Abstract
Objectives
This study measured and compared the pharmacokinetics of CMPD167, a small molecule antiretroviral CCR5 inhibitor with potential as an HIV microbicide, following vaginal, rectal and oral administration in rhesus macaques.
Methods
A vaginal hydroxyethylcellulose (HEC) gel, a rectal HEC gel, a silicone elastomer matrix-type vaginal ring and an oral solution, each containing CMPD167, were prepared and administered to rhesus macaques pretreated with Depo-Provera. CMPD167 concentrations in vaginal fluid, vaginal tissue (ring only), rectal fluid and blood plasma were quantified by HPLC–mass spectrometry.
Results
CMPD167 concentrations measured in rectal fluid, vaginal fluid and blood plasma were highly dependent on both the route of administration and the formulation type. Although rectal and vaginal fluid concentrations were highest when CMPD167 was administered locally (via either gel or ring), lower concentrations of the drug were also measured in these compartments following administration at the remote mucosal site or orally. CMPD167 levels in the vaginal and rectal fluid following oral administration were relatively low compared with local administration.
Conclusions
The study provides clear evidence for vaginal–rectal and rectal–vaginal drug transfer pathways and suggests that oral pre-exposure prophylaxis with CMPD167 may be less efficacious at preventing sexual transmission of HIV-1 than topically applied products.
Keywords: HIV microbicides, vaginal HEC gels, vaginal rings, rectal gels, pre-exposure prophylaxis, PrEP, CMPD167
Introduction
The development of topically applied microbicide formulations able to reduce the incidence of sexually acquired HIV-1 infection remains a priority within the prevention science field.1,2 The significant, albeit incomplete, protection provided by the vaginal administration of a water-based gel containing the reverse transcriptase inhibitor tenofovir (CAPRISA 004 trial) illustrates the potential of this approach.3 A major challenge for microbicide development is to increase the degree of protection seen in that trial. Possible improvements could come from the use of a different antiretroviral (ARV)4 or a combination of ARVs with different mechanisms of action,5 or by applying longer-lasting gels6,7 and/or sustained release devices such as vaginal rings.8–10 Rectal delivery of microbicides may also help protect both women and men against this route of sexual transmission. Recently, there has been considerable interest in administering ARVs orally to achieve the same goals [i.e. oral pre-exposure prophylaxis (PrEP)].11,12
Correlating efficacy data (from animal or human studies) with post-application ARV concentrations in relevant biological fluids and tissues is critical to understanding the drug levels required for protection and to guide improvements to the formulation. In pharmacokinetic (PK) studies of topically (vaginal or rectal) applied microbicide formulations, it is particularly important to measure ARV concentrations in the tissue and fluid compartments at the local site of product administration, since vaginal microbicides are primarily intended to prevent vaginal HIV-1 transmission and rectal microbicides rectal transmission. However, oral PrEP aims to deliver ARVs to both the vaginal and rectal compartments via the systemic circulation.12 Moreover, it is also possible that administering an ARV formulation to the vagina might protect against rectal transmission (and vice versa). Hence, we felt it would be useful to extend the measurement of drug concentrations to include biological compartments beyond the site of product application. Therefore, we carried out a series of PK experiments in rhesus macaques using the CCR5-targeted entry inhibitor CMPD167. This compound, from the same general class as the licensed drug maraviroc, provides substantial protection against vaginal challenge of macaques when delivered as a water-based vaginal gel or as oral PrEP.13,14
Methods
The International Partnership for Microbicides supplied CMPD167. Macaque studies were performed at the Tulane National Primate Research Center in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH and following approval from the Tulane University Institutional Animal Care and Use Committee. Each macaque (n = 24, in four groups of six animals) received a single 30 mg intramuscular injection of Depo-Provera 30 days prior to administration of CMPD167, to synchronize their menstrual cycles and thin the vaginal mucosa. Three different CMPD167 formulations were tested: a 3 mL volume of a 2.2% w/w hydroxyethylcellulose (HEC; grade HHX) gel containing 5 mM (∼3 mg/mL; 9 mg total dose) CMPD167 was administered either vaginally or rectally; a matrix-type silicone elastomer ring (overall diameter 25.0 mm, cross-sectional diameter 6.0 mm) containing 400 mg CMPD167 was inserted vaginally and removed after 28 days continuous use; and an aqueous CMPD167 tartrate buffer solution was given orally by gavage (20 mg/kg dose; mean macaque weight 7.0 kg; weight range 5.5–10.2 kg; CMPD167 oral dose range 110–204 mg). The ring and gels were prepared as described previously.8,15 Vaginal fluid (Weck-Cel inserted following wiping of the vaginal surface with moistened gauze), vaginal tissue (pinch biopsy) and blood were sampled at various times. Tissue biopsies were placed in 1 mL of distilled water and frozen within 30 min of sampling. CMPD167 concentrations were quantified using gradient reverse-phase HPLC–mass spectrometry according to previously described methods.8 Rectal fluid was similarly sampled by Weck-Cel and quantified using the following HPLC–mass spectrometry method. Internal standard (D5-CMPD167) was added to 150 μL of rectal fluid and proteins were precipitated by the addition of 450 μL of acetonitrile. After vortex mixing and centrifugation (3000 rpm, 10 min, 20°C), a 400 μL aliquot of the supernatant was evaporated to dryness under nitrogen at 40°C. Residues were reconstituted in 20 : 80 (v : v) methanol : 10 mM ammonium formate (pH 3.5) for analysis. Analysis was performed using a Shimadzu Prominence® HPLC system (Kyoto, Japan), a BDS Hypersil C8 column (50 × 2.1 mm, 5 μm; Thermo Scientific, Waltham, MA, USA) and an API3200® (AB Sciex, Framingham, MA, USA) triple quadrupole mass spectrometer. The API3200® mass spectrometer was used in positive TurboIonSpray® mode with a source temperature of 650°C. The mobile phase consisted of 50 : 50 10 mM ammonium formate (pH 3.5) : methanol with 0.1% formic acid, a 50%–95% organic gradient and a 0.5 mL/min flow rate. CMPD167 and the internal standard were detected using multiple reaction monitoring and the precursor → product ion transitions were m/z 575.5 → 444.3 for CMPD167 and 580.6 → 449.4 for D5-CMPD167. The linear range was 0.5–1000 ng/mL.
Where appropriate, data were statistically analysed using either a Mann–Whitney U-test or one-way ANOVA followed by post hoc analysis using the Tukey–Kramer multiple comparisons test. In all cases, a P value of <0.05 was considered significant. Analysis was conducted using GraphPad Prism.
Results and discussion
Vaginal fluid concentrations
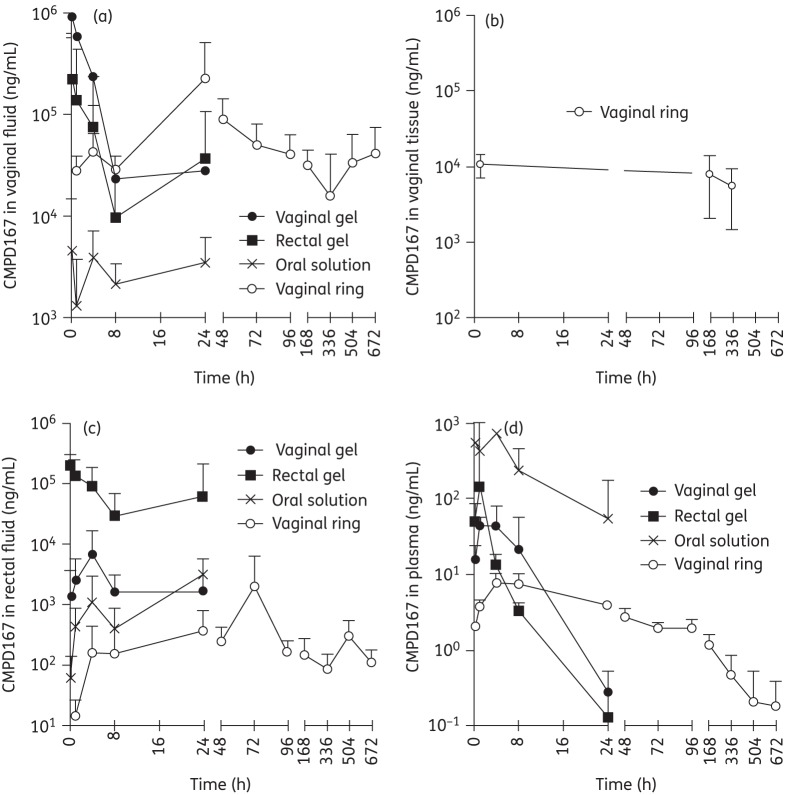
CMPD167 concentrations in vaginal fluid were highest in the vaginal gel group; they peaked at ∼106 ng/mL 15 min after gel application and steadily decreased to ∼2.8 × 104 ng/mL by 24 h (Figure 1a). The matrix-type vaginal ring device provided a similar mean CMPD167 vaginal fluid concentration (∼2.8 × 104 ng/mL) at the 1 h timepoint (the earliest sampled in this group) as the vaginal gel at 24 h and comparable concentrations (range: 1.6 × 104–2.3 × 105 ng/mL) were then sustained out to 672 h (28 days; final sampling timepoint). Rectal application of the same HEC gel also resulted in relatively high vaginal fluid CMPD167 concentrations (ranging from 2.2 × 105 at 1 h to 3.6 × 104 ng/mL at 24 h; Figure 1a), with values consistently, but not always significantly, lower than those obtained with the vaginal gel at each sampling timepoint. The vaginal gel AUC was 2.7-fold higher than that for the rectal gel (Table 1). Hence, there must be a transfer or diffusion of the ARV from the rectal to the vaginal compartment. Although this drug transfer mechanism is expected to be time dependent, the high variability in vaginal fluid concentrations following rectal gel application resulted in a Tmax value that was not statistically different (P = 0.18) from that for the vaginal gel (Table 1). Oral administration of a CMPD167 solution yielded only relatively low vaginal fluid concentrations that ranged from 1.3 × 103 to 4.6 × 103 ng/mL over the 24 h period. The time taken for vaginal fluid concentrations to reach peak levels (Tmax) was dependent on the route of administration and the type of formulation: vaginal gel (0.4 h) < rectal gel (4.1 h) < oral solution (9.4 h) <vaginal ring (24 h) (Table 1). However, the rank order of decreasing Cmax values was vaginal gel > vaginal ring > rectal gel > oral solution (Table 1).
Figure 1.
Vaginal fluid (a), vaginal tissue (b), rectal fluid (c) and plasma (d) concentrations (ng/mL) of CMPD167 following administration of a vaginal HEC gel, a rectal HEC gel, an oral solution and a silicone elastomer vaginal ring. Values are means ± SD (n = 6).
Table 1.
Pharmacokinetic parameters for CMPD167 concentrations measured in macaque rectal fluid, vaginal fluid and blood plasma following administration of vaginal gel, rectal gel, vaginal ring and oral solution
| Formulation type | CMPD167 dose/ring loading | Rectal fluid |
Vaginal fluid |
Blood plasma |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | AUC0–24 (ng · h/mL) | Cmax (ng/mL) | Tmax (h) | AUC0–24 (ng · h/mL) | Cmax (ng/mL) | Tmax (h) | AUC0–24 (ng · h/mL) | ||
| Vaginal gel | 9 mg | 6995 ± 9652 | 4.2 ± 2.2 | 58 420 ± 68 700 | 915 680 ± 267 810 | 0.4 ± 0.3 | 2 705 000 ± 617 500 | 55.98 ± 30.00 | 2.5 ± 1.6 | 447.4 ± 495.8 |
| Rectal gel | 9 mg | 207 700 ± 107 900 | 0.25 ± 0.0 | 1 434 000 ± 1 732 000 | 220 200 ± 347 200 | 4.1 ± 4.2 | 994 600 ± 2 016 000 | 144.0 ± 247.5 | 0.9 ± 0.3 | 370.0 ± 489.2 |
| Vaginal ring | 400 mg | 453 ± 456 | 18.0 ± 9.4 | 5084 ± 4410 | 225 200 ± 285 900 | 24.0 ± 0.0 | 2 282 000 ± 2 410 000 | 7.762 ± 2.500 | 4.0 ± 0.0 | 140.0 ± 37.6 |
| Oral solution | 110–204 mg (20 mg/kg)a | 3087 ± 2865 | 24.0 ± 0.0 | 33 330 ± 33 100 | 7920 ± 8745 | 9.4 ± 11.4 | 64 420 ± 37 970 | 841.3 ± 577.5 | 1.8 ± 1.8 | 4341 ± 4042 |
AUC0–24, area under the plasma concentration–time curve from 0 to 24 h; Cmax, maximum concentration; Tmax, time to reach the Cmax.
Values are means ± SD (n = 6).
aTotal administered oral dose was dependent on macaque weight (see the Methods section).
Rectal fluid concentrations
As expected, the highest concentrations of CMPD167 in rectal fluid were measured in animals receiving the rectal gel, with mean CMPD167 levels ranging from 2.9 × 104 ng/mL (8 h) to 2.0 × 105 ng/mL (15 min) (Figure 1c). In the other groups, rectal CMPD167 concentrations were lower, in the following rank order: vaginal gel (range: 1.4–6.7 × 103 ng/mL) > oral solution (6.1 × 101–3.1 × 103 ng/mL) > vaginal ring (1.4 × 101–1.9 × 103 ng/mL). Following rectal gel administration, the rectal and vaginal fluid concentrations were similar (compare Figure 1c and Figure 1a), producing rectal and vaginal AUC values of 1.4 × 106 and 9.9 × 105 ng · h/mL, respectively (Table 1). These data suggest there is an efficient drug transport pathway from the rectal to the vaginal compartment, more so than applies in the converse direction (see Figure 1c). With the vaginal ring device, rectal CMPD167 levels were maintained over the 14 day study period (Figure 1c), but were two to three orders of magnitude lower than vaginal fluid concentrations (Figure 1a). The relatively long Tmax value for CMPD167 concentrations in rectal fluids following oral administration (24 h; Table 1) suggests that Cmax is largely determined by gastrointestinal transit rather than diffusion from the systemic compartment, with implications for oral dosing studies measuring rectal tissue concentrations.
Plasma concentrations
Plasma levels of CMPD167 were, as expected, very much lower in the animals given the vaginal gel, rectal gel and vaginal ring, compared with oral administration (Figure 1d and Table 1). Plasma concentrations following oral and gel administration showed typical first-order elimination kinetics, while those following vaginal ring placement decreased relatively slowly (7.8 ng/mL at 4 h to 3.8 ng/mL at 24 h and 0.7 ng/mL at 672 h).
Vaginal tissue concentrations
CMPD167 tissue levels, only measured in vaginal ring recipients, were maintained in the range 2.29 × 103–1.72 × 104 ng/g (Figure 1b; levels were only measured out to 14 days). We have previously reported similar tissue levels in macaques with a CMPD167 ring.8 In that study, the PK parameters were highly dependent on Depo-Provera administration to the animals, which modestly reduced vaginal concentrations of CMPD167 while increasing transfer of the compound into the plasma (rectal concentrations were not measured).
Conclusions
Several points arise from this study. First, CMPD167 is effectively transferred from the rectum to the vagina (rectal gel) and from the vagina to the rectum (vaginal gel and ring), although the former route appears to be the more efficient. A simple mechanism accounting for the differential rate of drug transfer between the compartments is not immediately obvious. Although the thinner simple columnar epithelial tissue of the rectum should offer increased drug absorption from that compartment, thus facilitating the rectal to vaginal (and rectal to blood) drug transfer pathway, it is also likely to similarly effect drug diffusing in the other direction (vaginal to rectal). It is also possible that the concentration of drug at the mucosal tissue (and, in turn, the concentration gradient established across the tissue) is higher following rectal gel administration compared with vaginal gel administration, due to differences in how the gel spreads to cover the tissue and the different fluid volumes within each compartment. Finally, it is also possible that the intercompartmental transfer does not take place directly via the connecting tissue, but instead follows a mechanism similar to the first uterine pass effect observed for direct vaginal–uterine drug transfer and attributed to the overlapping network of blood vessels associated with the arteriovenous plexus.16,17
Thinning of the epithelial tissue has previously been postulated to increase transfer of CMPD167 from vaginal to systemic compartments when macaques were pretreated with Depo-Provera prior to ring administration.8 Hence, it is possible that rectal administration of an ARV microbicide formulation might provide protection against both rectal and vaginal transmission, a supposition supported by a recent macaque study of rectally and vaginally administered tenofovir gel.18 The influence of Depo-Provera on rectal absorption has not been reported.
Our second conclusion is that local concentrations of CMPD167 following vaginal and rectal application are typically one to two orders of magnitude greater than achieved by oral dosing. Similar differences have also been observed in women given vaginal gel or oral tablet formulations of tenofovir disoproxil fumarate.19 Assuming that protection is only mediated locally, these data suggest that it may be very difficult for orally dosed ARVs to achieve the relatively high vaginal/rectal concentrations obtained with mucosal dosing. The implication is that the efficacy of PrEP could be greater for vaginal/rectal dosing than for oral delivery, assuming similar adherence rates. Recently, weekly oral administration of two doses of maraviroc to macaques 24 h before and 2 h after rectal challenge provided negligible protection, despite concentrations in rectal fluid reaching as high as 105 ng/mL.12 A more sustained, multiday regimen of orally delivered CMPD167 was, however, significantly protective against vaginal challenge, suggesting that oral PrEP may not be limited to local activity.14 However, vaginal concentrations of CMPD167 were not measured in that study and the concentrations required for protection are unknown.
We also conclude that concentrations of CMPD167 in vaginal fluid during the first few hours after administration were greater for the vaginal gel compared with the ring, but they then decreased to below the more sustained concentrations provided by the ring. The critical unknown is whether the vaginal concentrations obtained with the ring are sufficient for protection. An answer would resolve whether the short-term high bolus effect of gels is more important than the longer PK tail seen with rings.
Additional PK and challenge studies in the macaque that compared various modes of ARV delivery, including, but not limited to, CCR5 inhibitors, would provide useful information on the short- and longer-term concentration requirements for protection from rectal and/or vaginal challenge. At present, mostly only inferences can be made by extrapolating across studies that yield partial datasets.
Funding
This work was supported by the National Institutes of Health (grant number U19 AI076982).
Transparency declarations
None to declare.
References
- 1.Friend DR, Kiser PF. Assessment of topical microbicides to prevent HIV-1 transmission: concepts, testing, lessons learned. Antiviral Res. 2013;99:391–400. doi: 10.1016/j.antiviral.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Shattock RJ, Rosenberg Z. Microbicides: topical prevention against HIV. Cold Spring Harb Perspect Med. 2012;2:a007385. doi: 10.1101/cshperspect.a007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karim QA, Karim SSA, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klasse PJ, Shattock R, Moore KP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Ann Rev Med. 2008;59:455–71. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 5.Balzarini J, Schols D. Combination of antiretroviral drugs as microbicides. Curr HIV Res. 2012;10:53–60. doi: 10.2174/157016212799304652. [DOI] [PubMed] [Google Scholar]
- 6.Forbes CJ, Lowry D, Geer L, et al. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J Control Release. 2011;156:161–9. doi: 10.1016/j.jconrel.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curran RM, Donnelly L, Morrow RJ, et al. Vaginal delivery of the recombinant HIV-1 clade-C trimeric gp140 envelope protein CN54gp140 within novel rheologically structured vehicles elicits specific immune responses. Vaccine. 2009;27:6791–8. doi: 10.1016/j.vaccine.2009.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malcolm RK, Veazey RS, Geer L, et al. Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob Agents Chemother. 2012;56:2251–8. doi: 10.1128/AAC.05810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416–23. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 10.Malcolm RK, Edwards K-L, Kiser P, et al. Advances in microbicide vaginal rings. Antiviral Res. 2010;88(Suppl 1):S30–9. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massud I, Aung W, Martin A, et al. Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol. 2013;87:8952–61. doi: 10.1128/JVI.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 14.Veazey RS, Springer MS, Marx PA, et al. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med. 2005;11:1293–4. doi: 10.1038/nm1321. [DOI] [PubMed] [Google Scholar]
- 15.Malcolm RK, Forbes CJ, Geer L, et al. Pharmacokinetics and efficacy of a vaginally administered maraviroc gel in rhesus macaques. J Antimicrob Chemother. 2013;68:678–83. doi: 10.1093/jac/dks422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073–9. doi: 10.1093/humrep/12.5.1073. [DOI] [PubMed] [Google Scholar]
- 17.Cicinelli E, de Ziegler D, Morgese S, et al. First uterine pass effect’ is observed when estradiol is placed in the upper but not lower third of the vagina. Fertil Steril. 2004;81:1414–6. doi: 10.1016/j.fertnstert.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Nuttall J, Kashuba A, Wang R, et al. Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrob Agents Chemother. 2012;56:103–9. doi: 10.1128/AAC.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]