal or hepatic dose adjustment [131].
Colesevelam should be used with caution in individuals with TG levels > 300 and is contraindicated in patients with a TG level > 500 [131,132]. Colesevelam is not recommended in patients with gastroparesis or GI motility disorders [131,133,134].
6. Bromocriptine mesylate
Bromocriptine mesylate (bromocriptine) is a central dopamine receptor agonist. Although the exact mechanism is unknown, animal and human studies suggest that bromocriptine may alter the hypothalamic circadian rhythm ultimately resulting in an insulin sensitive state with improved glucose tolerance [135,136]. Approved for treatment in the USA for type 2 diabetes in 2009, the quick release formulation of bromocriptine should be initiated at 1.6 mg/day and titrated to a maximum dose of 4.8 mg/day to be taken within 2 hours of waking. The clinical efficacy of bromocriptine has been studied as monotherapy and combination therapy with SU, metformin and insulin (Table 5).
Table 5.
Clinical efficacy trials of bromocriptine.
| Study | Duration (months) | Patients (n) | Background therapy | Intervention | Baseline HbA1C, mean (%) | Δ from placebo, HbA1c (%) | Δ from placebo, FPG (mg/dl) |
|---|---|---|---|---|---|---|---|
| Cycloset (package insert) [137] | 6 | 159 | Diet and exercise | Bromocriptine, maximum dose > 1.6 mg/day vs placebo | 9.0 | −0.4§ | −23§ |
| Cincotta et al. [138] | 6 | 154 | Diet and exercise | Bromocriptine, maximum dose 4.8 mg/day vs placebo | 8.95 | −0.4§ | −31§ |
| Cycloset (package insert) [137] | 6 | 249 | SU | Bromocriptine, maximum dose > 1.6 mg/day vs placebo | 9.3 | −0.5§ | −18§ |
| Cycloset (package insert) [137] | 6 | 245 | SU | Bromocriptine, maximum dose > 1.6 mg/day vs placebo | 9.3 | −0.6§ | −20§ |
| Cincotta et al. [138] | 6 | 485 | SU | Bromocriptine, maximum dose 4.8 mg/day vs placebo | 9.3 | −0.6§ | −23§ |
| Gaziano et al. [142] | 12 | 3070 | Usual care (1 -- 2 oral meds, insulin + 1 oral med or insulin alone) | Bromocriptine, maximum dose > 1.6 mg/day vs placebo | 8.3‡ | −0.5§ | Not recorded |
| Scranton et al. [143] | |||||||
| Forez et al. [144] |
Values shown for subgroup of patients (n = 559) with baseline HbA1c > 7.5%.
p ≤ 0.05.
FPG: Fasting plasma glucose; HbA1c: Glycosylated hemoglobin A1c; SU: Sulfonylurea.
6.1 Bromocriptine as monotherapy
In two 6-month trials, overweight or obese individuals with type 2 diabetes were randomized to receive bromocriptine monotherapy or diet [137]. When compared with diet alone, the average change in HbA1c and FPG from placebo was −0.4% and −27 mg/dl, respectively, in a population with an average baseline HbA1c of 8 – 9%. Bromocriptine-treated patients were more likely to achieve a 1% decline in HbA1c (28%) from baseline compared with diet alone (8%) and to have a reduction in PPG compared with diet (p < 0.002).
6.2 Bromocriptine in combination
Studies of bromocriptine added to SU therapy show further improvement in glycemic parameters compared with bromocriptine monotherapy [138]. In overweight patients receiving bromocriptine for 6 months (n = 730), with an average baseline HbA1c of ~ 9%, the mean reduction in HbA1c and FPG was 0.6% and 20 mg/dl, respectively, compared with placebo. The third and largest trial (n = 485) reported a significant reduction in PPG (p < 0.0002) [138]. Smaller studies have suggested more robust reduction in HbA1c from placebo (−1 to −1.8%), however, each was ≤ 40 patients over 12 – 16 weeks [139,140].
In a small study of 105 patients with type 2 diabetes (mean HbA1c of 7.8%), subjects were randomized to receive bromocriptine monotherapy, dual bromocriptine and metformin or metformin monotherapy over 12 weeks [141]. Metformin monotherapy was superior to bromocriptine monotherapy in reduction of HbA1c and FPG. When added to metformin, bromocriptine provided a small improvement in HbA1c and FPG compared with bromocriptine monotherapy (p-value not provided).
In the largest trial evaluating the safety and efficacy of bromocriptine therapy, 3070 type 2 diabetes patients were randomized to usual diabetes treatment plus once-daily bromocriptine or placebo for 52 weeks [142,143]. Usual diabetes care could include no more than 2 OHAs, insulin plus one OHA or insulin alone. Dosages of pre-trial diabetes therapy could be adjusted to avert hypoglycemia or added and/or titrated after week 12 to maintain glycemic control. At week 24, the mean change in HbA1c from baseline in the bromocriptine group was 0% and in the placebo group was −0.2% (no p-value provided). At enrollment, > 60% of the patients had HbA1c < 7%. The subgroup of patients with HbA1c ≥ 7.5% who received bromocriptine had a greater reduction in HbA1c (−0.5% change in HbA1c from baseline, p < 0.001). Bromocriptine added as adjunct to a TZD, using intention-to-treat analysis, had a 0.8% reduction in HbA1c from placebo (p = 0.001) [143,144]. Overall, there did not appear to be further efficacy of bromocriptine add-on therapy in patients who had already achieved HbA1c < 7%.
6.3 Safety and tolerability
In the large 52-week safety and efficacy trial (n = 3070), 47% of bromocriptine-treated patients discontinued therapy versus 32% of placebo-treated patients [142]. Nausea was the most prominent adverse event (8% with bromocriptine treatment vs 1% with placebo). This study and others document that nausea, attributed to bromocriptine treatment, was more likely to be seen at initial titration of bromocriptine and lasted about 2 weeks [138,139,142,143]. Hypotension and/or orthostatic hypotension were reported in the bromocriptine-treated groups (2.2, 0.3%, respectively) with 98% of the symptomatic patients on at least one antihypertensive medication [137]. Bromocriptine, as an adjunct to TZDs, did not show an increased risk of peripheral edema, weight gain or more cardiac events [144]. In post-marketing reports, there were no reports of hallucinations, psychotic or psychiatric disorders, serious fibrotic complications, stroke or neuroleptic-like malignant syndromes within bromocriptine-treated patients (n = 2500) [137].
The incidence of hypoglycemia (defined as symptoms suggestive of hypoglycemia that promptly resolved with appropriate care, symptoms with a measured glucose < 60 mg/dl or measured glucose < 49 mg/dl) was 6.9% in the bromocriptine-treated group versus 5.3% in the placebo-treated group [142].
The average change in body weight from placebo with bromocriptine monotherapy was +0.25 kg over 6 months [137,138]. A small but significant change in body weight relative to placebo (bromocriptine +1.2 kg, placebo +0.3 kg; p < 0.0002) was seen in bromocriptine-treated patients with a SU background [138].
The hazard ratio was 0.58 with a 42% relative risk reduction for the time to first occurrence of secondary cardiovascular end points when bromocriptine was compared with placebo suggesting a potential cardioprotective effect of bromocriptine [142]. Based on the Kaplan–Meier estimates, 79 patients would need to be treated to avoid one first important cardiovascular event.
Within the 52-week safety trial, 29% of subjects were ≥ 65 years of age [142]. There was no difference in observed safety and effectiveness between the elderly patients and patients ≤ 65 years of age [137]. Bromocriptine QR has not been studied in patients taking other dopamine receptor agonists or dopamine receptor antagonists; therefore, concomitant use is not recommended. There is no recommendation for renal or hepatic dosing, but consideration should be made to the extensive hepatic metabolism via cytochrome P4503A [137].
7. Conclusion
Metformin has excellent efficacy with favorable weight and lipid profiles supporting its use as first-line therapy. DPP-4 inhibitors are less efficacious than metformin but have proven non-inferior to SU treatment with a favorable side-effect profile and less risk for hypoglycemia. The meglitinides achieve similar efficacy to the longer-acting SU counterparts but are plagued with similar hypoglycemia with repaglinide use (less with nateglinide), a more frequent daily dosing scheme, and an unknown effect on cardiovascular preconditioning. AGIs can have good efficacy but use is limited due to adverse GI reactions and inconvenient t.i.d. dosing regimen. BAS and bromocriptine are less efficacious than metformin, SU and TZD therapy but also have unfavorable GI side-effect profiles, especially BAS. However, these agents are unlikely to cause hypoglycemia without concomitant SU or insulin therapy.
8. Expert opinion
Lifestyle modification should be recommended and reinforced to all patients being treated for type 2 diabetes. Metformin therapy is well studied, inexpensive and unlikely to cause hypoglycemia and should be used as first-line therapy in all patients who can tolerate it or have no contraindication. If the patient has not achieved the glycemic target, the practitioner now has a number of agents to consider as addon therapy. Herein lies the art of diabetes treatment. Efficacy and side-effect profiles of agents as well as patient age, preference, weight and comorbidities all play a role in determining which class to choose (Table 6, Figure 1). In addition, the literature on antidiabetic agents needs to be carefully examined to understand efficacy differences between classes. Certain factors such as baseline HbA1c as well as the duration of the trial need to be noted. As highlighted by Bloomgarden et al., baseline glycemic status strongly influences response to antidiabetic drug treatment with greater HbA1c and FPG improvements seen at higher baseline values [145]. Trials in more recent years, such as those involving DPP-4 inhibitors, have used participants with lower baseline HbA1c values and therefore may underestimate maximal response. On the other hand, older trials of metformin and SUs typically had higher baseline values and therefore showed greater response to these agents. It is therefore difficult to compare relative efficacies of classes unless there are head-to-head trials between them. Further, duration of the trial needs to be looked at as HbA1c response to pharmacologic intervention typically plateaus after 3 months.
Table 6.
Expert opinion of specific efficacy and safety profiles for OHA.
| Class | Ease of dosing | Dose adjustment for renal impairment | Risk of hypoglycemia | Weight |
|---|---|---|---|---|
| Metformin | ++ | Yes | No | ↓ |
| SU | ++ | Yes | Yes | ↑ |
| TZD | +++ | No | No | ↑ ↑ |
| DPP-4 inhibitor | +++ | Yes | No | ↔ |
| Meglitinides | + | Yes | Yes | ↑ or ↔ |
| AGIs | + | Yes | No | ↔ |
| BAS | ++ | No | No | ↓ or ↔ |
| Bromocriptine mesylate | +++ | No | No | ↓ or ↔ |
+: Unfavorable; ++: Moderately favorable; +++: Favorable; AGI: α-glucosidase inhibitor; BAS: Bile-acid sequestrants; DPP-4: Dipeptidyl peptidase-IV; OHA: Oral hypoglycemic agent; TZD: Thiazolidinedione.
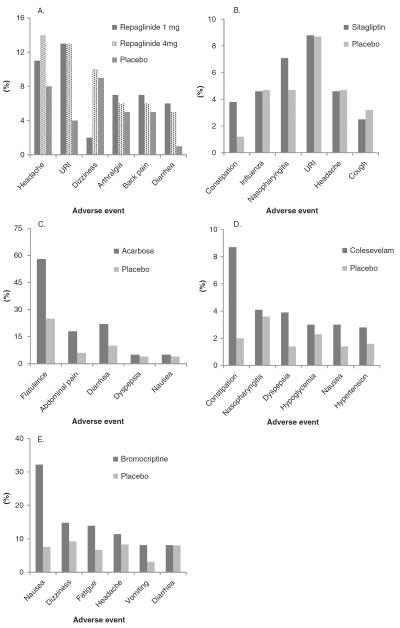
Figure 1.
Adverse events occurring in at least A) 5% of patients receiving repaglinide 1 or 4 mg or placebo [76]; B) 2% of patients receiving sitagliptin 100 mg/day or placebo [28]; C) 4% of patients receiving acarbose or placebo [112]; D) 2% of patients receiving colesevelam or placebo as add-on therapy with metformin, insulin or sulfonylurea [125]; E) 5% of patients receiving bromocriptine or placebo [137].
URI: Upper respiratory infection.
That being said, it is reasonable to consider a trial of SU therapy as add-on to metformin therapy or as monotherapy if metformin is contraindicated, but certain patient characteristics may challenge the use of this therapeutic class, including patient age and weight, renal function and cardiovascular status. TZDs have essentially fallen out of favor until the cardiovascular risk and cancer risk profiles have been either further clarified or disproven. As such, every physician treating patients with type 2 diabetes is faced with potential initiation of injection therapy which is often resisted by the patient.
DPP-4 inhibitors are well tolerated, have moderate HbA1c lowering and do not cause significant hypoglycemia. Both FPG and PPG are targeted via a non-β-cell mechanism. When actually comparing DPP-4 inhibitor with SU therapy, non-inferiority of gliptins has been shown. On the other hand, DPP-4 inhibitors offer less improvement in glycemic parameters than metformin. DPP-4 inhibitors are a good alternative when metformin treatment is limited by GI side effects or when SU treatment is complicated by significant hypoglycemia or weight gain. They should also be considered as add-on therapy when a patient is on maximally tolerated metformin, SU or TZD therapy but has not achieved full glycemic control. Dosing can be adjusted for renal impairment making them potential initial and/or add-on therapy in patients with stages 1 – 5 chronic kidney disease at risk for hypoglycemia. Cost could be a factor as this class is branded. DPP-4 inhibitors are equally expensive compared with meglitinides, but have a better side-effect profile and once-daily dosing.
Meglitinides should be considered in patients who have erratic meal times and need meal-time glycemic control in those with preserved pancreatic β-cell function. Consideration to this agent should be given in those with renal impairment. An expected improvement in HbA1c of 0.5 – 1.6% can be seen. Caution should be used with repaglinide in those at higher risk for hypoglycemia, in which case nateglinide would be the better option, despite less substantial improvements in glycemic control. Meglitinides should not be used in combination with SU therapy. As the cardiovascular risk is uncertain, meglitinides should be avoided in the same patients for whom one would avoid SU therapy for similar concerns. As all meglitinides are branded, preauthorization or consultation with pharmacy coverage and cost may need to be considered. In addition, the frequent dosing schedule may be a deterrent for some patients.
AGIs offer an alternative site of action (gut) and are essentially equally efficacious to DPP-4 inhibitors but are generally not well tolerated. The GI side effects are prohibitive to a good portion of patients despite lower cost compared with DPP-4 inhibitors and meglitinides. Therefore, AGIs may be most beneficial in patients suffering from constipation who are already taking other t.i.d. medications. In addition, consideration for use in elderly patients should be given as studies have shown its safety and improved insulin sensitivity in this population.
BAS and bromocriptine have the lowest efficacy in general. Not developed as primary therapeutic targets, the small initial glycemic benefits seen were noted in side-effect profiles in large studies where the medications were being used for alternative reasons. Neither has a risk of hypoglycemia unless used with other therapies like SU or insulin making them options in a patient at risk for hypoglycemia. A BAS could be considered in a patient who needs reductions in HbA1c as well as LDL cholesterol to achieve the target goals, but should be cautioned in those patients whose elevated TG have not been addressed. Bromocriptine could be considered in any patient in need of a small reduction in HbA1c, not on antihypertensive therapy, who may benefit from the potential cardioprotective effects.
The patient resistant to injection therapy has options provided that there is sufficient pancreatic β-cell reserve [146]. Studies already demonstrate the increased use of DPP-4 inhibitors as TZDs have been less utilized [147]. All of the medications discussed have better efficacy in patients with HbA1c > 8%, who are OHA-naïve, but it is often the patient who has achieved a HbA1c of 7.0 – 7.9% in whom the challenge of additional therapeutic options lie. Thus, the practitioner should be cognizant that use of these therapies in patients with HbA1c < 8% may not achieve ADA or EASD guideline reduction to < 7% alone.
As diabetes becomes an increasingly common disease, there continues to be interest in new pharmacologic targets. One class of OHA on the horizon is the sodium-glucose cotransporter 2 (SGLT2) inhibitors. This class of compounds targets SGLT2 on the proximal tubule of the kidney to block renal glucose reabsorption and thereby reduce blood glucose levels. Selectivity for SGLT2 was sought given its predominant role in glucose reabsorption and location mainly in the kidney [148,149]. SGLT1, on the other hand, plays a more minor role in glucose reabsorption and is not only expressed in the kidney but also the GI tract and heart, making it a less desirable target [150,151]. The mechanism for SGLT2 inhibitors is independent of insulin secretion or action and therefore less likely to cause hypoglycemia or to lose efficacy as β-cells lose their insulin secretory capacity. Dapagliflozin is a highly specific inhibitor of SGLT2 and is the most well-developed and studied agent in this class. Efficacy of dapagliflozin has been shown as monotherapy [152] and add-on therapy to metformin [153,154], SUs [155], TZDs [156] and insulin [157]. In treatment-naïve subjects at week 24, decreases in HbA1c from a mean baseline of 7.9% were 0.6, 0.8 and 0.9% for dapagliflozin 2.5, 5 and 10 mg compared with a decrease of 0.2% for placebo (p ≤ 0.005 vs placebo) [152]. No major episodes of hypoglycemia occurred, defined as symptoms of hypoglycemia, a blood glucose value < 54 mg/dl and requiring third-party assistance. Reports of signs or symptoms suggestive of urinary tract and genital infections were more common with dapagliflozin treatment than placebo. In a comparison of metformin XR plus dapagliflozin versus either agent as monotherapy in treatment-naïve subjects over 24 weeks, changes from baseline (9.1%) in HbA1c were significantly greater for combination therapy than monotherapy: −2.0% combination, −1.5% dapagliflozin 10 mg, −1.4% metformin (uptitrated to 2000 mg/day) (p < 0.0001) [154]. Dapagliflozin 10 mg was non-inferior to metformin monotherapy in HbA1c reduction and caused significantly greater weight loss. Excitement for SGLT2 inhibitors is gaining momentum with more studies proving efficacy and tolerability.
Another new class of agents for type 2 diabetes treatment is the dual PPAR modulators. The dual PPAR modular aleglitazar has affinity to PPAR-α and PPAR-γ and is in Phase III clinical trials. PPAR-α agonists (e.g., fibrates) are used for treatment of hyperlipidemia, specifically hypertriglyceridemia, while PPAR-γ agonists (e.g., TZDs) are used to improve glycemic control in type 2 diabetes [158]. The prospect of agents that work at both of these receptors is potentially attractive given the common co-occurrence of diabetes and cardiovascular disease. However, several attempts to develop dual PPAR agonists have failed given safety concerns. Some of these safety concerns include bladder cancer and hyperplasia with rapaglitazar and naveglitazar [159], renal dysfunction with tesaglitazar [160], and death, major cardiovascular events and congestive heart failure with muraglitazar [161]. In a study of subjects randomized to aleglitazar (20, 50, 100, 300, 600 or 900 μg) or placebo for 6 weeks after a 3-week washout period, dose-dependent decreases in levels of FPG, PPG and TG were seen with aleglitazar treatment with a seemingly balanced affinity for both α and γ receptors [162]. The well-known adverse reactions of PPAR-γ-related edema and weight gain and PPAR-α-related decreases in creatinine clearance were seen with aleglitazar treatment. In a 16-week study, subjects were randomized to placebo, pioglitazone 45 mg/day or aleglitazar 50, 150, 300 or 600 μg/day [163]. Significant dose-dependent decreases in HbA1c from baseline (8.0%) compared with placebo were seen with aleglitazar treatment from −0.4% with 50 μg (p = 0.048) to −1.4% with 600 μg (p < 0.001). Aleglitazar 50 μg and pioglitazone 45 mg produced similar glycemic effects. TG decreases and HDL increases were also significant with all doses of aleglitazar. The Phase III study ALECARDIO is currently ongoing to test the hypothesis that in patients with type 2 diabetes who suffered a recent acute coronary syndrome, aleglitazar 150 μg/day can reduce cardiovascular morbidity and mortality.
While SGLT2 inhibitors and dual-PPAR agonists are gathering the most excitement for new diabetes treatments, other agents being studied include 11 β-hydroxysteroid dehydrogenase type 1 inhibitors which minimize the antiglycemic effects of cortisol, and glycogen phosphorylase activators which increase hepatic glucose metabolism.
Article highlights
Metformin, sulfonylureas (SU) and thiazolidinediones (TZD) are the oldest and most well-studied classes of hypoglycemic/antihyperglycemic agents. HbA1c reduction of 1.0 – 1.5% should be expected with each of these agents.
Several other classes of oral agents have more recently been approved for type 2 diabetes management including: dipeptidyl peptidase-IV (DPP-4) inhibitors, meglitinide analogs, α-glucosidase inhibitors (AGIs), bile-acid sequestrants (BAS) and bromocriptine.
DPP-4 inhibitors are moderately efficacious and are well tolerated with a low risk of hypoglycemia. They should be considered as an alternative when metformin use is limited by gastrointestinal (GI) side effects or when SU use is limited by hypoglycemia.
Repaglinide has similar efficacy to SU, but is limited by its frequent dosing regimen and hypoglycemia (less with nateglinide).
AGIs can also have moderate efficacy but are limited mainly by their GI side-effect profile.
BAS and bromocriptine are the least efficacious but have a low risk of hypoglycemia.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: A patient-centered approach. position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–96. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 2.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–13. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2012;157:601–10. doi: 10.7326/0003-4819-157-9-201211060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–54. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 5.Deeg MA, Buse JB, Goldberg RB, et al. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2007;30:2458–64. doi: 10.2337/dc06-1903. [DOI] [PubMed] [Google Scholar]
- 6.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macroVascular events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 7.Tontonoz P, Nagy L, Alvarez JG, et al. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–52. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 8.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–56. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 10.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 11.Kahn SE, Zinman B, Lachin JM, et al. Rosiglitazone-associated fractures in type 2 diabetes: an analysis from A diabetes outcome progression trial (ADOPT) Diabetes Care. 2008;31:845–51. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- 12.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 13.Mcintyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet. 1964;2:20–1. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama T, Komatsu R, Namba M, et al. Glucagon-like peptide-1 (7–36 amide): a potent glucagonostatic and insulinotropic hormone. Diabetes Res Clin Pract. 1988;5:281–4. doi: 10.1016/s0168-8227(88)80063-9. [DOI] [PubMed] [Google Scholar]
- 15.Wettergren A, Schjoldager B, Mortensen PE, et al. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38:665–73. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]
- 16.Tourrel C, Bailbe D, Meile MJ, et al. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes. 2001;50:1562–70. doi: 10.2337/diabetes.50.7.1562. [DOI] [PubMed] [Google Scholar]
- 17.Perfetti R, Zhou J, Doyle ME, et al. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–5. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- 18.Holz GG, IV, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37) Nature. 1993;361:362–5. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–88. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab. 2010;12:648–58. doi: 10.1111/j.1463-1326.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 21.Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet. 2012;51:501–14. doi: 10.1007/BF03261927. [DOI] [PubMed] [Google Scholar]
- 22.Deacon CF, Holst JJ. Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2010;19:133–40. doi: 10.1517/13543780903463862. [DOI] [PubMed] [Google Scholar]
- 23.Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–88. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Dhillon S, Weber J. Saxagliptin. Drugs. 2009;69:2103–14. doi: 10.2165/11201170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51:411–27. doi: 10.2165/11630900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.He YL. Clinical pharmacokinetics and pharmacodynamics of vildagliptin. Clin Pharmacokinet. 2012;51:147–62. doi: 10.2165/11598080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Covington P, Christopher R, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther. 2008;30:499–512. doi: 10.1016/j.clinthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]; •• This large trial highlighted the efficacy of sitagliptin compared with placebo with regards to reductions in HbA1c, FPG and PPG.
- 29.Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–71. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]; •• This large trial highlighted the efficacy of sitagliptin compared with placebo with regards to reductions in HbA1c, FPG and PPG.
- 30.Rosenstock J, Aguilar-Salinas C, Klein E, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401–11. doi: 10.1185/03007990903178735. [DOI] [PubMed] [Google Scholar]
- 31.Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13:258–67. doi: 10.1111/j.1463-1326.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 32.Pi-Sunyer FX, Schweizer A, Mills D, et al. Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–8. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo RA, Fleck PR, Wilson CA, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: A randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315–17. doi: 10.2337/dc08-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aschner P, Katzeff HL, Guo H, et al. Efficacy and safety of monotherapy of sitagliptin compared with metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:252–61. doi: 10.1111/j.1463-1326.2009.01187.x. [DOI] [PubMed] [Google Scholar]
- 35.Schweizer A, Couturier A, Foley JE, et al. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naive patients with type 2 diabetes. Diabet Med. 2007;24:955–61. doi: 10.1111/j.1464-5491.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 36.Karagiannis T, Paschos P, Paletas K, et al. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. doi: 10.1136/bmj.e1369. [DOI] [PubMed] [Google Scholar]
- 37.Nauck MA, Meininger G, Sheng D, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 38.Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: A randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475–83. doi: 10.1016/S0140-6736(12)60691-6. [DOI] [PubMed] [Google Scholar]
- 39.Arechavaleta R, Seck T, Chen Y, et al. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2011;13:160–8. doi: 10.1111/j.1463-1326.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 40.Seck T, Nauck M, Sheng D, et al. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. Int J Clin Pract. 2010;64:562–76. doi: 10.1111/j.1742-1241.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein BJ, Feinglos MN, Lunceford JK, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–87. doi: 10.2337/dc07-0627. [DOI] [PubMed] [Google Scholar]; • This trial demonstrated the efficacy and safety of combination sitagliptin and metformin and also studied each drug as monotherapy.
- 42.DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649–55. doi: 10.2337/dc08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–45. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–68. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Vilsboll T, Rosenstock J, Yki-Jarvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–77. doi: 10.1111/j.1463-1326.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- 46.Fonseca V, Schweizer A, Albrecht D, et al. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–55. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 47.Scheen AJ, Charpentier G, Ostgren CJ, et al. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2010;26:540–9. doi: 10.1002/dmrr.1114. [DOI] [PubMed] [Google Scholar]
- 48.Richter B, Bandeira-Echtler E, Bergerhoff K, et al. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4:753–68. doi: 10.2147/vhrm.s1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marfella R, Barbieri M, Grella R, et al. Effects of vildagliptin twice daily vs. sitagliptin once daily on 24-hour acute glucose fluctuations. J Diabetes Complications. 2010;24:79–83. doi: 10.1016/j.jdiacomp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 50.De Meester I, Korom S, Van Damme J, et al. CD26, let it cut or cut it down. Immunol Today. 1999;20:367–75. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 51.Lankas GR, Leiting B, Roy RS, et al. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes. 2005;54:2988–94. doi: 10.2337/diabetes.54.10.2988. [DOI] [PubMed] [Google Scholar]
- 52.Engel SS, Williams-Herman DE, Golm GT, et al. Sitagliptin: Review of preclinical and clinical data regarding incidence of pancreatitis. Int J Clin Pract. 2010;64:984–90. doi: 10.1111/j.1742-1241.2010.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Profit L, Chrisp P, Nadin C. Vildagliptin: the evidence for its place in the treatment of type 2 diabetes mellitus. Core Evid. 2008;3:13–30. doi: 10.3355/ce.2008.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frederich R, Alexander JH, Fiedorek FT, et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med. 2010;122:16–27. doi: 10.3810/pgm.2010.05.2138. [DOI] [PubMed] [Google Scholar]
- 55.Johansen OE, Neubacher D, von Eynatten M, et al. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol. 2012;11:3. doi: 10.1186/1475-2840-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schweizer A, Dejager S, Foley JE, et al. Assessing the cardio-cerebrovascular safety of vildagliptin: Meta-analysis of adjudicated events from a large phase III type 2 diabetes population. Diabetes Obes Metab. 2010;12:485–94. doi: 10.1111/j.1463-1326.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 57.Sitagliptin cardiovascular outcome study (0431-082 AMI) (TECOS) [internet] [Internet] 2012 Available from: http://clinicaltrials.gov/ct2/show/NCT00790205?term=TECOS&rank=1.
- 58.Cardiovascular outcomes study of alogliptin in subjects with type 2 diabetes and acute coronary syndrome (EXAMINE) [Internet] 2012 Available from: http://clinicaltrials.gov/ct2/show/NCT00968708?term=EXAMINE&rank=1.
- 59.Cardiovascular outcome study of linagliptin versus glimepiride in patients with type 2 diabetes (CAROLINA) [internet] [Internet] 2012 Available from: http://clinicaltrials.gov/show/NCT01243424.
- 60.Scirica BM, Bhatt DL, Braunwald E, et al. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI) 53 study. Am Heart J. 2011;162:818–25. e6. doi: 10.1016/j.ahj.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Barzilai N, Guo H, Mahoney EM, et al. Efficacy and tolerability of sitagliptin monotherapy in elderly patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011;27:1049–58. doi: 10.1185/03007995.2011.568059. [DOI] [PubMed] [Google Scholar]
- 62.Patel CG, Li L, Girgis S, et al. Two-way pharmacokinetic interaction studies between saxagliptin and cytochrome P450 substrates or inhibitors: simvastatin, diltiazem extended-release, and ketoconazole. Clin Pharmacol. 2011;3:13–25. doi: 10.2147/CPAA.S15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheen AJ. Dipeptidylpeptidase-4 inhibitors (gliptins): focus on drug-drug interactions. Clin Pharmacokinet. 2010;49:573–88. doi: 10.2165/11532980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Chan JC, Scott R, Arjona Ferreira JC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab. 2008;10:545–55. doi: 10.1111/j.1463-1326.2008.00914.x. [DOI] [PubMed] [Google Scholar]
- 65.Nowicki M, Rychlik I, Haller H, et al. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract. 2011;65:1230–9. doi: 10.1111/j.1742-1241.2011.02812.x. [DOI] [PubMed] [Google Scholar]
- 66.Migoya EM, Stevens CH, Bergman AJ, et al. Effect of moderate hepatic insufficiency on the pharmacokinetics of sitagliptin. Can J Clin Pharmacol. 2009;16:e165–70. [PubMed] [Google Scholar]
- 67.Boulton DW, Li L, Frevert EU, et al. Influence of renal or hepatic impairment on the pharmacokinetics of saxagliptin. Clin Pharmacokinet. 2011;50:253–65. doi: 10.2165/11584350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 68.Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51:411–27. doi: 10.2165/11630900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 69.Polonsky KS, Given BD, Hirsch LJ, et al. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1988;318:1231–9. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 70.Gromada J, Dissing S, Kofod H, et al. Effects of the hypoglycaemic drugs repaglinide and glibenclamide on ATP-sensitive potassium-channels and cytosolic calcium levels in beta TC3 cells and rat pancreatic beta cells. Diabetologia. 1995;38:1025–32. doi: 10.1007/BF00402171. [DOI] [PubMed] [Google Scholar]
- 71.Bakkali-Nadi A, Malaisse-Lagae F, Malaisse WJ. Insulinotropic action of meglitinide analogs: concentration-response relationship and nutrient dependency. Diabetes Res. 1994;27:81–7. [PubMed] [Google Scholar]
- 72.Hu S, Wang S, Fanelli B, et al. Pancreatic beta-cell K(ATP) channel activity and membrane-binding studies with nateglinide: a comparison with sulfonylureas and repaglinide. J Pharmacol Exp Ther. 2000;293:444–52. [PubMed] [Google Scholar]
- 73.Owens DR, Luzio SD, Ismail I, et al. Increased prandial insulin secretion after administration of a single preprandial oral dose of repaglinide in patients with type 2 diabetes. Diabetes Care. 2000;23:518–23. doi: 10.2337/diacare.23.4.518. [DOI] [PubMed] [Google Scholar]
- 74.Jovanovic L, Dailey G, III, Huang WC, et al. Repaglinide in type 2 diabetes: a 24-week, fixed-dose efficacy and safety study. J Clin Pharmacol. 2000;40:49–57. doi: 10.1177/00912700022008694. [DOI] [PubMed] [Google Scholar]; •• This large trial highlighted the efficacy of repaglinide compared with placebo with regards to reductions in HbA1c and FPG.
- 75.Moses RG, Gomis R, Frandsen KB, et al. Flexible meal-related dosing with repaglinide facilitates glycemic control in therapy-naive type 2 diabetes. Diabetes Care. 2001;24:11–15. doi: 10.2337/diacare.24.1.11. [DOI] [PubMed] [Google Scholar]; •• This large trial highlighted the efficacy of repaglinide compared with placebo with regards to reductions in HbA1c and FPG.
- 76.Derosa G, Mugellini A, Ciccarelli L, et al. Comparison of glycaemic control and cardiovascular risk profile in patients with type 2 diabetes during treatment with either repaglinide or metformin. Diabetes Res Clin Pract. 2003;60:161–9. doi: 10.1016/s0168-8227(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 77.Madsbad S, Kilhovd B, Lager I, et al. Comparison between repaglinide and glipizide in type 2 diabetes mellitus: a 1-year multicentre study. Diabet Med. 2001;18:395–401. doi: 10.1046/j.1464-5491.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 78.Marbury T, Huang WC, Strange P, et al. Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract. 1999;43:155–66. doi: 10.1016/s0168-8227(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 79.Moses R, Slobodniuk R, Boyages S, et al. Effect of repaglinide addition to metformin monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 1999;22:119–24. doi: 10.2337/diacare.22.1.119. [DOI] [PubMed] [Google Scholar]
- 80.Jovanovic L, Hassman DR, Gooch B, et al. Treatment of type 2 diabetes with a combination regimen of repaglinide plus pioglitazone. Diabetes Res Clin Pract. 2004;63:127–34. doi: 10.1016/j.diabres.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Rosenstock J, Hassman DR, Madder RD, et al. Repaglinide versus nateglinide monotherapy: A randomized, multicenter study. Diabetes Care. 2004;27:1265–70. doi: 10.2337/diacare.27.6.1265. [DOI] [PubMed] [Google Scholar]; • This trial showed that repaglinide has greater efficacy with respect to glycemic control than nateglinide but with a higher risk of hypoglycemia.
- 82.NAVIGATOR Study Group Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–76. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- 83.Schwarz SL, Gerich JE, Marcellari A, et al. Nateglinide, alone or in combination with metformin, is effective and well tolerated in treatment-naive elderly patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:652–60. doi: 10.1111/j.1463-1326.2007.00792.x. [DOI] [PubMed] [Google Scholar]
- 84.Del Prato S, Heine RJ, Keilson L, et al. Treatment of patients over 64 years of age with type 2 diabetes: experience from nateglinide pooled database retrospective analysis. Diabetes Care. 2003;26:2075–80. doi: 10.2337/diacare.26.7.2075. [DOI] [PubMed] [Google Scholar]
- 85.Hatorp V, Hansen KT, Thomsen MS. Influence of drugs interacting with CYP3A4 on the pharmacokinetics, pharmacodynamics, and safety of the prandial glucose regulator repaglinide. J Clin Pharmacol. 2003;43:649–60. [PubMed] [Google Scholar]
- 86.Niemi M, Backman JT, Neuvonen M, et al. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2000;68:495–500. doi: 10.1067/mcp.2000.111183. [DOI] [PubMed] [Google Scholar]
- 87.Niemi M, Backman JT, Neuvonen M, et al. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003;46:347–51. doi: 10.1007/s00125-003-1034-7. [DOI] [PubMed] [Google Scholar]
- 88.Niemi M, Kajosaari LI, Neuvonen M, et al. The CYP2C8 inhibitor trimethoprim increases the plasma concentrations of repaglinide in healthy subjects. Br J Clin Pharmacol. 2004;57:441–7. doi: 10.1046/j.1365-2125.2003.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scheen AJ. Drug-drug and food-drug pharmacokinetic interactions with new insulinotropic agents repaglinide and nateglinide. Clin Pharmacokinet. 2007;46:93–108. doi: 10.2165/00003088-200746020-00001. [DOI] [PubMed] [Google Scholar]
- 90.Hasslacher C. Multinational Repaglinide Renal Study Group. Safety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal function. Diabetes Care. 2003;26:886–91. doi: 10.2337/diacare.26.3.886. [DOI] [PubMed] [Google Scholar]
- 91.Devineni D, Walter YH, Smith HT, et al. Pharmacokinetics of nateglinide in renally impaired diabetic patients. J Clin Pharmacol. 2003;43:163–70. doi: 10.1177/0091270002239825. [DOI] [PubMed] [Google Scholar]
- 92.Choudhury S, Hirschberg Y, Filipek R, et al. Single-dose pharmacokinetics of nateglinide in subjects with hepatic cirrhosis. J Clin Pharmacol. 2000;40:634–40. [PubMed] [Google Scholar]
- 93.Lopez-Garcia F, Borras J, Verdu C, et al. Cholestatic hepatitis associated with repaglinide. Diabetes Care. 2005;28:752–3. doi: 10.2337/diacare.28.3.752-a. [DOI] [PubMed] [Google Scholar]
- 94.Jaiswal S, Mehta R, Musuku M, et al. Repaglinide induced acute hepototoxicity. JNMA J Nepal Med Assoc. 2009;48:162–4. [PubMed] [Google Scholar]
- 95.Nan DN, Hernandez JL, Fernandez-Ayala M, et al. Acute hepatotoxicity caused by repaglinide. Ann Intern Med. 2004;141:823. doi: 10.7326/0003-4819-141-10-200411160-00024. [DOI] [PubMed] [Google Scholar]
- 96.Bischoff H. Pharmacology of alpha-glucosidase inhibition. Eur J Clin Invest. 1994;24(Suppl 3):3–10. [PubMed] [Google Scholar]
- 97.Lee A, Patrick P, Wishart J, et al. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab. 2002;4:329–35. doi: 10.1046/j.1463-1326.2002.00219.x. [DOI] [PubMed] [Google Scholar]
- 98.Takei I, Miyamoto K, Funae O, et al. Secretion of GIP in responders to acarbose in obese type 2(NIDDM) patients. J Diabetes Complications. 2001;15:245–9. doi: 10.1016/s1056-8727(01)00148-9. [DOI] [PubMed] [Google Scholar]
- 99.Enc FY, Imeryuz N, Akin L, et al. Inhibition of gastric emptying by acarbose is correlated with GLP-1 response and accompanied by CCK release. Am J Physiol Gastrointest Liver Physiol. 2001;281:G752–63. doi: 10.1152/ajpgi.2001.281.3.G752. [DOI] [PubMed] [Google Scholar]
- 100.Groop PH, Groop L, Totterman KJ, et al. Effects of acarbose on the relationship between changes in GIP and insulin responses to meals in normal subjects. Acta Endocrinol (Copenh) 1986;112:361–6. doi: 10.1530/acta.0.1120361. [DOI] [PubMed] [Google Scholar]
- 101.Derosa G, Maffioli P, Ferrari I, et al. Acarbose actions on insulin resistance and inflammatory parameters during an oral fat load. Eur J Pharmacol. 2011;651:240–50. doi: 10.1016/j.ejphar.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 102.Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, glibenclamide, or placebo in NIDDM patients. The Essen study. Diabetes Care. 1994;17:561–6. doi: 10.2337/diacare.17.6.561. [DOI] [PubMed] [Google Scholar]; •• This large trial showed no significant difference in reduction from baseline of HbA1c, FPG or PPG over 24 weeks in those treated with acarbose vs glibeclamide. However, there was a significant change vs placebo.
- 103.Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, metformin, or placebo in dietary-treated NIDDM patients: the Essen-II study. Am J Med. 1997;103:483–90. doi: 10.1016/s0002-9343(97)00252-0. [DOI] [PubMed] [Google Scholar]; •• This large trial showed no significant difference in reduction from baseline of HbA1c, FPG or PPG over 24 weeks in those treated with acarbose vs metformin. However, there was a significant change vs placebo.
- 104.Halimi S, Le Berre MA, Grange V. Efficacy and safety of acarbose add-on therapy in the treatment of overweight patients with type 2 diabetes inadequately controlled with metformin: a double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2000;50:49–56. doi: 10.1016/s0168-8227(00)00163-7. [DOI] [PubMed] [Google Scholar]
- 105.Rosenstock J, Brown A, Fischer J, et al. Efficacy and safety of acarbose in metformin-treated patients with type 2 diabetes. Diabetes Care. 1998;21:2050–5. doi: 10.2337/diacare.21.12.2050. [DOI] [PubMed] [Google Scholar]
- 106.Phillips P, Karrasch J, Scott R, et al. Acarbose improves glycemic control in overweight type 2 diabetic patients insufficiently treated with metformin. Diabetes Care. 2003;26:269–73. doi: 10.2337/diacare.26.2.269. [DOI] [PubMed] [Google Scholar]
- 107.Costa B, Pinol C. Acarbose in ambulatory treatment of non-insulin-dependent diabetes mellitus associated to imminent sulfonylurea failure: a randomised-multicentric trial in primary health-care. diabetes and acarbose research group. Diabetes Res Clin Pract. 1997;38:33–40. doi: 10.1016/s0168-8227(97)00083-1. [DOI] [PubMed] [Google Scholar]
- 108.Chiasson JL, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994;121:928–35. doi: 10.7326/0003-4819-121-12-199412150-00004. [DOI] [PubMed] [Google Scholar]
- 109.Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. prospective diabetes study 44) Diabetes Care. 1999;22:960–4. doi: 10.2337/diacare.22.6.960. [DOI] [PubMed] [Google Scholar]
- 110.van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–63. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 111.Van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003639. doi: 10.1002/14651858.CD003639.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coniff RF, Shapiro JA, Seaton TB. Long-term efficacy and safety of acarbose in the treatment of obese subjects with non-insulin-dependent diabetes mellitus. Arch Intern Med. 1994;154:2442–8. [PubMed] [Google Scholar]
- 113.Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 114.Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–94. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 115.Josse RG, Chiasson JL, Ryan EA, et al. Acarbose in the treatment of elderly patients with type 2 diabetes. Diabetes Res Clin Pract. 2003;59:37–42. doi: 10.1016/s0168-8227(02)00176-6. [DOI] [PubMed] [Google Scholar]
- 116.Meneilly GS, Ryan EA, Radziuk J, et al. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care. 2000;23:1162–7. doi: 10.2337/diacare.23.8.1162. [DOI] [PubMed] [Google Scholar]
- 117.Miura T, Ueno K, Tanaka K, et al. Impairment of absorption of digoxin by acarbose. J Clin Pharmacol. 1998;38:654–7. doi: 10.1002/j.1552-4604.1998.tb04474.x. [DOI] [PubMed] [Google Scholar]
- 118.Precose (package insert) [Internet] Bayer Health Care Pharmaceuticals Inc.; Wayne, New Jersey: 2008. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020482s023lbl.pdf. [Google Scholar]
- 119.Garg A, Grundy SM. Cholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Ann Intern Med. 1994;121:416–22. doi: 10.7326/0003-4819-121-6-199409150-00004. [DOI] [PubMed] [Google Scholar]
- 120.Staels B. A review of bile acid sequestrants: potential mechanism(s) for glucose-lowering effects in type 2 diabetes mellitus. Postgrad Med. 2009;121:25–30. doi: 10.3810/pgm.2009.05.suppl53.290. [DOI] [PubMed] [Google Scholar]
- 121.Shang Q, Saumoy M, Holst JJ, et al. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419–24. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 122.Bays HE, Goldberg RB, Truitt KE, et al. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168:1975–83. doi: 10.1001/archinte.168.18.1975. [DOI] [PubMed] [Google Scholar]
- 123.Fonseca VA, Rosenstock J, Wang AC, et al. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479–84. doi: 10.2337/dc08-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goldberg RB, Fonseca VA, Truitt KE, et al. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–40. doi: 10.1001/archinte.168.14.1531. [DOI] [PubMed] [Google Scholar]
- 125.Goldfine AB, Fonseca VA, Jones MR, et al. Long-term safety and tolerability of colesevelam HCl in subjects with type 2 diabetes. Horm Metab Res. 2010;42:23–30. doi: 10.1055/s-0029-1241195. [DOI] [PubMed] [Google Scholar]; •• This was a 52-week open-label extension trial examining the long-term efficacy, safety and tolerability of colesevelam.
- 126.Rigby SP, Handelsman Y, Lai YL, et al. Effects of colesevelam, rosiglitazone, or sitagliptin on glycemic control and lipid profile in patients with type 2 diabetes mellitus inadequately controlled by metformin monotherapy. Endocr Pract. 2010;16:53–63. doi: 10.4158/EP09146.OR. [DOI] [PubMed] [Google Scholar]
- 127.Ovalle F, Philis-Tsimikas A, Truitt K, Colesevelam HCL. improves glycemic control, independent of age in patients with type 2 diabetes uncontrolled on metformin, sulfonylurea, or insulin-based therapy. Diabetes. 2007;56:A536. [Google Scholar]
- 128.Donovan JM, Stypinski D, Stiles MR, et al. Drug interactions with colesevelam hydrochloride, a novel, potent lipid-lowering agent. Cardiovasc Drugs Ther. 2000;14:681–90. doi: 10.1023/a:1007831418308. [DOI] [PubMed] [Google Scholar]
- 129.Brown KS, Armstrong IC, Wang A, et al. Effect of the bile acid sequestrant colesevelam on the pharmacokinetics of pioglitazone, repaglinide, estrogen estradiol, norethindrone, levothyroxine, and glyburide. J Clin Pharmacol. 2010;50:554–65. doi: 10.1177/0091270009349378. [DOI] [PubMed] [Google Scholar]
- 130.Weitzman SP, Ginsburg KC, Carlson HE. Colesevelam hydrochloride and lanthanum carbonate interfere with the absorption of levothyroxine. Thyroid. 2009;19:77–9. doi: 10.1089/thy.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wechol (package insert) [Internet] Sankyo Pharma Inc.; New York, New York: 2008. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021176s014lbl.pdf. [Google Scholar]
- 132.Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;39:709–16. doi: 10.1097/01.mcg.0000173929.60115.b4. [DOI] [PubMed] [Google Scholar]
- 133.Merten DF, Grossman H. Intestinal obstruction associated with cholestyramine therapy. AJR Am J Roentgenol. 1980;134:827–8. doi: 10.2214/ajr.134.4.827. [DOI] [PubMed] [Google Scholar]
- 134.Lloyd-Still JD. Cholestyramine therapy and intestinal obstruction in infants. Pediatrics. 1977;59:626–7. [PubMed] [Google Scholar]
- 135.Luo S, Meier AH, Cincotta AH. Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters. Neuroendocrinology. 1998;68:1–10. doi: 10.1159/000054344. [DOI] [PubMed] [Google Scholar]
- 136.Cincotta AH, Schiller BC, Meier AH. Bromocriptine inhibits the seasonally occurring obesity, hyperinsulinemia, insulin resistance, and impaired glucose tolerance in the Syrian hamster, Mesocricetus auratus. Metabolism. 1991;40:639–44. doi: 10.1016/0026-0495(91)90057-4. [DOI] [PubMed] [Google Scholar]
- 137.Cycloset (package insert) [Internet] Pantheon Inc.; Cincinnati, Ohio: 2009. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020866lbl.pdf. [Google Scholar]
- 138.Cincotta AH, Meier AH, Cincotta M., Jr Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: A new approach in the treatment of diabetes. Expert Opin Investig Drugs. 1999;8:1683–707. doi: 10.1517/13543784.8.10.1683. [DOI] [PubMed] [Google Scholar]
- 139.Pijl H, Ohashi S, Matsuda M, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000;23:1154–61. doi: 10.2337/diacare.23.8.1154. [DOI] [PubMed] [Google Scholar]
- 140.Aminorroaya A, Janghorbani M, Ramezani M, et al. Does bromocriptine improve glycemic control of obese type-2 diabetics? Horm Res. 2004;62:55–9. doi: 10.1159/000078932. [DOI] [PubMed] [Google Scholar]
- 141.Ramteke KB, Ramanand SJ, Ramanand JB, et al. Evaluation of the efficacy and safety of bromocriptine QR in type 2 diabetes. Indian J Endocrinol Metab. 2011;15:S33–9. doi: 10.4103/2230-8210.83062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gaziano JM, Cincotta AH, O'Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care. 2010;33:1503–8. doi: 10.2337/dc09-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This 52-week study examined the safety and cardiovascular outcomes in patients randomized to bromocriptine or placebo in addition to their usual diabetes care. The frequency of serious adverse events was similar in the bromocriptine and placebo groups and a potential cardioprotective effect of bromocriptine was suggested.
- 143.Scranton RE, Gaziano JM, Rutty D, et al. A randomized, double-blind, placebo-controlled trial to assess safety and tolerability during treatment of type 2 diabetes with usual diabetes therapy and either cycloset or placebo. BMC Endocr Disord. 2007;7:3. doi: 10.1186/1472-6823-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Forez H, Scranton R, Farwell WR, et al. Randomized clinical trial assessing the efficacy and safety of bromocriptine-QR when added to ongoing thiazolidinedione therapy in patients with type 2 diabetes mellitus. J Diabetes Metabolism. 2011;2:142. [Google Scholar]; • This study showed the efficacy of bromocriptine in combination with TZD, with the greatest benefit seen in those with a baseline HbA1c ≥ 7.5% with a reduction of 0.8% compared with placebo.
- 145.Bloomgarden ZT, Dodis R, Viscoli CM, et al. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: A meta-regression analysis. Diabetes Care. 2006;29:2137–9. doi: 10.2337/dc06-1120. [DOI] [PubMed] [Google Scholar]
- 146.Neutel CI, Campbell NR, Morrison HI. Trends in diabetes treatment in Canadians, 1994–2004. Chronic Dis Can. 2010;30:107–11. [PubMed] [Google Scholar]
- 147.Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: Quality and cost implications. Am J Med. 2012;125:302.e1–302.e7. doi: 10.1016/j.amjmed.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kanai Y, Lee WS, You G, et al. The human kidney low affinity Na+/glucose cotransporter SGLT2. delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93:397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10–18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- 150.Chen J, Williams S, Ho S, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010;1:57–92. doi: 10.1007/s13300-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Banerjee SK, Wang DW, Alzamora R, et al. SGLT1, a novel cardiac glucose transporter, mediates increased glucose uptake in PRKAG2 cardiomyopathy. J Mol Cell Cardiol. 2010;49:683–92. doi: 10.1016/j.yjmcc.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–24. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: A randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–22. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Henry RR, Murray AV, Marmolejo MH, et al. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66:446–56. doi: 10.1111/j.1742-1241.2012.02911.x. [DOI] [PubMed] [Google Scholar]
- 155.Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: A randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928–38. doi: 10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 156.Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473–8. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilding JP, Norwood P, T'joen C, et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: Applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–62. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet. 1999;354:141–8. doi: 10.1016/S0140-6736(98)10364-1. [DOI] [PubMed] [Google Scholar]
- 159.Long GG, Reynolds VL, Lopez-Martinez A, et al. Urothelial carcinogenesis in the urinary bladder of rats treated with naveglitazar, a gamma-dominant PPAR alpha/gamma agonist: lack of evidence for urolithiasis as an inciting event. Toxicol Pathol. 2008;36:218–31. doi: 10.1177/0192623307311757. [DOI] [PubMed] [Google Scholar]
- 160.Wilding JP, Gause-Nilsson I, Persson A, et al. Tesaglitazar, as add-on therapy to sulphonylurea, dose-dependently improves glucose and lipid abnormalities in patients with type 2 diabetes. Diab Vasc Dis Res. 2007;4:194–203. doi: 10.3132/dvdr.2007.040. [DOI] [PubMed] [Google Scholar]
- 161.Rubin CJ, Ledeine JM, Fiedorek FT. Improvement of glycaemic and lipid profiles with muraglitazar plus metformin in patients with type 2 diabetes: An active-control trial with glimepiride. Diab Vasc Dis Res. 2008;5:168–76. doi: 10.3132/dvdr.2008.028. [DOI] [PubMed] [Google Scholar]
- 162.Sanwald-Ducray P, Liogier D'ardhuy X, Jamois C, et al. Pharmacokinetics, pharmacodynamics, and tolerability of aleglitazar in patients with type 2 diabetes: Results from a randomized, placebo-controlled clinical study. Clin Pharmacol Ther. 2010;88:197–203. doi: 10.1038/clpt.2009.259. [DOI] [PubMed] [Google Scholar]
- 163.Henry RR, Lincoff AM, Mudaliar S, et al. Effect of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): a phase II, randomised, dose-ranging study. Lancet. 2009;374:126–35. doi: 10.1016/S0140-6736(09)60870-9. [DOI] [PubMed] [Google Scholar]