Abstract
Research in social neuroscience has uncovered a social knowledge network that is particularly attuned to making social judgments. However, the processes that are being performed by both regions within this network and those outside of this network that are nevertheless engaged in the service of making a social judgment remain unclear. To help address this, we drew upon research in semantic memory, which suggests that making a semantic judgment engages 2 distinct control processes: A controlled retrieval process, which aids in bringing goal-relevant information to mind from long-term stores, and a selection process, which aids in selecting the information that is goal-relevant from the information retrieved. In a neuroimaging study, we investigated whether controlled retrieval and selection for social information engage distinct portions of both the social knowledge network and regions outside this network. Controlled retrieval for social information engaged an anterior ventrolateral portion of the prefrontal cortex, whereas selection engaged both the dorsomedial prefrontal cortex and temporoparietal junction within the social knowledge network. These results suggest that the social knowledge network may be more involved with the selection of social information than the controlled retrieval of it and incorporates lateral prefrontal regions in accessing memory for making social judgments.
Keywords: neuroimaging, prefrontal cortex, semantic memory, social cognition, theory of mind
Introduction
One of the most important jobs for humans is to understand each other. Our ability to do so begs a classic question in social psychology: How do we make judgments about other people's attributes, feelings, beliefs, and personalities? Neuroscience research addressing this question over the last decade has focused primarily on identifying the systems that are involved in making such judgments. To this effect, a core network supporting such judgments has been identified that has been variously referred to as the mental state attribution, theory of mind, or social cognition network (Fletcher et al. 1995; Grady and Keightley 2002; Gallagher and Frith 2003; Ochsner 2004; Amodio and Frith 2006; Van Overwalle 2009). Here, we use the term “social knowledge network” to refer to this set of areas because it captures a key feature common to all accounts of it—this group of regions is engaged when accessing and making use of social knowledge in general, be it for the sake of mental state attribution, trait inference, person memory, impression formation, and so on (Gallagher and Frith 2003; Saxe and Kanwisher 2003; Lieberman et al. 2004; Ochsner 2004; Olsson and Ochsner 2008; Mitchell 2009; Zaki et al. 2009; Spunt et al. 2011). The social knowledge network is centered around the dorsomedial prefrontal cortex (putative Brodmann's area 9), which is the region most commonly associated with making person judgments, and may include a variety of posterior cortical regions as well, including the precuneus, temporal-parietal junction, and temporal pole (Grady and Keightley 2002; Ochsner 2004; Van Overwalle 2009).
While we know that this network (or some portion of it) is engaged when making judgments about people, 2 key questions about this network remain unanswered. First, it is not yet clear what specific processes each region within this network carries out when making person judgments. Secondly, areas beyond those typically thought to comprise the social knowledge network are also active when one makes judgments about people, and the contributions of these regions also remain unclear. For example, parts of ventrolateral prefrontal cortex (vlPFC, which includes the inferior frontal gyrus), whose activity is often found in studies of “cold” nonsocial/nonaffective cognition, have also been engaged (Gorno-Tempini and Price 2001; Grabowski et al. 2001; Shah et al. 2001; Kelley et al. 2002; Tsukiura et al. 2002; Heberlein and Saxe 2005; Mitchell et al. 2005; Zahn et al. 2007; Rameson et al. 2010; Simmons et al. 2010; Cloutier et al. 2011). These activations often are not the focus of research in the neuroscience of person judgments in part because the domain general functions of the lateral prefrontal cortex (Miller and Cohen 2001; Simmons et al. 2010) have not been central to the theme of what is distinct about social cognition (Sergent et al. 1992; Mitchell et al. 2002; Saxe and Powell 2006). As such, these regions are most often left in the background in the person judgment literature and have only received a modest amount of speculative attention (Grabowski et al. 2001; Tsukiura et al. 2002; Lieberman et al. 2004; Ochsner et al. 2004, 2005; Cloutier et al. 2011). An alternative, complimentary approach to studying the neural systems supporting person judgments is not to ask which neural systems are “distinctly” social, but instead to characterize neural regions based on what “kinds” of processes are engaged when making social judgments (Mitchell 2009). Doing so may help us understand the roles that both medial and lateral portions of the prefrontal cortex play in social cognition.
In pursuing this course, we began by asking what making person judgments and making judgments about nonperson objects have in common. We reasoned that both judgments depend upon retrieving information from memory. Further, we noted that the tasks used to study semantic retrieval (Thompson-Schill et al. 1997; Wagner et al. 2001) are in many ways similar to tasks used in studies of person judgments. In both cases, it is common to be given a target stimulus and make judgments about its attributes—be they semantic or social. To date, what has been emphasized in the literature is the fact that when it is a person, there are different kinds of attributes and judgments being made than when it is not a person. These differences in content have inspired the hypothesis that these regions engage “processes” that may be unique to social cognition (Sergent et al. 1992; Mitchell et al. 2002; Van Overwalle 2009; Contreras et al. 2011). However, though differences in content have clearly been found, there may nevertheless be similarities in terms of the kinds of processes that access information about nonsocial things as well as for people.
Studies of semantic memory suggest that the act of accessing information may involve 2 separate control processes (Thompson-Schill et al. 1997; Wagner et al. 2001; Badre et al. 2005). The first is a “controlled retrieval” process that enables us to search for and retrieve information that may be of relevance. The second is a “selection” process—another kind of control process—that picks the most goal-appropriate item from among all the information that has been retrieved. For example, suppose one is trying to remember a specific song sung by a pop star. The name of the pop star might automatically retrieve the information that is most strongly associated with her, perhaps her image or the latest gossip. If the song title is not automatically retrieved, however, a controlled retrieval process may be engaged to produce more associated information. In this way, controlled retrieval enables one to retrieve more information from memory, however weakly it may be associated with the initial retrieval cue. As controlled retrieval brings to mind the various pieces of information about the pop star, a second control process, “selection,” must pick out the information that is most relevant to the goal (i.e. the specific song) and ignore retrieved information that is irrelevant to it (e.g. other song titles or gossip that may be irrelevant to the song). As such, selection is a form of conflict resolution (Miller and Cohen 2001). Notably, we use the term "controlled retrieval" to distinguish it from more automatic forms of retrieval, but we use the term "selection" because there is no automatic form of selection; rather selection always involves the use of control processes to resolve conflict between competing alternatives (Miller and Cohen 2001).
In most situations, accessing information from memory involves both controlled retrieval and selection. However, the contributions of controlled retrieval and selection to neural activity can be assessed by taking 2 principles into account. The first principle is that the relative involvement of controlled retrieval and selection processes in making a judgment is driven by different features (Fig. 1). Controlled retrieval is driven by the extent to which goal-relevant information is not automatically produced (i.e. more controlled retrieval is required if goal-relevant information is not automatically produced). Selection is driven by the competition among retrieved items, regardless of whether they were retrieved via automatic or controlled mechanisms (i.e. more competition between items places more demands on selection). The second principle is that these features, and hence controlled retrieval and selection processes, interact with each other. For instance, this may play out as follows: As controlled retrieval brings more goal-associated information to mind, selection has a more difficult time choosing which associates are goal-relevant and which are not. As selection demand increases with competition of the items, controlled retrieval may be further employed to access more information to help resolve this competition. In both cases, increasing the demands placed on controlled retrieval also increases demands placed on selection and vice versa. This is critical, for it indicates that task manipulations cannot selectively increase the involvement of controlled retrieval or selection—increasing demands placed on controlled retrieval will have an influence on selection and vice versa. Thus, to measure the contribution of these processes to social knowledge retrieval requires breaking the correlation between these factors.
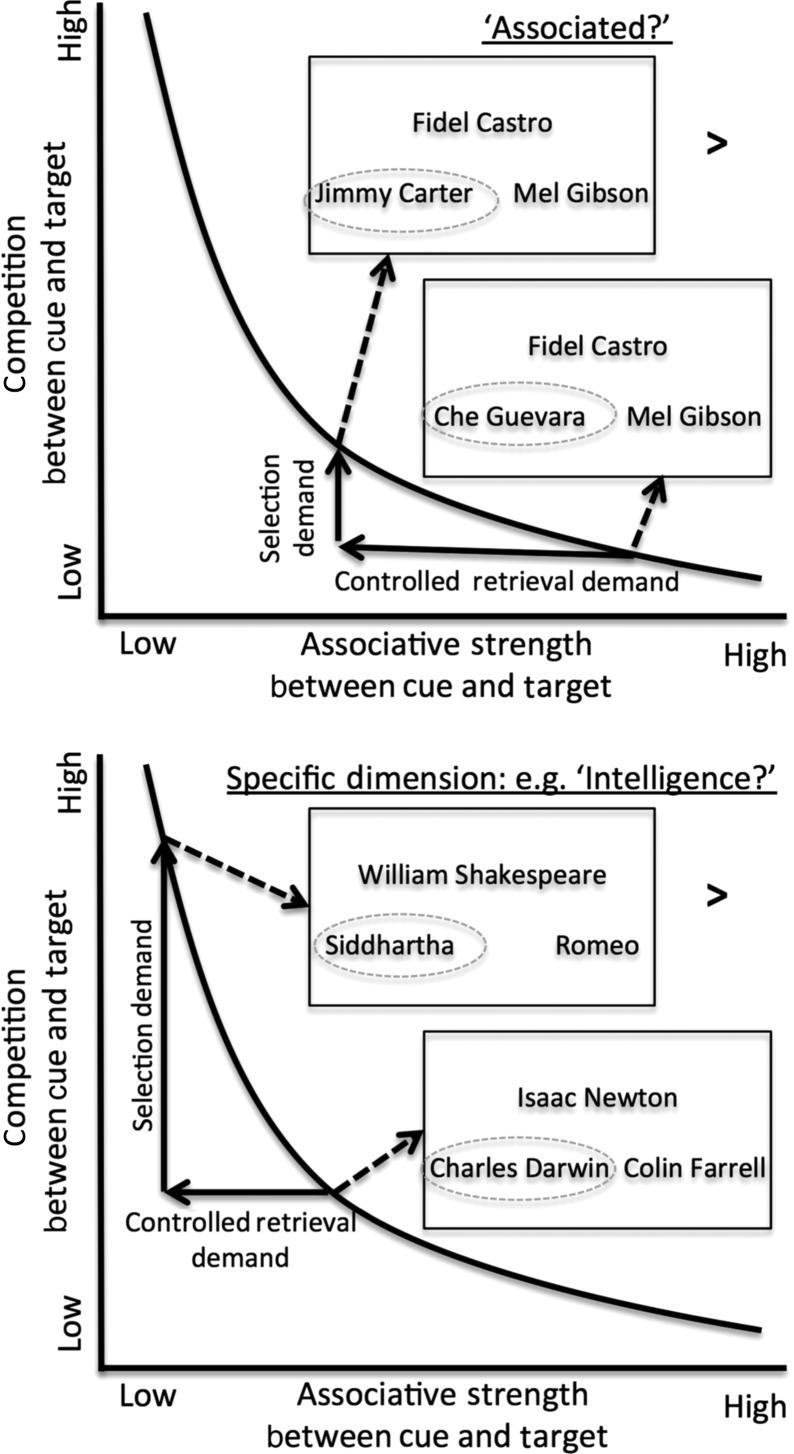
Figure 1.
Making social judgments involves accessing goal-relevant information from memory. This access relies on 2 processes: Controlled retrieval and selection. The demand for these processes in turn depends on 2 features, the associative strength between the cue and target (depicted along the x-axis) and the competition between the cue and target (depicted along the y-axis). The curvilinear line illustrates that these features are nonindependent: As associative strength between a cue and a target decreases, the competition or interference also increases (e.g. because weakly associated targets of a cue must compete against more strongly associated probes that may be retrieved surreptitiously). Thus, to separate out the contributions of controlled retrieval and selection in making social judgments, participants engaged in 2 conditions. For one condition, which is illustrated in the top portion of the graph, participants made an “Associated?” judgment by matching the cue name (e.g. “Fidel Castro”) with the more associated probe name. The probe was either more strongly associated (e.g. “Che Guevara”) or more weakly associated (e.g. “Jimmy Carter”; gray circles were not shown in the task, but are included here to indicate the correct probe response). Comparing trials with weaker associative strength to trials with stronger associative strength increases demands placed on controlled retrieval. However, it may also increase the demands placed on selection (e.g. because matching Fidel Castro with Jimmy Carter may nevertheless bring information about stronger associates, such as Che Guevara, to mind surreptitiously, and this information must be selected against). Thus, a second condition is involved, which is illustrated in the bottom portion of the graph, in which a matching judgment is made along a specific dimension (e.g. “Intelligence?”). Comparing trials with higher selection demand (e.g. having to match the cue “William Shakespeare” with the probe “Siddhartha” for intelligence involves selecting against the stronger but irrelevant probe “Romeo”) versus those that have low selection demand (i.e. trials in which the more associated probe is also the better match for the given dimension, in this case for “Isaac Newton,” “Charles Darwin” is both more associated and the correct match). With these conditions, the difference between the conditions in the top graph and in the bottom graph reveals whether neural regions are more associated with controlled retrieval or selection.
With this in mind, we adapted a matching task from prior semantic memory research that allows for these processes to be partially uncoupled during retrieval (Fig. 1; Badre et al. 2005). In this task, participants see a cue word (e.g. “candle”) and judge which of 2 probe words (e.g. “flame” and “pencil”) is most associated with it (in this case, flame). The demand for controlled retrieval is varied by manipulating how strongly associated the cue and target are. For example, similar to tasks examining semantic priming (e.g. Gold et al. 2006), when the cue and target are weakly associated (e.g. presenting candle with “halo”), this places greater demands on controlled retrieval when compared with when they are strongly associated (e.g. presenting candle with flame, which is more strongly associated with candle). Notably, this manipulation will also putatively increase demands on selection as competition will necessarily covary with additional retrieval on weak trials. The demand for selection is manipulated by asking participants to match along a dimension (e.g. “shape”) that requires selecting the typically nonassociated probe (i.e. pencil, which now matches the cue candle better than flame for shape) over the typically associated probe. The critical analysis is to compare these task conditions, and hence the demands placed on controlled retrieval and selection, against each other. Imaging studies using these methods have found that controlled retrieval and selection depend upon distinct regions of the vlPFC (Badre et al. 2005; Badre and Wagner 2007). Here, we asked whether the controlled retrieval and selection of social information depend upon the prefrontal regions implicated in mental state attribution, such as the dorsomedial prefrontal cortex (Fletcher et al. 1995; Grady and Keightley 2002; Gallagher and Frith 2003; Ochsner 2004; Amodio and Frith 2006), prefrontal regions implicated in accessing information from semantic memory, such as the vlPFC, or a combination of both.
To address this question, we adapted the experimental logic described above for use with social information (Fig. 1). We generated a normed body of social information consisting of famous people and fictional characters using norming methods similar to those in semantic memory research. Using these stimuli, participants were asked to match a cue name with 1 of the 2 probe names. To vary demands for the controlled retrieval of social information, participants were shown the instruction, “Associated” which indicated that the cue name was to be matched with the more associated name. Controlled retrieval demands were greater for trials in which the target name was weakly associated with the cue name relative to those in which the target name was more strongly associated with the cue name (see Materials and Methods, for example). To vary demands for the selection of social information, participants were shown one of several different selection dimensions (e.g. “Age,” “Intelligence,” “Gender,” etc.) on which the cue name could be matched to one of the probe names. Selection demands were greater for trials where the correct response required choosing the probe name that was more weakly associated with the probe name overall (and selecting against the more associated but incorrect probe name) relative to trials in which the selection cue required choosing the strongly associated name (see Materials and Methods, for example). With these conditions in place, we first contrasted them to examine which neural regions were preferentially engaged by controlled retrieval or selection (Fig. 1). We then combined them to examine which neural regions are commonly involved when increasing demands are placed on both controlled retrieval and selection.
Materials and Methods
Participants
Thirty-three (aged 18–35; 11 males) healthy, native English speaking, right-handed participants provided informed consent following Columbia University's IRB guidelines. They received US$25/hour in compensation. Participants were screened to have no ferromagnetic metals in their body and no metals that could influence the magnetic resonance imaging (MRI) signal, not to be claustrophobic, and for female participants not to be pregnant.
Stimuli
Across 4 pilot studies, we developed a normed body of social information (famous people and fictional characters) using procedures similar to those implemented in studies of semantic memory (Postman and Keppel 1970; see Supplementary Materials). In brief, participants were shown cue names and responded with the first associated person or character that came to mind. From this list, frequencies were tabulated and associate strength ratings were obtained (e.g. for the cue name “Arnold Schwarzenegger,” the most frequently and hence strongly associated name was “The Terminator” and a less frequently and hence weakly associated name was “Maria Shriver”). The overall ratio of strong-to-weak associative strength was 15.2:1; that is, on average people would retrieve the strong associate 15.2 times more often than the weak associate when presented with the cue name (this is comparable with studies in semantic memory, which have ranged in their strong:weak ratio between 5:1 and 22:1, for example, Badre et al. 2005). Based on these pilot studies, 4 lists of 18 items each were generated, balanced for familiarity ratings, number of letters, and number of syllables. Each item was composed of a cue name, a strongly associated probe name, a weakly associated probe name, an unrelated probe name, and a selection instruction cue. Lists were counterbalanced across the 4 conditions (see below) across subjects.
Design and Task
The goal of this experiment was to manipulate controlled retrieval and selection demands for social information (Fig. 1). In one condition, participants were shown the instruction word “Associated” for 1 s, followed by a blank screen interstimulus interval (ISI) jittered for 2–8 s (see below for details on the jitter). A triad of names consisting of the cue name and 2 probe names then appeared. The cue name was presented in the top center of the screen, and the 2 probe names—one associated (either strongly or weakly) with the cue and one unrelated to the cue—were presented on the bottom left and right of the screen counterbalanced for the target position. Participants were instructed to match the cue with the probe name that was more globally associated with it, and the need for controlled retrieval was varied by manipulating how strongly associated the cue was to the target. For half the trials, the cue name (e.g. “Karl Marx”) was to be matched with a weakly associated target name (e.g. “John Locke”) over a nonassociated name (e.g. “Wonder Woman”), whereas for the other half, the cue name was to be matched with a strongly associated target name (e.g. “Vladimir Lenin”) over the nonassociated name (Fig. 1). The triad remained on the screen until a response was made or upon a 5-s time-out and was followed by a blank screen intertrial interval (ITI) jittered for 4–10 s (see below for details on the jitter).
In another condition, the trial structure was essentially identical except that, instead of the Associated instruction, each trial started with 1 of 6 instruction words that indicated the dimension of comparison for cue and probe names to be used as the basis of selection: “Intelligence,” “Authority,” “Gender,” “Age,” “Healthiness,” or “Craziness” (Supplementary Materials). The need for selection was manipulated by asking participants to match along a dimension that required selecting the typically nonassociated probe over the typically associated probe (Fig. 1). Thus, on high selection demand trials, given a specific selection dimension (e.g. Gender), the cue name (e.g. “Hugh Grant”) was to be matched with the nonassociated target name (e.g. “Chewbacca”) rather than an associated name (e.g. “Elizabeth Hurley”). But on low selection demand trials, given a specific selection dimension (e.g. Gender), the cue name (e.g. “Martha Stewart”) was to be matched with an associated target name (e.g. “Julia Child”) rather than the nonassociated target name (e.g. “Popeye”).
To determine the duration of jittered intervals during the ISI and ITI periods and the arrangement of the trials, a design optimization algorithm was implemented (Dale 1999). The algorithm generated designs randomly with the constraints that the total ISI and ITI times granted to each experimental condition were the same, and that the experimental conditions had a maximum of 3 successive trials for a given condition, were each represented across 18 successive trials, and were evenly represented across the 2 functional runs. From 500 generated designs, we selected the design that optimally separated the time course signals for the comparisons of high versus low retrieval demand conditions, high versus low selection demand conditions, and activity related to the instruction cue phase from the triad of names. The task was divided across 2 functional runs, each lasting 7 min 24 s.
Procedure
Participants were prescreened over the telephone. Upon arrival at the Neurological Institute, participants completed the consent process, completed a standardized battery of demographic and individual differences questionnaires (not used in the current study), completed practice versions of the scanner tasks, and were then placed in the MRI scanner where 12 min of structural scanning was completed followed by 2 runs of the present experimental task and 3 runs of a different task related to another study (the other experiment investigated the neural bases of emotion using a completely different task paradigm). After exiting the scanner, participants completed a debriefing questionnaire and were compensated for participation.
Apparatus
Scanning was conducted on a GE TwinSpeed 1.5-T scanner equipped with an 8-channel head coil. Functional scans were obtained using a spiral in/out spoiled gradient echo (SPGR) pulse sequence (time repetition, TR = 2000 ms, slice thickness = 4.5 mm, gap = 0, flip = 84, field of view (FOV) = 22.4 cm, matrix = 64 × 64, voxel size = 3.5, 3.5, 4.5 mm, interleaved bottom-to-top acquisition). A structural SPGR sequence was also obtained (TR = 19 s, time to echo = 5 ms, slice thickness = 1 mm, gap = 0, flip = 20, FOV = 25.6, matrix = 256 × 256, voxel size = 1, 1, 1, interleaved bottom-to-top acquisition). Stimulus presentation and behavioral data collection were administered with a desktop PC and Matlab software with the Psychophysics toolbox (Brainard 1997; Pelli 1997). Stimuli were projected on a screen visible in a mirror attached to the head coil. Responses were made with a scanner compatible button response pad. Participants wore earplugs to reduce scanner noise, and pads and a strip of tape were used to reduce head motion.
Data Analysis
For behavioral data, a correct response was defined as choosing the probe name that best matched the cue name on the dimension given by the instruction cue. Trials that were responded to faster than 200 ms were recoded as a nonresponse. Mean reaction times were calculated for correct responses.
Functional images were preprocessed in SPM5, and statistical models were implemented using custom open-source software (www.neuroelf.com). Images were coregistered, corrected for motion and slice-timing, normalized to the MNI-ICBM152 template, resliced to 3-mm resolution voxels, and smoothed (6-mmfull-width at half-maximum). First-level models included regressors for: High controlled retrieval demand, low controlled retrieval demand, high selection demand, low selection demand, the associative instruction, the selection instruction, incorrect and nonresponse trials combined, and a Fourier high-pass filter (120 s cutoff). Events were modeled using sticks of 1 s duration and convolved with a canonical hemodynamic response function. Robust regressions were performed at the first level to reduce the influence of outliers in estimating the fit of the model. For second-level analyses, subjects were modeled as a random variable. Robust t-tests were performed across the brain to reduce the potential influence of outlier subjects in the analyses (Wager et al. 2005). For statistical thresholding, an AlphaSim Monte Carlo simulation as implemented in AFNI (Forman et al. 1995; smoothing kernel estimated at 8.4 mm from the data) was used to determine an extent threshold given a height (P= 0.01) threshold to identify clusters of activation that were significant across a whole-brain corrected threshold [whole-brain family-wise error rate (FWER), α < 0.05; see Zaki et al. 2009; Kober et al. 2010; Somerville et al. 2010, for similar uses of AlphaSim]. This indicated a cluster extent of k= 84.
Results
Behavioral Results
Reaction times and error rates were analyzed to confirm that both the controlled retrieval and selection manipulations increased the demands on each type of process in the expected way. For controlled retrieval demand, when making an associated judgment, significantly longer reaction times were found for trials that involved having to choose the weaker associate (M = 2661 ms, SD = 439) than for trials that involved having to choose the stronger associate (M = 2415 ms, SD = 407, t(32) = 7.03, P < 10−7). The error rates were not significantly different between the 2 conditions (for the weak associates, M = 6.9%, SD = 7.9%; for the strong associates, M = 4.5%, SD = 6.7%; t(32) = 1.68, P = 0.10). For selection demand, when making a judgment for a specific dimension (e.g. Authority), reaction times were longer for trials involving having to choose the nonassociated probe (M = 2687 ms, SD = 532) than for trials involving having to choose the associated probe (M = 2587 ms, SD = 497, t(32) = 2.11, P < 0.05), and error rates were higher as well (select nonassociate: M = 37.4%, SD = 14%; select associate: M = 7.5%, SD = 7%; t(32) = 12.06, P < 10−7). In both selection and controlled retrieval conditions, speed and accuracy were in the same direction, inconsistent with a tradeoff between speed and accuracy. Overall, the behavioral results confirmed that demands were increased in both conditions, similar to studies of semantic memory (Badre et al. 2005).
Neural Regions Responsive Specifically to the Controlled Retrieval or Selection of Social Information
To examine which neural regions were specifically responsive to the controlled retrieval or selection of social information, we compared (1) conditions that increased the demands placed on controlled retrieval with (2) conditions that increased the demands placed on selection. For (1), we took trials in which participants made an associated judgment and among those contrasted trials in which the target name was weakly associated with the cue name (and hence requires more controlled retrieval) versus trials in which target name was strongly associated with the cue name (and hence requires less controlled retrieval). For (2), we took trials in which participants made a judgment along a given dimension (e.g. Age) and we compared trials in which the correct choice was the nonassociated name (and hence places more demand on selection to select for the relevant information against the more associated information) with those in which the correct choice was also the associated name (and hence places less demand on selection since the associated information promotes the same response). To isolate regions that are more associated with controlled retrieval, we compared (1) > (2), and to isolate regions that are more associated with selection, we compared (2) > (1).
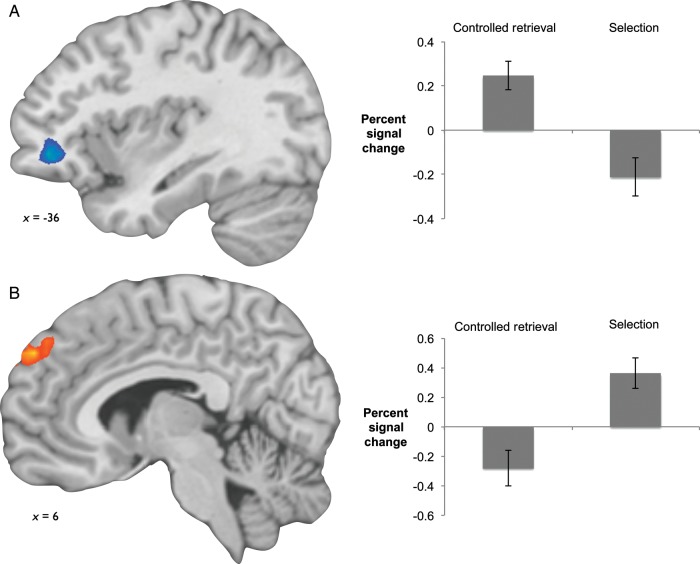
Results are displayed in Figure 2. For the controlled retrieval of social information, no regions showed greater activation. Relaxing the extent threshold revealed a cluster in the left anterior prefrontal cortex showing greater activity to controlled retrieval demand than to selection demand (Montreal Neurological Institute, MNI = [−36, 42, −6], k= 25, tcluster(32) = 3.07, uncorrected), but no activations in regions associated with the social knowledge network. For the selection of social information, activity was found in the dorsomedial prefrontal cortex [putative Brodman's area (∼BA) 9; MNI = [6, 54, 36], tcluster(32) = 2.90, k = 187], the lateral temporal cortex (MNI = [60, −27, 3], tcluster(32) = 2.96, k = 162; MNI = [54, −6, −12], tcluster(32) = 2.90, k = 100), the right temporal-parietal junction (local maxima, MNI = [57, −51, 33], tcluster(32) = 2.95, k = 62), and the inferior parietal lobule (MNI = [42, −78, 39], tcluster(32) = 3.08, k = 91; MNI = [48, −54, 48], tcluster(32) = 2.96, k = 123), respectively.
Figure 2.
Neural regions specific to controlled retrieval demands (A) and selection demands (B) for social information. The left anterior vlPFC (A) showed greater activity to controlled retrieval demand than to selection demand for social information, but at an uncorrected threshold. The dorsomedial prefrontal cortex (B) showed greater activity to both selection demand alone and in the comparison of selection demand with controlled retrieval demand (P< 0.05, FWER corrected).
Neural Regions Engaged by Increasing Demands for the Controlled Retrieval and Selection of Social Information
Next, we examined which regions were responsive to increasing demands for both controlled retrieval and selection combined (i.e. (1) + (2) using the description above). This showed activity in the lateral prefrontal cortex bilaterally (left hemisphere MNI = [−39, 27, 18], k = 107, tcluster(32) = 3.22; right hemisphere MNI = [51, 33, 24], k = 113, tcluster(32) = 2.92), the striatum (MNI = [9, 15, 0], k = 175, tcluster(32) = 3.07), the primary motor cortex (MNI = [−33, −21, 57], k = 279, tcluster(32) = 2.83), the cuneus (MNI = [12, −78, 12], k = 91, tcluster(32) = 2.93), and the lingual gyrus (MNI = [−18, −84, −9], k = 90, tcluster(32) = 2.90; all activations FWER corrected).
Discussion
This study began with the observation that research in social cognitive neuroscience has identified neural systems involved in making judgments about other people—and has differentiated these systems from the systems involved in generally nonsocial cognition—but it has not yet clarified the nature of the processes that these systems carry out. We helped address this issue by drawing an analogy between person judgments and semantic memory judgments more generally, which suggested that making judgments about social information may involve 2 kinds of control processes: Controlled retrieval, which retrieves more information from memory, and selection, which resolves conflict between competing alternatives to select the goal-relevant option (Thompson-Schill et al. 1997; Wagner et al. 2001; Badre et al. 2005; Badre and Wagner 2007). To examine the neural correlates of these processes as deployed in the social domain, we designed a task that manipulated controlled retrieval and selection demands when making judgments about other people. The results showed that selection over controlled retrieval engaged the dorsomedial prefrontal cortex and the temporoparietal junction within the social knowledge network (Fig. 2). A tentative finding was that controlled retrieval over selection was associated with activity in the left anterior vlPFC. This finding is bolstered by several studies in semantic memory, which have implicated the anterior vlPFC in controlled retrieval (Badre and Wagner 2007) using priming tasks (Wagner et al. 1997; Gold et al. 2006), paired-associate learning tasks (e.g. Danker et al. 2008), and studies that show impairment in controlled retrieval following disruption to the anterior vlPFC (e.g. Gough et al. 2005). Further, we found that increasing demands for both controlled retrieval and selection engaged the portions of mid-vlPFC among other regions (Fig. 3).
Figure 3.

Sagittal (A) and coronal (B) views of lateral prefrontal neural regions that respond to increasing memory retrieval demands for social information (controlled retrieval and selection demands combined; P < 0.05, FWER corrected).
Implications for Studying the Neural Basis of Person Judgments
The present results were motivated by the question of how distinct neural systems work together when making judgments about people. Prior studies often have focused on the question of which regions are distinctly involved in mental state or trait attribution in particular, or social cognition more broadly. The present study offers a complementary approach to fractionating the various processes that are involved in making a person judgment. It places equal importance on neural regions associated specifically with social cognition, such as dorsomedial prefrontal cortex, and on neural regions that are not so uniquely associated with social cognition, but are nevertheless activated when making social cognitive judgments, such as the vlPFC.
The findings suggest that the psychological act of making a social cognitive judgment relies on both of these systems, but for different reasons. Globally, we found portions of the vlPFC to be involved in using control processes to access and select information for social judgments. This suggests that these regions may involve selection, controlled retrieval, or both. The portions of these lateral prefrontal regions have also been associated with reasoning (Goel et al. 1997; Prabhakaran et al. 1997; Prado et al. 2011), including making causal inferences (Satpute et al. 2005), which may also be relied upon when making social judgments. More specific to controlled retrieval and selection, however, we found that an anterior portion of vlPFC may support control processes used to retrieve information about people. In contrast, the social knowledge network, centered on the dorsomedial prefrontal, was particularly associated with the selection of information that is most relevant to the social judgment at hand.
Prior studies in the neuroscience of person judgments have often compared conditions where participants make social judgments with conditions where they make nonsocial judgments (Kelley et al. 2002; Mason 2004; Mitchell et al. 2005; Zahn et al. 2007), or have directly compared 2 kinds of social judgment (Gorno-Tempini et al. 1998; Shah et al. 2001; Lieberman et al. 2004; Ochsner et al. 2004, 2005; Lieberman et al. 2007; Rameson et al. 2010; Spunt et al. 2011; Denny et al. 2012). In general, such studies have found activation differences in both medial and lateral portions of the prefrontal cortex. While a role for the dorsomedial prefrontal cortex in memory for social information has been proposed in earlier work (Mitchell et al. 2002; Lieberman et al. 2004), the present findings extend this idea by proposing that the dorsomedial prefrontal cortex is specifically engaged by the selection of social information rather than the controlled retrieval of social information.
The results also showed that selection engaged other regions commonly associated with the social knowledge network, too, such as the temporoparietal junction (Saxe and Wexler 2005). Indeed, we did not find that different regions in the social knowledge network were differentially responsive to controlled retrieval and selection. This finding suggests that regions in the social knowledge network may function more cohesively in selecting for social information.
Implications for the Cognitive Neuroscience of Semantic Memory
A current theme in the semantic memory literature is that strategic access to semantic memory involves distinct control processes (i.e. controlled retrieval and selection of semantic information), and that these processes are dissociable in the brain (Badre et al. 2005; Badre and Wagner 2007). Our study drew on the theory and the methods used in that research to extend this distinction to the social domain. Broadly, our findings support the view that, as with semantic memory, controlled retrieval and selection processes also engage different neural circuitry when making social judgments—although the nature of these differences varies across domains.
Consider, for example, that semantic memory studies (Badre and Wagner 2007) have found that controlled retrieval is associated with an anterior portion of the vlPFC. We found that a region of the left anterior vlPFC was similarly engaged by the controlled retrieval of social information. These results suggest that the controlled retrieval of social and semantic information may both rely on the anterior vlPFC. Studies of semantic memory also have found that selection is associated with a more dorsal area in the mid-vlPFC. While we found this area to be activated by selection and controlled retrieval for social information, we found in particular that selection for social information engaged the dorsomedial prefrontal cortex and temporoparietal junction in the social knowledge network.
Such potential differences are intriguing since a current focus of research in social cognitive neuroscience is the degree to which dorsomedial prefrontal cortex uniquely processes social information (Sergent et al. 1992; Mitchell et al. 2002; Van Overwalle 2009; Contreras et al. 2011). On the one hand, our findings suggest that the selection of social information engages dorsomedial prefrontal cortex may support the idea of greater functional specialization for this region. On the other hand, our findings also suggest that what places dorsomedial prefrontal cortex apart is not necessarily the presence of a distinct social cognitive process per se, but a difference in the kind of information being selected. That is, in accessing social or nonsocial/semantic information, the same kinds of processes (i.e. selection or controlled retrieval) may be engaged, but what differentiates these neural systems is the kind of semantic information that is being selected for be it social or nonsocial.
This perspective offers new insights into current themes on the organization of social knowledge in the brain. For instance, a recent meta-analysis of imaging studies on judgments about the self versus of others (Denny et al. 2012) showed that both kinds of judgments activate dorsal and ventromedial prefrontal cortices, but that the dorsomedial prefrontal cortex is more frequently activated for judgments about others and ventromedial prefrontal cortex for judgments about the self (also see Mitchell et al. 2005). The present results suggest that judgments about others may depend more than that about the self on the controlled selection of social information. However, we did not see any differences in controlled retrieval or selection in the ventromedial prefrontal cortex. One possibility is that ventromedial prefrontal cortex is specifically involved in the selection of emotional and/or bodily state information (Fellows and Farah 2003; Lieberman et al. 2007; Ochsner et al. 2009; Schiller and Delgado 2010). This possibility could be tested in future work. Indeed, though the present study was designed to compare controlled retrieval and selection demands within the domain of social knowledge, a future study that directly compares these demands across the domains of social, semantic, and bodily/affective information, would be of interest.
Implications for Clinical Populations
Several clinical populations show abnormal processing of social information including autism spectrum disorder (Baron-Cohen et al. 2001), borderline personality disorder (Benjamin and Wonderlich 1994), and schizophrenia (Penn et al. 1997). Intriguingly, these populations also show abnormal activity in both the dorsomedial prefrontal cortex and vlPFC (Hadjikhani and de Gelder 2003; Ochsner 2008; Pinkham et al. 2008). The present study makes 2 key suggestions for future studies of these clinical groups. The first concerns the fact that much of the literature has focused on relating activity in the social knowledge network to social cognitive deficits in clinical populations. Our findings suggest that future work could look beyond the social knowledge network as well, to examine the ways in which social cognitive deficits also may also stem from domain general retrieval mechanisms in vlPFC. The second is that deficits in social cognitive abilities may be thought of as arising from deficits in specific constituent processes in the controlled retrieval or selection of social information. As such, the findings of this study may help generate new hypotheses for why some populations have difficulties in making social judgments and may help to better characterize what the underlying cognitive nature of these deficits may be.
Conclusions and Future Directions
Overall, this study suggests that our ability to make judgments about and understand others involves not only regions implicated in the social knowledge network that are used to select task-appropriate social information, but also involves domain general control mechanisms used to retrieve this information in the first place. Apart from these insights, the study also highlights future questions to be addressed and the possibility that social cognitive deficits are related to decrements in these specific processes. Future studies may benefit from the approach of manipulating the same processing demands across both social and nonsocial semantic knowledge domains to determine how neural activity in both domain general and domain specific neural systems plays a central role in the ability to make judgments about people.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by National Institute of Health (1 R01 MH076137-01 A1) awarded to K.O.
Supplementary Material
Notes
We thank Leor Hackel and Isabelle Carren-LeSauter for assistance in stimulus preparation and pilot testing, Andrew Kogan for assistance in MRI data acquisition, and Jochen Weber for assistance in MRI data analysis. Conflict of Interest: None declared.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Benjamin LS, Wonderlich SA. Social perceptions and borderline personality disorder: the relation to mood disorders. J Abnorm Psychol. 1994;103:610–624. doi: 10.1037//0021-843x.103.4.610. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Cloutier J, Kelley WM, Heatherton TF. The influence of perceptual and knowledge-based familiarity on the neural substrates of face perception. Soc Neurosci. 2011;6:63–75. doi: 10.1080/17470911003693622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JM, Banaji MR, Mitchell JP. Dissociable neural correlates of stereotypes and other forms of semantic knowledge. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr053. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Gunn P, Anderson JR. A rational account of memory predicts left prefrontal activation during controlled retrieval. Cereb Cortex. 2008;18:2674–2685. doi: 10.1093/cercor/bhn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of "theory of mind" in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind”. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. The seats of reason? An imaging study of deductive and inductive reasoning. Neuroreport. 1997;8:1305–1310. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings: a functional neuroimaging study of semantically unique items. Brain. 2001;124:2087–2097. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS. The neural systems sustaining face and proper-name processing. Brain. 1998;121:2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Keightley ML. Studies of altered social cognition in neuropsychiatric disorders using functional neuroimaging. Can J Psychiatry. 2002;48:327–336. doi: 10.1177/070674370204700403. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, de Gelder B. Seeing fearful body expressions activates the fusiform cortex and amygdala. Curr Biol. 2003;13:2201–2205. doi: 10.1016/j.cub.2003.11.049. [DOI] [PubMed] [Google Scholar]
- Heberlein AS, Saxe RR. Dissociation between emotion and personality judgments: convergent evidence from functional neuroimaging. Neuroimage. 2005;28:770–777. doi: 10.1016/j.neuroimage.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self ? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Satpute AB. Evidence-based and intuition-based self-knowledge: an FMRI study. J Pers Soc Psychol. 2004;87:421–435. doi: 10.1037/0022-3514.87.4.421. [DOI] [PubMed] [Google Scholar]
- Mason MF. Thinking about actions: the neural substrates of person knowledge. Cereb Cortex. 2004;14:209–214. doi: 10.1093/cercor/bhg120. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell J. Inferences about mental states. Philos Trans Roy Soc B. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA. 2002;99:15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Macrae N, Banaji MR. Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26:251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Current directions in social cognitive neuroscience. Curr Opin Neurobiol. 2004;14:254–258. doi: 10.1016/j.conb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64:48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JD. Neural systems supporting the control of affective and cognitive conflicts. J Cogn Neurosci. 2009;21:1842–1855. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121:114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postman L, Keppel G. Norms of word association. New York (NY): Academic Press; 1970. [Google Scholar]
- Prabhakaran V, Smith JA, Desmond JE, Glover GH, Gabrieli JD. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven's Progressive Matrices Test. Cognitive Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Prado J, Chadha A, Booth JR. The brain network for deductive reasoning: a quantitative meta-analysis of 28 neuroimaging studies. J Cogn Neurosci. 2011;23:3483–3497. doi: 10.1162/jocn_a_00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson LT, Satpute AB, Lieberman MD. The neural correlates of implicit and explicit self-relevant processing. Neuroimage. 2010;50:701–708. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Fenker DB, Waldmann MR, Tabibnia G, Holyoak KJ, Lieberman MD. An fMRI study of causal judgments. Eur J Neurosci. 2005;22:1233–1238. doi: 10.1111/j.1460-9568.2005.04292.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in "theory of mind". Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It's the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;1:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124:804–815. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20:813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cereb Cortex. 2010;20:3005–3013. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Satpute AB, Lieberman MD. Identifying the what, why, and how of an observed action: an fMRI study of mentalizing and mechanizing during action observation. J Cogn Neurosci. 2011;23:63–74. doi: 10.1162/jocn.2010.21446. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Fujii T, Fukatsu R, Otsuki T, Okuda J, Umetsu A, Suzuki K, Tabuchi M, Yanagawa I, Nagasaka T, et al. Neural basis of the retrieval of people's names: evidence from brain-damaged patients and fMRI. J Cogn Neurosci. 2002;14:922–937. doi: 10.1162/089892902760191144. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JD. Semantic repetition priming for verbal and pictorial knowledge: a functional MRI study of left inferior prefrontal cortex. J Cogn Neurosci. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proc Natl Acad Sci USA. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.