Abstract
OBJECTIVE
To characterize white matter abnormalities in adolescents with early onset schizophrenia (EOS) relative to three comparison groups (adolescents at clinical high risk for developing schizophrenia [CHR], adolescents with cannabis use disorder [CUD], and healthy controls [HC]), and to identify neurocognitive correlates of white matter abnormalities in EOS.
METHOD
We used diffusion tensor imaging and tractography methods to examine fractional anisotropy (FA) of the cingulum bundle, superior longitudinal fasciculus, corticospinal tract (CST), inferior longitudinal fasciculus (ILF), inferior fronto-occipital fasciculus (IFOF), and uncinate fasciculus in adolescents with EOS (n=55), CHR (n=21), CUD (n=31), and HC (n=55). FA in tracts that were significantly altered in EOS was correlated with neurocognitive performance.
RESULTS
EOS and CHR groups had significantly lower FA than HC in four tracts: bilateral CST, left ILF, and left IFOF. CUD had lower FA than HC in left IFOF. Lower FA in left IFOF and left ILF predicted worse neurocognitive performance in EOS.
CONCLUSIONS
This study identified left ILF and left IFOF as possible biomarkers of vulnerability for developing schizophrenia. Lower FA in these tracts may disrupt functioning of ventral visual and language streams, producing domain-specific neurocognitive deficits that interfere with higher order cognitive abilities.
Keywords: adolescent, diffusion tensor imaging (DTI), Diffusion tensor imaging, inferior longitudinal fasciculus (ILF), schizophrenia
INTRODUCTION
Schizophrenia is a disease characterized in part by white matter (WM) abnormalities that alter brain connectivity.1 A large but variable body of literature exists on the topographic location of WM deficits in adult-onset schizophrenia.2 Adolescents with early-onset schizophrenia (EOS) (onset by age 18 years) represent an ideal population to examine WM alterations as they may represent a subgroup with high genetic loading3 that is less affected by chronic exposure to antipsychotic medication.4
Diffusion tensor imaging (DTI) is a magnetic resonance imaging technique that capitalizes on the propensity of water molecules to diffuse along, rather than across, WM tracts. Fractional anisotropy (FA) is a DTI measure that reflects tract integrity and coherence.5 Region-of-interest (ROI) and voxel-based analyses have reported reduced FA in EOS across widespread brain regions and fiber tracts including left posterior hippocampus,6 bilateral cerebral peduncles,7 anterior and posterior corpus callosum,7 right anterior corona radiata,7 inferior frontal WM,8 occipital WM,8 corticospinal tracts,9 parietal WM,7,10 left anterior cingulate,11 corpus callosum12 and left inferior longitudinal fasciculus (ILF).13
Several hypotheses have been proposed to explain the variable WM findings in EOS. First, underlying WM abnormalities may be subtle2 and non–spatially-overlapping,14 making anomalies difficult to detect. However, a recent meta-analysis of adults with first-episode schizophrenia identified lower WM FA in the left deep temporal lobe, corresponding to left ILF and left IFOF.15 In another study, these two major left hemisphere fiber tracts showed specific myelination deficits and FA reduction in adults with chronic schizophrenia, which correlated with reductions in processing speed,16 a well-known cognitive abnormality in schizophrenia.
Second, exposure to antipsychotic medication could potentially affect the pattern of WM alterations in EOS. To address this problem, the study of adolescents with subthreshold psychotic symptoms (CHR) could be informative: these adolescents are at elevated risk for conversion to psychosis in adulthood, but tend to have no or very limited exposure to antipsychotic medications.4 Also, prior history of cannabis misuse17 may affect the pattern of WM alterations within EOS. Adolescent cannabis exposure also increases the risk of developing a psychotic disorder,18 and is associated with cognitive deficits.19 Data from adults with first episode schizophrenia indicate that patterns of WM alterations may be affected by cognitive function.20
In the present study, we investigated WM integrity in adolescents with EOS relative to three other comparison groups: (1) CHR, (2) nonpsychotic adolescents with cannabis-use disorders (CUD), and (3) healthy comparison participants (HC). We used probabilistic tractography, a methodological refinement compared to previous DTI studies in EOS, which localizes FA variations to specific fiber tracts as compared to brain regions. We also investigated the relationship between FA and neurocognitive performance in EOS.21 Based on recent studies in adults with schizophrenia15,16,22 and previous studies in EOS, we hypothesized that (1) patients with EOS would have lower FA than HC in the left temporal lobe, specifically in left ILF8,13 and left IFOF; (2) CHR and CUD would have some shared WM abnormalities with EOS, based on non-specific phenotypic characteristics; (3) deficits in executive function and motor skills would be related to FA in fiber tracts that were aberrant in EOS.20
METHOD
Participants
The details of the clinical protocol have been described in detail elsewhere.23 In brief, 162 participants ranging age 10 to 23 years were recruited from clinical programs at the University of Minnesota under an approved Institutional Review Board protocol. For participants under age 18, informed consent was obtained from parents and assent was obtained from the child. Participants over age 18 provided their own consent and their parents were consented for a collateral interview. These parents were consented so that they could provide information about pertinent family psychiatric history and collateral data to aid in the diagnostic formulation and assessment of premorbid function24 of their children.
Participants with EOS met criteria for schizophrenia (n=43), schizoaffective (n=5), or schizophreniform disorder (n=7), and reported an onset of psychotic symptoms prior to age 18 years. Thirty-four participants with EOS had no past or current DSM-IV diagnosis for substance or alcohol-use disorders. Twenty-one of the 55 participants with EOS met lifetime criteria for a co-occurring cannabis use disorder (CUD) of abuse or dependence. In EOS, participants with co-occurring CUD were included if a history of psychotic symptoms was present when there was no evidence of substance misuse or withdrawal. Forty-seven of the 55 participants with EOS were taking second-generation antipsychotic medications (SGAs) at the time of scanning, which included risperidone (n=15), aripiprazole (n=12), quetiapine (n=10), clozapine (n=4), olanzapine (n=3), ziprasidone (n=2), and paliperidone (n=1). Chlorpromazine equivalent (CPZ) dose and lifetime exposure were calculated from the dose and duration of antipsychotics received using a standardized method.25
Nonpsychotic adolescents with CUD (n=31) were recruited from programs for chemical dependency. As many of the adolescents with schizophrenia-spectrum disorders tested positive for cannabis at the time of scan, the CUD group allowed for examination of the effect of repeated exposure to cannabis on white matter microstructure in the absence of psychosis. Adolescents were selected who reported cannabis as their drug of choice with significant cannabis exposure by age 17 years (>50 exposures to cannabis), and who did not meet lifetime criteria for abuse of or dependence on other illicit drugs with the exception of alcohol abuse or nicotine dependence. Exclusion criteria for the CUD group included a lifetime diagnosis of bipolar disorder or schizophrenia-spectrum disorder. However, as this was a treatment-seeking clinical population, the presence of other psychopathology was permitted. Fourteen of the 31 participants with CUD were taking psychotropic medication at the time of scanning, which included stimulants (n=1), antidepressants (n=13), mood stabilizers (n=2), and SGAs (n=6). SGAs were prescribed in this group to target sleep disturbance (n=4) and irritability (n=2), both frequent problems in this group during the early stages of sobriety, in order to enhance program retention.
Treatment-seeking adolescents at clinical high risk for developing schizophrenia (CHR) (n=21) were recruited from an outpatient university clinic developed to evaluate youth who might be at risk for developing a psychotic illness. Participants were included in the CHR group if they met one or more of three sets of criteria as described in detail elsewhere:26 (1) Brief intermittent psychotic syndrome; (2) Attenuated positive symptom syndrome; or (3) genetic risk and deterioration syndrome. Participants at CHR were excluded if they had ever met DSM-IV criteria for an Axis I psychotic disorder. Eleven of 21 participants at CHR were taking psychotropic medication at the time of scanning, which included stimulants (n=5), antidepressants (n=11), mood stabilizers (n=4), and second-generation antipsychotic agents (n=11). SGAs were prescribed in this group to reduce emotional distress and behavioral problems associated with transient psychotic symptoms.
A total of 55 HC were recruited from the same geographic area in response to flyers and by word of mouth to match the EOS group on age, sex, and handedness. Controls were excluded if they had any current or past DSM-IV diagnosis (with the exception of minor anxiety disorders), prior or current treatment with psychotropic medications, history of psychological counseling, reported history of more than five lifetime exposures to any illicit drug (with the exception of alcohol), and/or history of schizophrenia or psychosis in a first-degree relative.
General exclusion criteria for all participants included any contraindication to magnetic resonance imaging (MRI), positive pregnancy test, history of a DSM-IV diagnosis of mental retardation, a neurological disorder, head injury with loss of consciousness for more than 30 seconds, or active medical illness that could potentially affect brain structure.
Clinical Measures
Diagnoses of Axis I disorders including schizophrenia and of substance use disorders were made using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (<18 years of age) or the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (>=18 years of age) using multiple data sources including school records, past psychiatric evaluations and hospitalizations. These data were also used to retrospectively assess premorbid function. We used the Structured Interview for Prodromal Syndromes (SIPS)27 to determine whether the subjects met criteria for CHR.28
Urine toxicology tests were performed on the day of the MRI scan using the K012B 12 Panel Drug Screen Test (THC sensitivity 50 ng/ml) from Drug Test Systems (Dover, NH).
To streamline the protocol, psychopathology was assessed using ratings of negative29 and positive30,31 symptoms of schizophrenia for EOS and using the Scale of Prodromal Symptoms (SOPS)27 for CHR. The Wide Range Achievement Test–3rd Edition (WRAT)32 Reading Subtest was administered to establish reading decoding.
For purposes of comparison to adults with a first episode of schizophrenia20 we administered the following subtests to examine neuropsychological function for correlative analyses: (1) Executive function: number-letter switching, category-switching, Twenty Questions;33 (2) Verbal memory: long-delay free recall;34 (3) Motor speed and dexterity: Grooved Pegboard Test;35 and (4) Attention: d-prime.36
Premorbid function was assessed using the Cannon-Spoor Premorbid Adjustment Scale.24
MRI Data Acquisition
Imaging data were acquired on a Siemens 3 Tesla Trio scanner (Erlangen, Germany) at the University of Minnesota’s Center for Magnetic Resonance Research. T1-weighted images were acquired in the coronal plane using a 3D magnetization prepared rapid gradient echo sequence (MPRAGE, TR=2530ms, TE=3.65ms, TI=1100ms, 224 slices, voxel size 1×1×1mm, FOV=256mm2). DTI data were acquired axially using a dual spin echo, single shot, pulsed gradient, echo planar imaging (EPI) sequence (TR=8500ms, TE=98ms, 64 slices, voxel size=2×2×2mm, 0mm skip, FOV=256mm2, 1 average, b value=1000s/mm2). Thirty non-collinear diffusion-encoding volumes, and six b=0s/mm2 volumes, were collected to compute the tensor. Field maps were acquired (TR=700ms, TE=4.62ms/7.08ms, flip angle=90 degrees, voxel parameters identical to the DTI, magnitude and phase difference contrasts) and used to correct the DTI data for geometric distortion.
Fourteen participants (3 HC; 1 CUD; 3 CHR; 7 EOS) were scanned but not included in this analysis due to (1) technical reasons including fiber tracts that could not be tracked (2 HC; 1 CUD; 3 CHR; 1 EOS), (2) claustrophobia and refusal to participate (1 HC; 3 EOS); and (3) excessive motion artifacts leading to poor quality data (3 EOS).
Anatomical Data Processing
T1-weighted images were processed using the FreeSurfer analysis suite, which is documented and freely available to download online (http://surfer.nmr.mgh.harvard.edu). Processing steps included removal of non-brain tissue, segmentation of deep structures, tessellation of the gray-white matter boundary, topology correction, surface deformation, parcellation of the cerebral cortex, and classification of WM into subunits. FreeSurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths.37 Finally, these tools have been widely used in adults and have been shown to be suitable for use in children in this age-range.38
DTI Data Processing
The diffusion tensor was computed using the Diffusion Toolbox (FDT) from the FMRIB software library39 (FSL, http://www.fmrib.ox.ac.uk/). Each diffusion weighted volume was aligned to the first b=0 image using an affine transformation to correct for the distortions caused by eddy currents,40 and the entire diffusion series was corrected for geometric distortion caused by magnetic field inhomogeneity using FUGUE (FMRIB). The diffusion tensor was derived from the b=0 images and the thirty aligned, distortion-corrected diffusion weighted images. FA maps were created from the diffusion tensor.
Selection of Tractography Seeds and Targets
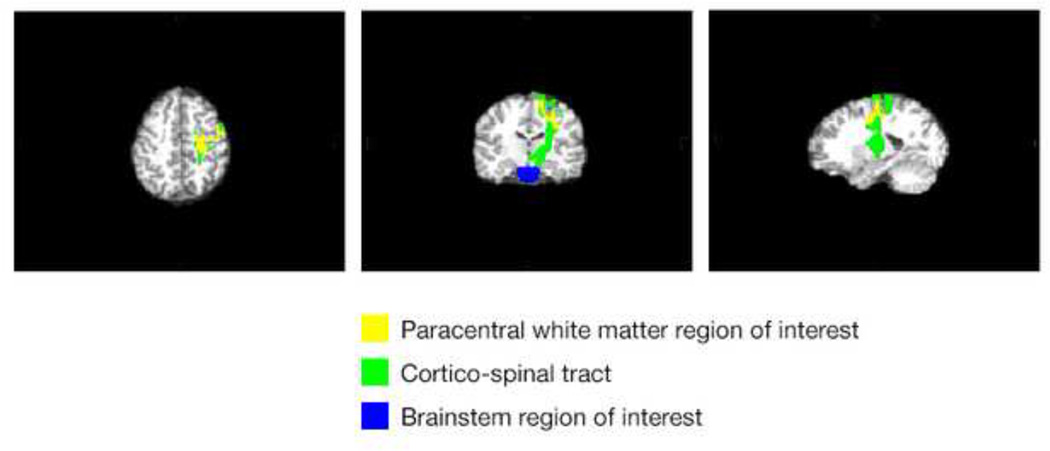
In order to reliably and automatically initiate and terminate fiber tracking for each participant without rater bias,41 FreeSurfer WM parcellations were used as seed and target regions of interest in this analysis. Selection of appropriate and robust ROIs for all participants was done by examining the overlap between each FreeSurfer WM ROI with six tracts of interest selected from the Johns Hopkins University (JHU) Tractography atlas. ROIs showing high overlap with the distal ends of tracts from the JHU atlas were selected as seed and target regions of interest (ROI) for tractography analyses. Figure 1 shows the corticospinal tract and the corresponding FreeSurfer WM regions used for initiation and termination of tracking. In certain cases, multiple ROIs were added together to form a single seed or target. The T1-weighted brain was aligned to the FA map of each participant using a 6 degree of freedom linear registration.
Figure 1.
FreeSurfer white matter regions used for tracking initiation and termination of corticospinal tract.
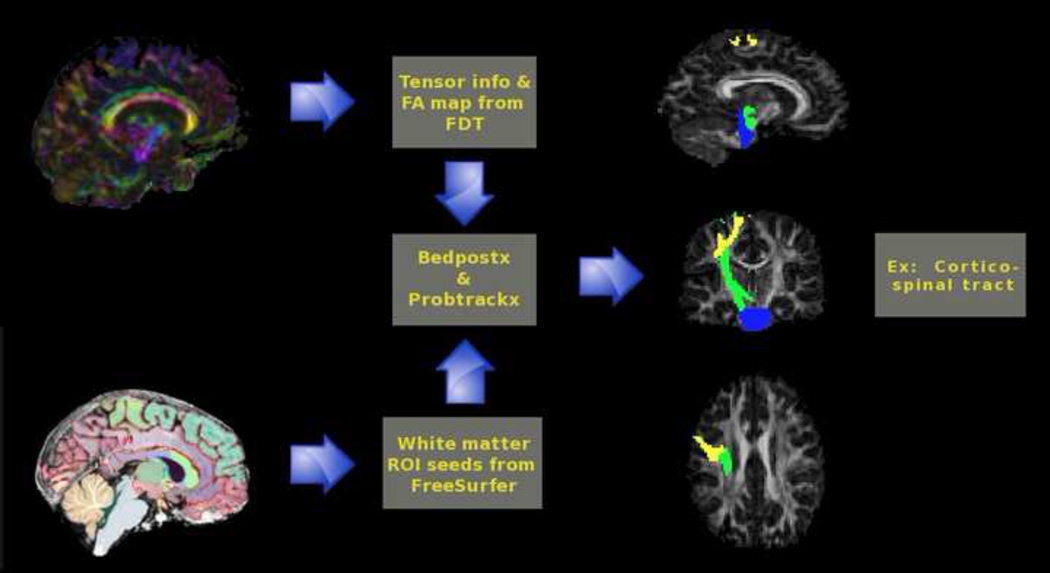
Tractography
The Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques, accounting for crossing fibers (BEDPOSTX) and Probtrackx42 were used to generate connectivity distributions between the seed and target ROIs as shown in Figure 2. The diffusion series of each subject, corrected for eddy currents and geometric distortions, was used as the input for BEDPOSTX. Connectivity distributions were generated by sending 10,000 samples from each voxel within seed masks. Constraints on tracking required voxels to have an FA value >0.15 and minimum angle between voxels 80°. Only samples reaching the target mask were included in the final connectivity distributions, which were normalized based on the number of successful seed-to-target attempts (streamlines). A threshold was subsequently applied to these maps to reduce outlier-induced noise by dropping voxels <5% of samples relative to the total number of streamlines. This threshold dropped much of the noise and is similar to what has been previously reported.43 Post-threshold connectivity distributions were converted into binary masks, and mean FA was computed along each tract.
Figure 2.
Bedpostx and Probtrackx were used to generate connectivity distributions between the seed and target regions of interest (ROIs). Note: FA=Fractional Anisotropy; FDT=FMRIB Diffusion Toolbox.
Data Analysis
Statistical analyses were performed with SPSS version 21 (SPSS Inc., Chicago, IL).
FA values were multiplied by 1000. FA was analyzed across six fiber bundles with an omnibus multivariate analysis of covariance (MANCOVA) with a between-subjects factor group (HC, CUD, CHR, and EOS). As all of the patient groups had lower reading decoding scores (an estimate of premorbid intelligence) compared to healthy controls, this variable was included as a covariate in all analyses. In addition, age, sex, and urine toxicology were included as covariates. The fiber tracts of interest included: cingulum, superior longitudinal fasciculus, corticospinal tract (CST), ILF, IFOF, and uncinate fasciculus. Post hoc pairwise group comparisons using analysis of covariance (ANCOVA) were conducted with the same covariates to clarify any significant effect of group.
Spearman rank correlations were used to test relationships between those WM tracts that were significantly altered in participants with EOS and neurocognitive variables and other clinical measures of interest. Correlations in EOS, the primary group of interest for this study, were planned for two specific measures of cognition (i.e., number-letter switching,44 Grooved Pegboard Test45) that had been previously shown to affect the pattern of WM alterations in adults with first-episode schizophrenia.20 Specifically, we predicted that in EOS, worse number-letter switching performance would be related to lower FA in the left IFOF and left ILF and that worse Grooved Pegboard Test performance would be related to lower FA in the left IFOF.20 Because these hypotheses were a priori, a statistical threshold of p<.05 (two-tailed) was used to determine significance. Additional exploratory analyses were conducted to examine whether FA was related to other measures of neurocognitive performance and premorbid function. For these exploratory analyses, a statistical threshold of p < 0.01 (two-tailed) was used to determine significance.
RESULTS
Demographics and Substance Use
Demographic and clinical characteristics are presented in Table 1. There were no significant differences in age or handedness among the four groups. However, CHR differed in terms of gender distribution (the majority of participants were male) compared with other groups. CUD and CHR were phenotypically similar to EOS in terms of having lower reading decoding scores compared to HC and elevated rates of comorbid mood and anxiety disorders. Twenty-four participants tested positive for cannabis on the day of MRI scanning (12 CUD; 4 CHR; 8 EOS); this was addressed in follow-up analyses (below). All of the participants who tested positive for cannabis on the day of scan were previously known to have recently used cannabis prior to hospitalization based on diagnostic interviews. As expected, participants with EOS had considerably more antipsychotic exposure compared to CUD and CHR subjects.
TABLE 1.
Demographic Features and Clinical Characteristics of Subjects
| HC (n=55) |
CUD (n=31) |
CHR (n=21) |
EOS (n=55) |
χ2 or F | |
|---|---|---|---|---|---|
| Demographic Features | |||||
| Mean age, y (SD) | 16.5 (2.6) | 17.6 (2.4) | 16.1 (3.3) | 16.9 (1.7) | 1.9 |
| Sex, male | 27 | 22 | 18 | 31 | 10.4* |
| Handedness, right | 47 | 30 | 18 | 47 | 2.95 |
| Ethnicity, Caucasian | 46 | 23 | 11 | 25 | 20.2*** |
| Median SESb, High (low) | 2 | 2 | 3 | 2 | 7.2 |
| Mean WRATc score (SD) | 113 (16.3) | 102.3 (16.3)a | 98.4 (14.4)a | 99.6 (16.9)a | 7.8*** |
| Cannon-Spoor24 premorbid adjustment (SD) | − | − | − | 9.0 (3.8) | − |
| Clinical Characteristics, Means | |||||
| Psychosis age of onset, years (SD) | − | − | − | 13.3 (3.4) | − |
| BPRSd total score (SD) | − | − | − | 22.4 (13.1) | − |
| SANSe total score (SD) | − | − | − | 32.1 (26.9) | − |
| SOPSf negative | − | − | 10.6 | − | − |
| SOPSf positive | − | − | 6.8 | − | − |
| Currentg CPZh antipsychotic dose, mg (SD) | 0 | 148 (71.3) | 161 (186) | 437 (422)c | 3.5* |
| Lifetimei CPZh exposure, 1,000s mg (SD) | 0 | 36 (108) | 273 (590) | 1364 (1748)b,c | 19.19*** |
| Comorbid diagnoses | |||||
| Externalizingj disorders, yes | 0 | 13 | 2 | 14 | 27.32*** |
| Moodk disorders, yes | 0 | 6 | 6 | 13 | 16.02** |
| Anxietyl disorders, yes | 1 | 6 | 4 | 3 | 11.34 |
| Cannabis use disorder, yes | 0 | 31 | 2 | 21 | 95.44** |
| Positive UAm on day of scan, yes | 0 | 12 | 4 | 8 | 23.89*** |
| Alcohol use, yes | 8 | 20 | 0 | 14 | 35.1 |
| Tobacco use, yes | 1 | 14 | 0 | 8 | 34.8 |
Note: CHR = clinical high-risk for schizophrenia; EOS=early-onset schizophrenia, with and without comorbid cannabis use disorder (CUD); HC=healthy controls.
Significant difference from HC, p<.05.
Socioeconomic status (SES), Hollingshead system,55 where 1–3 = “high” and 4–5 = “low”
Wide Range Achievement Test (WRAT)32
Scale for Assessment of Negative Symptoms (SANS)29
Scale of Prodromal Symptoms (SOPS)27
Current dose based on participants taking antipsychotic medications at time of scan (6 CUD, 11 CHR, 47 EOS);
Chlorpromazine (CPZ) equivalents25
Estimates of lifetime CPZ exposure includes all members of patient groups
Externalizing disorders=attention-deficit/hyperactivity disorder, conduct disorder, or oppositional defiant disorder
Mood disorders=depressive disorder not otherwise specified (NOS), dysthymia, major depressive disorder, or mood disorder NOS
Anxiety disorders=anxiety disorder NOS, generalized anxiety disorder, posttraumatic stress disorder, social phobia, obsessive compulsive disorder
UA=urine analysis for cannabis.
p<.05;
p<.01;
p<.001
Analysis of FA Values
Twenty-one of 55 participants with EOS were diagnosed as having a comorbid CUD. To determine if these 21 participants were representative of the larger group of EOS participants, a MANCOVA was used to test the association between FA and CUD status (+/−). The overall MANCOVA was not significant (F12,38=0.83, p=0.62). Based on this finding, we did not divide the EOS patient group based on cannabis status for subsequent analyses.
The MANCOVA examining FA of all six fiber bundles showed a significant main effect of group (F36,423=1.46, p=0.047). Means and standard deviations of raw FA values for each fiber bundle are shown in Table 2. Planned pairwise group comparisons were conducted using ANCOVA with age, sex, WRAT scores and urine toxicology screen results as covariates to clarify group differences in each fiber tract. Reading decoding scores were not found to be a significant covariate (F12,143=1.27, p=0.24) in this model, suggesting that group differences in this variable had a limited impact on the results.
TABLE 2.
Means and SD of Fractional Anisotropy (x1000) in Fiber Bundles
| HC (n=55) M (SD) |
CUD (n=31) M (SD) |
CHR (n=21) M (SD) |
EOS (n=55) M (SD) |
F (df=3, 162) | |
|---|---|---|---|---|---|
| Fiber Tract | |||||
| L. cingulum | 456 (34) | 480 (29) | 458 (38) | 459 (35) | |
| R. cingulum | 441 (32) | 451 (30) | 438 (34) | 445 (26) | |
| L. superior longitudinal fasciculus | 468 (29) | 468 (22) | 458 (21) | 464 (23) | |
| R. superior longitudinal fasciculus | 472 (32) | 470 (29) | 462 (29) | 466 (27) | |
| L. corticospinal | 599 (24) | 599 (25) | 583 (25)a,b | 581 (29)a,b | 5.80** |
| R. corticospinal | 590 (23) | 587 (19) | 570 (28)a,b | 576 (26)a,b | 6.26*** |
| L. inferior longitudinal fasciculus | 480 (24) | 474 (24) | 462 (27)a,b | 463 (26)a | 4.62** |
| R. inferior longitudinal fasciculus | 486 (30) | 476 (26) | 476 (24) | 473 (27) | |
| L. inferior fronto-occipital fasciculus | 529 (25) | 522 (22)a | 511 (23)a | 513 (26)a | 5.77** |
| R. inferior fronto-occipital fasciculus | 534 (22) | 530 (23) | 523 (24) | 524 (29) | |
| L. uncinate | 382 (36) | 383 (30) | 376 (24) | 384 (22) | |
| R. uncinate | 410 (26) | 415 (28) | 405 (29) | 411 (28) |
Note: Multivariate tests (Wilk’s lambda): Wide Range Achievement Test (WRAT) score32: F=1.22, p=.21; Sex: F=1.05, p=.41; Age: F=1.33, p=.21; Positive urine analysis for cannabis: F=1.15, p=.32. CHR=clinical high-risk for schizophrenia; EOS=earlyonset schizophrenia, with and without comorbid cannabis use disorder (CUD); L=left; R=right.
Significant difference from healthy controls (HC): p<.05.
Significant difference from CUD: p<.05.
p<.01;
p<.001
Post-hoc analyses indicated that EOS and CHR had lower FA in bilateral CST compared to HC and nonpsychotic adolescents with CUD and lower FA values in left ILF and left IFOF compared to HC. Adolescents with CUD only had lower FA values in left IFOF compared to HC.
To further assess the effects of cannabis on white matter microstructure, the above analyses were repeated excluding participants with a comorbid CUD diagnosis in the EOS (n=21) and CHR (n=1) groups. The overall MANCOVA remained significant (F36,361=1.6, p=0.04), and pairwise group comparisons revealed significant group effects for the four fiber tracts identified in Table 2.
The above analyses were also repeated excluding CUD participants who had been prescribed SGAs (n=6). The significance of the overall MANCOVA declined to a trend level (F36,406=1.4, p=0.05) and pairwise group comparisons revealed lower FA in left IFOF than HC at a trend level (p=0.06).
To assess the effects of antipsychotic medications on FA in four fiber tract showing significant group effects, univariate ANCOVAs were conducted with lifetime antipsychotic medication exposure as a covariate. For each fiber tract, the ANCOVAs revealed a significant main effect of group.
Neurocognitive Performance
Nine participants (2 HC; 3 CUD; 2 CHR; 2 EOS) were unable to complete the neurocognitive battery due to scheduling conflicts and/or lack of subject cooperation. As expected, participants with EOS had significantly lower scores across all neurocognitive measures included in this test battery compared to HC and CUD as shown in Table 3. By contrast, deficits in CHR were limited to the domains of executive function and motor dexterity and were milder in severity. CUD was phenotypically similar to CHR in terms of having lower number-letter switching scores compared to HC.
TABLE 3.
Neuropsychological Tests: Scaled Scores, Estimated Means, and Standard Errors.
| HC (n=53d)M (SE) |
CUD (n=28d) M (SE) |
CHR (n=19d) M (SE) |
EOS (n=53d) M (SE) |
F (df=5, 141) | |
|---|---|---|---|---|---|
| Hypothesized to be significant | |||||
| Number-letter switchinge | 11.1 (.5) | 8.7 (.6)a | 7.8 (.7)a | 6.6 (.4)a,b | 17.79*** |
| Grooved pegboardf, dominant hand | 98.6 (5.3) | 84.2(7.1) | 58.5 (8.6)a,b | 56.0 (5.0)a,b | 12.45*** |
| Exploratory analyses | |||||
| CVLTg long delay free recall | .01 (.2) | −.60 (.2)a | −.17 (.3) | −1.1 (.2)a,b,c | 9.09*** |
| CPTh d-prime 4 | 1.3 (.1) | 1.14 (.1) | 1.42 (.1) | .85 (.1)a,b,c | 5.29** |
| 20 questions achievement scorei | 11.6 (.46) | 10.4 (.6) | 9.0 (.73)a | 8.8 (.43)a, b | 7.21*** |
| Category switching correct responsesi | 12.1 (.46) | 11.4(.61) | 11.2 (.74) | 8.8 (.43)a,b,c | 10.08*** |
| Cannon-Spoor premorbid adjustment24 | − | − | − | 9.0 (3.8) | − |
Note: Multivariate tests (Wilk’s lambda): Wide Range Achievement Test (WRAT) score32: F=9.65, p<.001; Sex: F=.95, p=.450; Age: F=13.00, p< .001; Positive urine analysis for cannabis: F=2.46, p=.036. EOS=early-onset schizophrenia, with and without comorbid.
Significant difference from healthy controls (HC), p<.05.
Significant difference from cannabis use disorder (CUD), p<.05.
Significant difference from clinical high-risk for schizophrenia (CHR), p<.05.
Not all participants completed neuropsychological testing
Subtest of Trail Making Test in Delis-Kaplan Executive Function System (D-KEFS)33
Grooved Pegboard Test35
Continuous Performance Test36
Subset of D-KEFS.33
p<.01
p<.001
Correlational Analyses
Planned Spearman rho correlations between neurocognitive measures and fiber tracts for EOS are presented in Table 4. As predicted, results showed significant associations between FA values in left ILF and scores on the number-letter switching subtest. However, no significant correlations were found between FA values in the left IFOF and scores on the Grooved Pegboard Test.
Table 4.
Spearman Rho Correlations Between Fiber Tract and Neurocognitive Measures in Early-Onset Schizophrenia (EOS)
| L. inferior longitudinal fasciculus |
L. inferior fronto-occipital fasciculus |
L. corticospinal | R. corticospinal | |
|---|---|---|---|---|
| Hypothesized to be significant relationships | ||||
| Number-letter switchinga (n=54) | .28* | .23 | ||
| Grooved pegboardb, dominant hand (n=54) | −.05 | −.19 | ||
| Exploratory relationships | ||||
| CVLTc long delay free recall (n=54) | .22 | .11 | −.14 | .05 |
| CPTd d-prime 4 (n=54) | .31 | .21 | −.17 | −.09 |
| 20 questions achievement scoree (n=54) | .40** | .32 | .05 | .09 |
| Category switching correct responsese (n=54) | .37** | .38** | .004 | −.02 |
| Cannon-Spoor premorbid adjustment24 (n=53) | −.30 | −.29 | −.15 | −.13 |
| WRATf score (n=54) | −.10 | .11 | −.26 | −.31 |
| Lifetime CPZg exposure (n=55) | −.07 | .09 | .22 | .04 |
| Current CPZg exposure (n=55) | .03 | −.11 | .20 | .29 |
Note: EOS with and without comorbid cannabis use disorder (CUD); L=left; R=right.
Subtest of trail-making test in Delis-Kaplan Executive Function System (D-KEFS)33
Grooved Pegboard Test35
Continuous Performance Test36
Subtest of D-KEFS33
Wide Range Achievement Test (WRAT)32
Chlorpromazine (CPZ) equivalents25
p<.05
p<.01
Exploratory correlational analyses between FA values and other measures of executive function were performed to generate hypotheses for future studies, and revealed significant relationships between lower FA values in left ILF and left IFOF and worse executive function (Table 4).
DISCUSSION
This study identified four WM tracts that were significantly altered in EOS compared to age-matched HC, replicating previous findings of alterations in WM microstructure in adolescents and adults with schizophrenia. These four tracts were the bilateral CST, left ILF, and left IFOF. Alterations in bilateral CST tracts in EOS replicate an earlier finding by Douaud and colleagues.9 CST alterations may fade as adolescents with EOS mature into adulthood.7,41 However, the presence of these abnormalities in the CHR group suggests that these abnormalities may not be specific to EOS. The finding that left ILF is altered in EOS also replicates earlier studies in EOS13 and in adults with schizophrenia, including first-episode,15 and never-medicated patients.46 Although to our knowledge, WM alterations in left IFOF have not been previously reported in EOS, abnormalities in this tract have been observed in adults with schizophrenia,16,47 including a recent meta-analysis of first-episode patients,15 and never-medicated patients.46
The left ILF and left IFOF are implicated in visual and auditory processing that may be relevant to the pathophysiology of psychotic disorders during adolescence. The ILF connects occipital and temporal lobes, while the IFOF connects the occipital and temporal lobes with the orbitofrontal cortex. Together, ILF and IFOF are thought to form the ventral visual stream, which enables recognition and discrimination of visual shapes and objects.48 These tracts are also thought to be part of a ventral language stream, which converts sound to meaning and serves as an interface between sound-based representations of speech and widely distributed conceptual representations.49
In this study, the same four tracts—bilateral CST, left ILF, and left IFOF—were also significantly altered in CHR with a magnitude comparable to alterations observed in EOS. This finding is important because CHR had lower doses of antipsychotic medication prescribed at time of MRI scan and less cumulative exposure to antipsychotic medication.4 Lower FA in left ILF has been previously reported in a younger cohort of children aged 11–13 years with subclinical psychotic symptoms.50 Patients with 22q.11.2 deletion syndrome, who are at genetic high risk for developing schizophrenia, also showed lower FA values in left IFOF.51 Together, these data suggest that the observed WM tract alterations may represent a risk factor for development of psychosis.
The present study included a comparison group of nonpsychotic adolescents with CUD. We detected a shared decrease in FA among CUD, CHR, and EOS in left IFOF, relative to HC. In addition, we did not find differences between EOS and CUD in planned post-hoc analyses of WM in left IFOF and left ILF. These data support the notion suggested by James and colleagues52 that prior exposure to cannabis may contribute to the variance in FA abnormalities observed in patients with EOS.
The present study replicated a finding that lower FA in left ILF was associated with worse performance on the Trailmaking Test Part B in adults with a first-episode of schizophrenia who had received limited antipsychotic medication.20 The present study extended this by showing an association between lower FA in left ILF with lower performance on 2 other measures of executive function:34 20 Questions Achievement and Category Switching in the Verbal Fluency Subtest. Given the putative roles of ILF and IFOF in vision and language processing, these findings are compatible with a hypothesis that alterations in fiber tracts thought to subserve domain-specific functions may have general effects on higher-order cognitive processing.53 The absence of a significant relationship between FA in fiber tracts in visual processing regions in the left hemisphere and cognition in the other groups suggests that FA in left IFOF or ILF is unlikely to contribute to cognitive performance in these adolescents and/or may reflect issues related to power due to smaller sample size.
The findings of this study should be considered in the context of several limitations. Many of the patients in the study were receiving antipsychotic medications. However, the presence of similar abnormalities in CHR participants makes it unlikely that the observed alterations in WM in EOS are primarily due to antipsychotic medication exposure. Also, higher antipsychotic medication dose at time of scan was found to be related to higher FA in the right corticospinal tract in EOS patients which is contrary to what was expected.
Second, all patient groups had lower reading decoding scores (an estimate of premorbid intelligence) compared to healthy controls. This is likely to be an artifact of screening out subjects with any psychopathology. However, differences in reading decoding scores did not appear to have a significant impact on white matter microstructure as this covariate was not significant in our model and specific aspects of cognition were found to predict FA in the expected direction better than reading decoding scores in adolescents with EOS.
Finally, there is evidence that some white matter abnormalities in schizophrenia appear as widely-dispersed, focal reductions in FA called “potholes” that vary spatially among individuals.14 These focal abnormalities may be more amenable to detection by the pothole method of analysis, which looks for within-subject clusters of white-matter voxels with reduced FA.54
In summary, this study found lower FA in four WM tracts in EOS, two of which mapped onto neurocognitive deficits in EOS. The results here suggest that adolescents with CHR, but not with CUD, have similar WM abnormalities. Future work is needed to confirm these findings and further characterize the relationship between WM abnormalities and neurocognitive impairment in high-risk groups before and after illness onset.
Supplementary Material
Acknowledgments
This research was funded by National Institute of Mental Health Grant MH073150-05 (Cannabis and Schizophrenia; to S.K.).
Dr. Lee served as the statistical expert for this research.
The authors acknowledge Lois Laitinen, MBA, MM, of the University of Minnesota for her support.
Dr. Kumra has received research support from the National Alliance for Research on Schizophrenia and Depression, and Otsuka Pharmaceutical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Cullen, Lee, and Mueller, and Ms. Epstein report no biomedical financial interests or potential conflicts of interest.
References
- 1.Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch. Gen. Psychiatry. 2003 May;60(5):443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 2.Melonakos ED, Shenton ME, Rathi Y, Terry DP, Bouix S, Kubicki M. Voxel-based morphometry (VBM) studies in schizophrenia-can white matter changes be reliably detected with VBM? Psychiatry Res. 2011 Aug 30;193(2):65–70. doi: 10.1016/j.pscychresns.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asarnow RF, Nuechterlein KH, Fogelson D, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. Arch. Gen. Psychiatry. 2001 Jun;58(6):581–588. doi: 10.1001/archpsyc.58.6.581. [DOI] [PubMed] [Google Scholar]
- 4.Cannon M, Caspi A, Moffitt TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch. Gen. Psychiatry. 2002 May;59(5):449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 5.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging. 2001 Apr;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 6.White T, Kendi AT, Lehericy S, et al. Disruption of hippocampal connectivity in children and adolescents with schizophrenia--a voxel-based diffusion tensor imaging study. Schizophr. Res. 2007 Feb;90(1-3):302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res. 2010 Mar 30;181(3):193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumra S, Ashtari M, McMeniman M, et al. Reduced frontal white matter integrity in early-onset schizophrenia: a preliminary study. Biol. Psychiatry. 2004 Jun 15;55(12):1138–1145. doi: 10.1016/j.biopsych.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007 Sep;130(Pt 9):2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 10.Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol. Psychiatry. 2008 Mar 1;63(5):519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Kumra S, Ashtari M, Cervellione KL, et al. White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. J. Am. Acad. Child Adolesc. Psychiatry. 2005 Sep;44(9):934–941. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- 12.Henze R, Brunner R, Thiemann U, et al. White matter alterations in the corpus callosum of adolescents with first-admission schizophrenia. Neurosci. Lett. 2012 Apr 4;513(2):178–182. doi: 10.1016/j.neulet.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Ashtari M, Cottone J, Ardekani BA, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch. Gen. Psychiatry. 2007 Nov;64(11):1270–1280. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- 14.White T, Ehrlich S, Ho BC, et al. Spatial characteristics of white matter abnormalities in schizophrenia. Schizophr. Bull. 2013 Sep;39(5):1077–1086. doi: 10.1093/schbul/sbs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao L, Lui S, Liao Y, et al. White matter deficits in first episode schizophrenia: An activation likelihood estimation meta-analysis. Prog. Neuropsychopharmacol. Bol. Psychiatry. 2013 May 3;45C:100–106. doi: 10.1016/j.pnpbp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Palaniyappan L, Al-Radaideh A, Mougin O, Gowland P, Liddle PF. Combined white matter imaging suggests myelination defects in visual processing regions in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 Aug;38(9):1808–1815. doi: 10.1038/npp.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J. Psychiatr. Res. 2009 Jan;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters BD, de Haan L, Vlieger EJ, Majoie CB, den Heeten GJ, Linszen DH. Recent-onset schizophrenia and adolescent cannabis use: MRI evidence for structural hyperconnectivity? Psychopharmacol. Bull. 2009;42(2):75–88. [PubMed] [Google Scholar]
- 19.Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003 Apr 1;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am. J. Psychiatry. 2010 Apr;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 21.Rhinewine JP, Lencz T, Thaden EP, et al. Neurocognitive profile in adolescents with early-onset schizophrenia: clinical correlates. Biol. Psychiatry. 2005 Nov 1;58(9):705–712. doi: 10.1016/j.biopsych.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009 Mar;108(1-3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Kumra S, Robinson P, Tambyraja R, et al. Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012 Feb;51(2):171–180. doi: 10.1016/j.jaac.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr. Bull. 1982;8(3):470–484. doi: 10.1093/schbul/8.3.470. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010 Feb 1;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol. Psychiatry. 2009 Sep 15;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 28.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA psychiatry. 2013 Jan;70(1):107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch. Gen. Psychiatry. 1982 Jul;39(7):784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 30.Overall J, Gorham D. The brief psychiatric rating scale. Psychol. Rep. 1962;10:799–812. [Google Scholar]
- 31.Lachar D, Randle SL, Harper RA, et al. The brief psychiatric rating scale for children (BPRS-C): validity and reliability of an anchored version. J. Am. Acad. Child Adolesc. Psychiatry. 2001 Mar;40(3):333–340. doi: 10.1097/00004583-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson G. Wide Range Achievement Test, 3rd Edition (WRAT-3) Manual. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- 33.Delis D, E K, Kramer J. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 34.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test: Second Edition. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 35.Matthews CKH. Instruction Manual for the Adult Neuropsychological Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- 36.Cornblatt B, Obuchowski M, Schnur DB, O'Brien JD. Attention and clinical symptoms in schizophrenia. Psychiatr. Q. 1997 Winter;68(4):343–359. doi: 10.1023/a:1025495030997. [DOI] [PubMed] [Google Scholar]
- 37.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006 Aug 1;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 38.Walhovd KB, Moe V, Slinning K, et al. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage. 2007 Jul 15;36(4):1331–1344. doi: 10.1016/j.neuroimage.2007.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 40.Haselgrove JC, Moore JR. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn. Reson. Med. 1996 Dec;36(6):960–964. doi: 10.1002/mrm.1910360620. [DOI] [PubMed] [Google Scholar]
- 41.Draganski B, May A. Training-induced structural changes in the adult human brain. Behav. Brain Res. 2008 Sep 1;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007 Jan 1;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciccarelli O, Behrens TE, Altmann DR, et al. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain. 2006 Jul;129(Pt 7):1859–1871. doi: 10.1093/brain/awl100. [DOI] [PubMed] [Google Scholar]
- 44.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 45.Mathews CG, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- 46.Liu X, Lai Y, Wang X, et al. Reduced white matter integrity and cognitive deficit in nevermedicated chronic schizophrenia: A diffusion tensor study using TBSS. Behavioural brain research. 2013 Sep 1;252:157–163. doi: 10.1016/j.bbr.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberger G, Kubicki M, Nestor PG, et al. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr. Res. 2008 Jul;102(1-3):181–188. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in neurosciences. 1992 Jan;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 49.Axer H, Klingner CM, Prescher A. Fiber anatomy of dorsal and ventral language streams [published online ahead of print May 23 2012] [Accessed May 23];Brain Lang. 2012 doi: 10.1016/j.bandl.2012.04.015. http://www.ncbi.nlm.nih.gov/pubmed/22632814. [DOI] [PubMed]
- 50.Jacobson S, Kelleher I, Harley M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11-13 year old schoolchildren. Neuroimage. 2010 Jan 15;49(2):1875–1885. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Kikinis Z, Makris N, Finn CT, et al. Genetic contributions to changes of fiber tracts of ventral visual stream in 22q11.2 deletion syndrome [published online ahead of print April 24 2013] Brain imaging and behavior. 2013 doi: 10.1007/s11682-013-9232-5. http://www.ncbi.nlm.nih.gov/pubmed/23612843. [DOI] [PMC free article] [PubMed]
- 52.James A, Hough M, James S, et al. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS) Schizophr. Res. 2011 May;128(1-3):91–97. doi: 10.1016/j.schres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annual review of clinical psychology. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White T, Schmidt M, Karatekin C. White matter 'potholes' in early-onset schizophrenia: a new approach to evaluate white matter microstructure using diffusion tensor imaging. Psychiatry Res. 2009 Nov 30;174(2):110–115. doi: 10.1016/j.pscychresns.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hollingshead AdB, Redlich FC. Social class and mental illness; a community study. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Overall JE, Gorham DR, Shawver JR. Basic dimensions of change in the symptomatology of chronic schizophrenics. Journal of abnormal and social psychology. 1961 Nov;63:597–602. doi: 10.1037/h0039893. [DOI] [PubMed] [Google Scholar]
- 57.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test--Children's Version. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.