Abstract
The motivation of getting rewards or avoiding punishments reinforces learning behaviors. Although the neural mechanisms underlying the effect of rewards on episodic memory have been demonstrated, there is little evidence of the effect of punishments on this memory. Our functional magnetic resonance imaging (fMRI) study investigated the effects of monetary rewards and punishments on activation during the encoding of source memories. During encoding, participants memorized words (item) and locations of presented words (source) under 3 conditions (Reward, Punishment, and Control). During retrieval, participants retrieved item and source memories of the words and were rewarded or penalized according to their performance. Source memories encoded with rewards or punishments were remembered better than those without such encoding. fMRI data demonstrated that the ventral tegmental area and substantia nigra and nucleus accumbens activations reflected both the processes of reward and punishment, whereas insular activation increased as a linear function of punishment. Activation in the hippocampus and parahippocampal cortex predicted subsequent retrieval success of source memories. Additionally, correlations between these reward/punishment-related regions and the hippocampus were significant. The successful encoding of source memories could be enhanced by punishments and rewards, and interactions between reward/punishment-related regions and memory-related regions could contribute to memory enhancement by reward and/or punishment.
Keywords: functional magnetic resonance imaging, punishment, reward, source memory
Introduction
The motivation of receiving rewards or avoiding punishments enhances learning performance in experimental animals (Rolls 2007). Although human functional neuroimaging studies have demonstrated the neural mechanisms related to the effect of the motivation of receiving rewards on episodic memory (Adcock et al. 2006; Shigemune et al. 2010), there is little evidence of the effect of the motivation of avoiding punishment on this memory. This functional magnetic resonance imaging (fMRI) study investigated the effects of the motivation of monetary rewards and punishments on neural activation during the encoding of item and source memories.
The reward-related enhancement of episodic memory could be explained by the modulatory effect of the dopaminergic pathway on memory-related brain regions. Previous neurophysiological studies suggest that the dopaminergic neurons could code the predictions and prediction errors of rewards, and that these processes could reinforce the approaching behaviors to rewards (Schultz et al. 1997; Schultz 1998). The reward-related information is processed in the dopaminergic pathway, where the dopaminergic neurons in the ventral tegmental area and substantia nigra (VTA/SN) are projected to the nucleus accumbens (NA) and medial orbitofrontal cortex (mOFC; Olds and Milner 1954; Haber and Knutson 2010). There is also anatomical evidence that the VTA/SN dopaminergic neuron maintains direct projections to the hippocampus as a memory-related region (Carter and Fibiger 1977; Amaral and Cowan 1980; Swanson 1982; Samson et al. 1990; Gasbarri et al. 1991; Gasbarri, Packard, et al. 1994; Gasbarri, Verney, et al. 1994; Lewis et al. 2001). Functional neuroimaging studies have shown that these reward-related regions are activated together with the memory-related hippocampal region, which is involved in the vivid remembering of memory details including source or contextual information of events (Wittmann et al. 2005; Adcock et al. 2006; Tsukiura and Cabeza 2008; Wittmann et al. 2008; Shigemune et al. 2010; Tsukiura and Cabeza 2011). The interaction between the reward-related VTA/SN region and the hippocampus has been identified in animal studies, where dopaminergic neurons in the VTA/SN modulate hippocampal activities, and the interaction contributes to the hippocampus-dependent learning and memory (Gasbarri et al. 1996; O'Carroll et al. 2006; Martig and Mizumori 2011; Nazari-Serenjeh et al. 2011). Interactions between reward-related regions, including VTA/SN, NA, or mOFC, and recollection-related hippocampal regions could be important in the enhancement of memory details by rewards.
The motivation of avoiding punishments and receiving rewards reinforces learning behaviors (Seymour et al. 2007). As memory enhancement by monetary rewards is modulated by an interaction between reward-related and memory-related brain regions, punishment-related enhancement of episodic memory could be explained by interactions between regions associated with the processing of punishments and regions associated with the processing of memory. Previous neuroimaging studies have demonstrated the involvement of VTA/SN and NA in the prediction of both rewards and punishments (Knutson et al. 2000; Samanez-Larkin et al. 2007; Wrase et al. 2007; Carter et al. 2009). Thus, VTA/SN and NA could show significant activations during the prediction of both punishments and rewards.
Another region that may be associated with the prediction of receiving punishments is the insula. There is cognitive neuroscience evidence that insular activation is induced by the processing of pain or aversion, and that the activation predicts subsequent avoidance behaviors (Knutson and Greer 2008). Neuroscientific studies involving experimental animals have demonstrated that the insular cortex is anatomically and functionally connected with the hippocampus (Pribram and Maclean 1953; Friedman et al. 1986; Rutecki et al. 1989), and that the insular cortex collaborates with the hippocampus during memorizing aversive tastes (Yefet et al. 2006). Functional neuroimaging studies involving humans have reported that greater activations in the insular cortex have been found in the successful encoding of aversive events or faces with bad impressions (Rasch et al. 2009; Tsukiura et al. 2012). Taken together, these results may indicate that memory enhanced by the motivation of avoiding punishments could be modulated by interactions between the VTA/SN, NA, or insular regions, which are associated with the prediction of punishments, and the hippocampus, which is associated with the processing of memory details.
In view of the previous studies mentioned above, we hypothesized that the enhancing effects on episodic memory due to the motivation of receiving rewards and avoiding punishments could reflect an influence of the VTA/SN, NA, mOFC, and insular regions, which are involved in the prediction of rewards and/or punishments, on the hippocampal and parahippocampal memory system and, in particular, on a region strongly associated with the successful encoding of memory for contextual details (recollection): The hippocampus and parahippocampal cortex (PHC; Yonelinas 2002; Davachi 2006; Diana et al. 2007; Eichenbaum et al. 2007). In functional neuroimaging studies, where study-phase activation is analyzed as a function of memory performance in the later test (subsequent memory paradigm), study-phase hippocampal and parahippocampal activations predicted subsequent memory for item with source as contextual details (Davachi et al. 2003; Ranganath et al. 2004; Sommer et al. 2005; Peters et al. 2007), or for items “Remembered” in the Remember/Know paradigm (Uncapher and Rugg 2005; Johnson and Rugg 2007; Otten 2007). The study-phase hippocampal activation also predicts subsequent retrieval success with high confidence (Kim and Cabeza 2007), which is an indication of vivid remembering or recollection. Thus, we predicted that the recollection of episodic details could be enhanced by the prediction of rewards and punishments, and that the memory enhancement could be mediated by interactions between the hippocampal region associated with the successful encoding of memory details and the VTA/SN, NA, mOFC, and insular regions associated with the processing of rewards and/or punishments.
To investigate our hypothesis, participants were scanned during the encoding of both words (item) and places where the words were presented (source) under 3 different conditions (Reward, Control, and Punishment; Fig. 1). Participants were rewarded or penalized in relation to their subsequent memory performance of item and source retrieval. On the basis of the aforementioned research, we made 3 predictions. First, VTA/SN, NA, and mOFC would be identified as common regions of activation in predicting both rewards and punishments, and the insula would show significant activations specific to the prediction of punishments. Second, activation in the hippocampus and PHC would increase as a function of subsequent retrieval performance of both item and source memories. Third, interaction between these reward/punishment-related and memory-related hippocampal regions would be significant during the successful encoding of item and source memories.
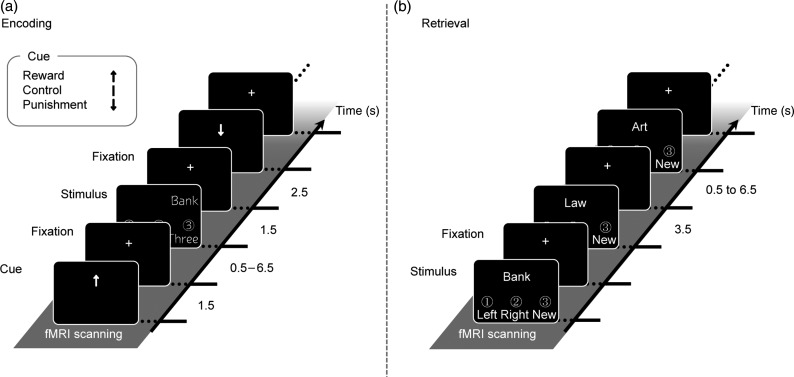
Figure 1.
Experimental design of encoding and retrieval blocks. (a) During encoding, Japanese words were presented on the left or right side of the screen in 3 different font types. Participants were instructed to memorize the words (item) and on which side (left or right) they were presented (source) by judging font types. The cues indicated the 3 different conditions (Reward, Punishment, and Control). Participants were told that they would be rewarded when they could remember item and/or source encoded in the Reward condition, and that they would be penalized when they could not remember item and/or source encoded in the Punishment condition in the subsequent retrieval blocks. (b) During retrieval, old and new words were randomly presented in the center of the screen. Participants were required to judge whether the words had been previously presented and on which side they were presented during encoding. All labels were actually presented in Japanese. English is used here for illustration purposes only.
Materials and Methods
Participants
Thirty right-handed, college-aged participants (8 males and 22 females; mean age 20.7 years; range 19–24 years) were recruited from the Tohoku University community and were paid for their participation. All participants were healthy, native Japanese speakers, with no history of neurological or psychiatric disorders. The data from 5 participants were excluded from analyses because 2 fell asleep during fMRI scanning and 3 showed low memory performance, in which 1 participant yielded higher false alarm rates than hit rates in item memories and 2 produced higher false alarm rates than hit rates in source remembering. Thus, our analyses included 25 participants (5 males and 20 females; mean age 20.7 years; range 19–24 years). All participants gave informed consent to a protocol approved by the Institutional Review Board of Tohoku University School of Medicine.
Experimental Tasks
Experimental tasks in this study included encoding and retrieval phases, which were alternated across 6 blocks, each retrieval block testing memory encoded in the previous encoding block (i.e., 3 encoding-retrieval sessions). Figure 1 illustrates examples of the stimuli in the encoding and retrieval phases. fMRI activations were measured in blocks of both encoding and retrieval, which were separated by an approximately 1-min interval. In each block, our fMRI experiment was designed by an event-related procedure. In each trial during the encoding blocks, first, a cue which indicated the conditions of Reward (upward arrow), Control (vertical bar), or Punishment (downward arrow) was presented on the screen for 1500 ms. These conditions were randomly assigned to each of the encoding trials. After the presentation of the cue, a fixation interval was presented for variable durations (jittered from 500 to 6500 ms), and then a Japanese word (Itsukushima et al. 1991) was presented for 1500 ms on the right or left side of the screen. In this phase, participants were required to encode the words (item) and the side (left or right) on which the words were presented (source) by judging the font types of target words. For the judgment of font types, 3 Japanese font types were prepared, and participants judged which font was the same as that of the target word in each case by pressing 1 of the 3 buttons (font types: HG gothic M, MS mincyo, and MOTOYA birch1). This task of font judgment was done to maintain the participants' attention on the target words. In this encoding phase of each block, participants were required to learn 90 Japanese words and the side on which each word was presented. The 3 word lists, which were used in each condition of Reward, Control, and Punishment, were matched in terms of frequency, imagery, and concreteness scores and were counterbalanced across participants.
During the retrieval phase of each block, participants were randomly presented with 90 learned and 45 new words one by one for 3500 ms (interstimulus interval was jittered from 500 to 6500 ms) and were required to judge whether or not each word had been learned in the previous encoding block and, if the words were learned, which side, left or right, of the screen they had been shown on during the previous encoding block. Participants were rewarded if they could successfully retrieve item and/or source information of target words when they were encoded in the Reward condition (correct retrieval of item: +50 yen and correct retrieval of source: +50 yen for 1 trial). If participants failed to retrieve item and/or source information of the target words, which were encoded in the Punishment condition, they were penalized (incorrect retrieval of item: −50 yen and incorrect retrieval of source: −50 yen for 1 trial). For words encoded in the Control condition, participants were neither rewarded nor penalized, whether or not they could remember item and/or source information of the target words. For new words, participants were rewarded for correct rejection responses and were penalized for false alarm responses (correct rejection: +66.7 yen and false alarm: −33.3 yen for 1 trial). This process prevented the participants from relying on a strategy of obtaining rewards and avoiding punishments by pressing only the “old” buttons. Participants started the experimental tasks with 0 yen, and then earned or lost real money as reward or punishment according to their retrieval performance.
fMRI Data Acquisition and Analysis
All MRI data acquisition was conducted with a 3-T Philips Achieva scanner. Stimuli were visually presented through a projector and back-projected onto a screen. Participants viewed the stimuli via a mirror attached with the head coil of an MRI scanner. Behavioral responses were recorded using a 4-button fiber optic response box (Current Designs, Inc., Philadelphia, PA, USA). Scanner noise was reduced with earplugs, and head motion was minimized using foam pads and a headband. Anatomical scans began by first acquiring a T1-weighted sagittal localizer series. Second, functional images were acquired utilizing echo-planar functional images (EPIs) sensitive to blood oxygenation level-dependent contrast [64 × 64 matrix, repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 70°, field of view (FOV) = 24 cm, 34 slices, 3.75 mm slice thickness]. Finally, high-resolution T1-weighted structural images (magnetization prepared rapid acquisition with gradient echo, 240 × 240 matrix, TR = 6.5 ms, TE = 3 ms, FOV = 24 cm, 162 slices, 1.0 mm slice thickness) were collected.
The preprocessing and statistical analyses for all images were performed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (www.mathworks.com). In the preprocessing analysis, images were corrected for slice-timing and head motion, and then spatially normalized into the Montreal Neurological Institute (MNI) EPI template. High-resolution T1-weighted structural images were not used in the spatial normalization process. After the spatial normalization, fMRI images were reformatted into isometric voxels (3.75 × 3.75 × 3.75 mm), and spatially smoothed using a Gaussian kernel of 8-mm full-width at half-maximum.
The fMRI analyses focused on data from the encoding phase in this study. Retrieval-related activations will be reported elsewhere. Statistical fMRI analyses were performed first at the subject level and then at the group level. At the subject level, fixed-effect analyses were performed. Stimulus onsets, but not cue onsets, were modeled as delta functions convolved with a canonical hemodynamic response function in the context of the general linear model (GLM). Confounding factors (head motion and magnetic field drift) were also included in the model. Activations associated with the prediction of reward/punishment and with the subsequent memory (Paller and Wagner 2002) were identified using the parametric analyses with 2 independent models. In the case of reward/punishment-related activation, all encoding trials were divided into 3 conditions of Reward, Control, and Punishment. Reward-related increase of activation was identified with a linear regressor of reward-related increase (Reward: 3, Control: 2, and Punishment: 1), whereas Punishment-related increase of activation was identified with a reverse pattern of the linear regressor (Reward: 1, Control: 2, and Punishment: 3). Increasing activation associated with the processing of both reward and punishment was computed with a V-shaped regressor (Reward: 2, Control: 1, and Punishment: 2). These analyses yielded t-contrasts reflecting reward-related and/or punishment-related activations at the subject level. In the case of subsequent retrieval performance of item and source memories, all encoding trials were categorized into 3 conditions of the successful encoding of item with source (IWS), successful encoding of item only (IO), and missed encoding (ME). Previous studies have shown that the recollection-based responses are primarily associated with the highest level of perceived oldness, whereas the familiarity-based responses increase gradually as a function of perceived oldness (Yonelinas 2001; Yonelinas et al. 2002; Wixted and Stretch 2004). Thus, encoding-success activation associated with the successful retrieval of IWS was identified with a quasiexponential regressor (ME: 1, IO: 2, and IWS: 9), which was employed in the previous fMRI studies (Daselaar et al. 2006; Tsukiura and Cabeza 2011). This analysis also yielded a t-contrast reflecting the subsequent retrieval success of item and source memories at the subject level.
At the group-level, random-effect analysis, by using contrast images from the subject-level, fixed-effect analyses, we conducted models of the 1-sample t-test for 3 contrasts of reward-/punishment-related activations, and for 1 contrast of encoding-success activations. To dissociate 3 patterns of activations associated with both reward and punishment, reward only, and punishment only, we employed a 2-step analysis. First, activations reflecting functions of reward-related increase, punishment-related increase, and V-shaped increase for both reward and punishment were analyzed by three 1-sample t-tests, where we employed the threshold of P < 0.005 (uncorrected) with a minimum cluster size of 10 contiguous voxels. Second, to find activations selective to reward or punishment, by using the exclusive masking methods, we removed activations reflecting the V-shaped regressor from activations associated with linear increases for reward or punishment. In this analysis, we employed a threshold of P < 0.01 as a masking contrast and a threshold of P < 0.005 (uncorrected) with a minimum cluster size of 10 contiguous voxels as masked contrast. The cluster size in these analyses corresponds to around P < 0.1 for cluster extent corrections for multiple comparisons by the Monte Carlo simulations of spatially correlated data (https://www2.bc.edu/sd-slotnick/scripts.htm). To avoid type I errors in activations, activations in VTA/SN, NA, mOFC, and insula, which were defined as regions-of-interest (ROIs) based on previous studies, were corrected by the small volume correction (SVC) method (Worsley et al. 1996) with a sphere of 15 mm radius [P < 0.05, family wise error (FWE) correction]. The MNI coordinates of VTA/SN, NA, and mOFC ROIs were decided by previous functional neuroimaging studies investigating the reward-related enhancement of memory (left VTA: −4 −15 −12; right VTA: 5 −14 −10; left NA: −11 4 0; right NA: 10 3 −7; and left mOFC: −8 28 −26; Adcock et al. 2006; Shigemune et al. 2010). The insular ROI coordinates of MNI for the SVC procedure were defined by the previous functional neuroimaging studies investigating the activation during anticipating monetary loss (left insular cortex: −45 −6 9 and right insular cortex: 45 −6 15; Wrase et al. 2007). The coordinates reported by the Talairach space were transformed into the MNI space by the tal2mni tool (http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/tal2mni.m). This procedure provided us with 3 dissociable activation patterns associated with the processing of both reward and punishment, reward only, and punishment only. In addition, to examine whether activation in these reward-related and/or punishment-related regions was affected by a successful encoding factor, we extracted mean activations (effect sizes) during reward and/or punishment conditions from clusters identified in this analysis and compared them among 3 successful encoding conditions (IWS, IO, and ME) by a 1-way repeated-measure analysis of variance (ANOVA).
To identify brain regions reflecting the subsequent retrieval success of memory for IWS information, a 1-sample t-test for contrasts of encoding-success activation was performed. In this analysis, we also employed the threshold of P < 0.005 (uncorrected) with a minimum cluster size of 10 contiguous voxels, which corresponds to around P < 0.1 for cluster extent corrections for multiple comparisons by the Monte Carlo simulations of spatially correlated data (https://www2.bc.edu/sd-slotnick/scripts.htm). In addition, on the basis of an a priori hypothesis, we employed a more lenient threshold of P < 0.005 with a minimum cluster size of 3 voxels within the hippocampus and PHC, which were defined as ROIs by the WFU PickAtlas (http://www.fmri.wfubmc.edu) and the AAL ROI package (Tzourio-Mazoyer et al. 2002). To avoid type I errors in activations, activations identified in these ROIs were corrected by the SVC method with a sphere of 15 mm radius (P < 0.05, FWE correction), in which the MNI coordinates of a center voxel (left hippocampus: −20 −11 −21; right hippocampus: 21 −12 −22; right entorhinal cortex: 20 −10 −33; and left PHC: −34 −43 −8) were defined by a previous fMRI study (Adcock et al. 2006). Original coordinates in the Talairach space were transformed into the MNI space by the tal2mni tool (http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/tal2mni.m). Mean activations (effect sizes) of Reward, Control, and Punishment conditions during IWS, which were extracted from the hippocampal and PHC clusters identified in this analysis, were also analyzed by a 1-way repeated-measures ANOVA with a factor of reward/punishment (Reward, Control, and Punishment). In this ANOVA analysis, we investigated whether activation in memory-related regions was also modulated by the effect of reward/punishment during encoding.
To examine the interaction between reward-/punishment-related and memory-related regions, which were identified in the prior analyses, we conducted a 2-step correlation analysis between activations in these regions across participants. In this analysis, during the successful encoding of IWS memories, we examined how reward-/punishment-related regions were functionally interacted to memory-related regions, because we hypothesized that memory enhancement by rewards and punishments could be observed only in recollection, not in familiarity, and that interactions between reward-/punishment-related and recollection-related regions could contribute to the memory enhancement by rewards and punishments. First, we defined activation clusters identified in both the reward-/punishment-related and recollection-related analyses as ROIs, from which mean activations (effect sizes) were extracted during IWS in all participants. Second, by using the values of activations in IWS conditions, we computed the Pearson correlations between these ROIs, and then examined whether or not the correlation coefficients (r) were significant in each correlation.
Moreover, to examine whether correlations between reward-/punishment-related and memory-related regions during the successful encoding of IWS memories are modulated separately by the effects of rewards or punishments during encoding, we performed additional correlation analyses with 2 steps. First, we extracted mean activations (effect sizes) from ROIs, which were defined as an activation cluster in the 2 models of parametric modulation analysis, in each condition of Reward, Punishment, and Control during IWS by each participant. Second, using the values of activations in each condition of Reward, Punishment, and Control during IWS, we computed the Pearson correlation coefficients between reward-/punishment-related and memory-related ROIs separately in these conditions, and then examined whether or not the correlation coefficients (r) across participants were significant in each condition. Previous neuroimaging studies investigating the effects of emotion on episodic memory have employed similar correlation analysis and have shown a potential interaction between emotion-related regions such as the amygdala and memory-related regions such as the hippocampus (Dolcos et al. 2004; Richardson et al. 2004).
To further investigate interactions between memory-related and reward/punishment-related regions in each condition of Reward, Punishment, and Control during IWS, we employed a 3-step correlation analysis based on individual trial activations (Rissman et al. 2004; Daselaar et al. 2006; Ritchey et al. 2008; St Jacques et al. 2009). First, we created a GLM, in which each individual trial was modeled by a separate covariate. This yielded different parameter estimates for each individual trial in each individual participant. Second, parameter estimates from the seed voxels of the hippocampus and PHC, which were identified in our parametric modulation analysis, were correlated with trial-by-trial parameter estimates from all other voxels, and then contrast images reflecting correlation patterns related to each seed voxel of the hippocampus and PHC for each trial type (Reward, Punishment, and Control in IWS) were generated in individual participants. Finally, to investigate the effects of reward/punishment and memory-related region factors on correlation patterns associated with the seed voxels, using the contrast images reflecting correlations in each participant, we conducted a 2-way repeated-measures ANOVA with the factors of reward/punishment (Reward, Control, and Punishment) and memory-related region (hippocampus and PHC).
In this analysis, first, to identify regions showing higher correlation in both Reward and Punishment than in Control, a main effect (F-contrast) of reward/punishment was masked inclusively with 2 t-contrasts of Reward versus Control and Punishment versus Control. Similarly, to identify regions showing higher correlation in Reward than in Punishment and Control, a main effect (F-contrast) of reward/punishment was masked inclusively with 2 t-contrasts of Reward versus Control and Reward versus Punishment, whereas regions related to higher correlation selectively in Punishment were identified in a main effect (F-contrast) of reward/punishment masked inclusively with 2 t-contrasts of Punishment versus Control and Punishment versus Reward. Second, we explored regions showing higher correlation associated with the hippocampus than with the PHC. This analysis was performed in the main effect (F-contrast) of memory-related region masked inclusively with a t-contrast of hippocampus versus PHC. Third, to identify regions showing both reward-related and punishment-related enhancement of correlation only with the hippocampus, an interaction (F-contrast) between reward/punishment factor and memory-related region factor was masked inclusively with 2 t-contrasts of Reward versus Control and Punishment versus Control in the hippocampus-related correlations. Regions reflecting a reward-related increase of correlation with the hippocampus only were analyzed in an interaction (F-contrast) between the 2 factors masked inclusively with 2 t-contrasts of Reward versus Control and Reward versus Punishment in the hippocampal correlations, whereas regions related to punishment-related increase of correlation with the hippocampus only were identified in an interaction (F-contrast) between the 2 factors masked inclusively with 2 t-contrasts of Punishment versus Control and Punishment versus Reward in the hippocampal correlations. Statistical thresholds were set in P < 0.05 for both F-contrasts (uncorrected with a minimum cluster size of 2 contiguous voxels) and t-contrasts. Regions showing a significant correlation were masked with ROIs as a sphere of 15 mm radius, in which the coordinates of center voxels were defined in our results (right VTA/SN: 8 −11 −8; right NA: 11 8 −15; and insula: 45 0 19), or in previous studies (left VTA: −4 −15 −12; right VTA: 5 −14 −10; left NA: −11 4 0; right NA: 10 3 −7; left mOFC: −8 28 −26; left insular cortex: −45 −6 9; and right insular cortex: 45 −6 15; Adcock et al. 2006; Wrase et al. 2007; Shigemune et al. 2010). The SVC for these regions showing significant correlations was performed with a sphere of 15 mm radius with center at the coordinate of peak voxel (P < 0.05).
Results
Behavioral Results
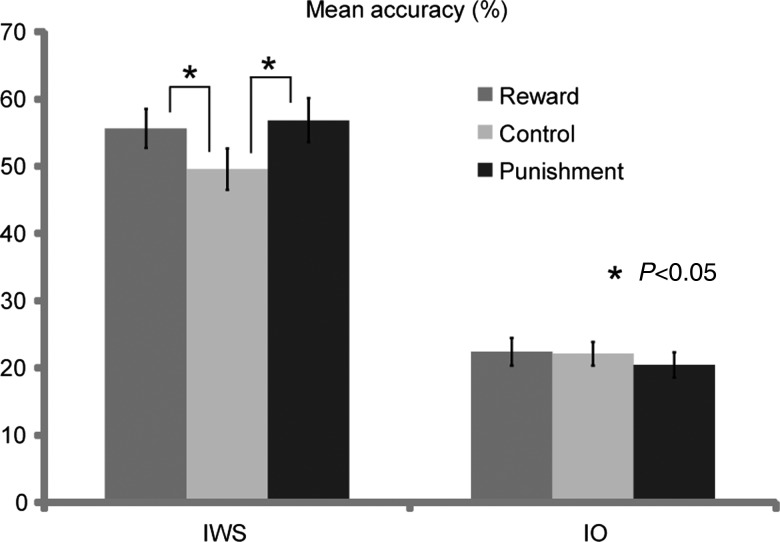
Table 1 shows accuracy in the retrieval phase and response times (RTs) in the encoding and retrieval phases. The application of reward and punishment during encoding enhanced the retrieval accuracy of IWS memories, but this enhancement was not found in the retrieval of IO memories. A 2-way repeated-measures ANOVA, which included 2 factors of reward/punishment (Reward, Control, and Punishment) and memory performance (IWS and IO), demonstrated significant main effects of reward/punishment (F2,48 = 6.23, P < 0.01) and memory performance (F1,24 = 63.44, P < 0.01), and a significant interaction between the 2 factors (F2,48 = 6.93, P < 0.01). Post hoc tests showed a significant difference in accuracy between Reward and Control (P < 0.05), and Punishment and Control (P < 0.05) in the IWS condition, but not in the IO condition (Fig. 2). This finding is consistent with our assumption that the enhancing effect of reward and/or punishment on memory is mediated by the retrieval of memory details or recollection.
Table 1.
Behavioral results
| IWS | IO | ME | |
|---|---|---|---|
| Number of trials | |||
| Reward | 47.5 (2.6) | 18.7 (1.8) | 18.7 (1.8) |
| Control | 41.9 (2.9) | 20.0 (1.8) | 23.5 (2.5) |
| Punishment | 50.0 (3.0) | 17.6 (1.5) | 17.8 (1.8) |
| Proportion of trials (%) | |||
| Reward | 55.8 (2.9) | 22.0 (2.0) | 24.4 (2.4) |
| Control | 48.9 (3.3) | 23.4 (2.1) | 26.5 (2.5) |
| Punishment | 58.0 (3.1) | 20.7 (1.8) | 23.9 (2.6) |
| RT during encoding (ms) | |||
| Reward | 1204.1 (22.2) | 1202.3 (28.7) | 1214.3 (24.9) |
| Control | 1212.1 (23.3) | 1191.7 (24.9) | 1210.7 (27.8) |
| Punishment | 1213.0 (24.0) | 1203.1 (23.6) | 1204.2 (25.8) |
| RT during retrieval (ms) | |||
| Reward | 1553.2 (50.6) | 1867.7 (77.0) | 1794.5 (79.0) |
| Control | 1615.6 (57.5) | 1910.3 (77.8) | 1773.4 (59.0) |
| Punishment | 1556.2 (53.2) | 1890.7 (74.3) | 1764.5 (74.6) |
All values are mean (SE).
IWS, item with source; IO, item only; ME, missed encoding.
Figure 2.
Mean proportion of retrieval accuracy for both the IWS and IO memories in the Reward, Punishment, and Control conditions. Error bars represent standard error. *P < 0.05.
RTs in the encoding and retrieval phases were compared by 2-way ANOVAs with 2 factors of reward/punishment (Reward, Control, and Punishment) and memory performance (IWS and IO). In the encoding phase, this analysis showed no significant main effect of each factor (reward/punishment: F2,48 = 0.23, P = 0.80 and memory performance: F1,24 = 1.19, P = 0.29) and no significant interaction between the 2 factors (F2,48 = 0.41, P = 0.67). In the retrieval phase, this analysis showed a significant main effect of memory performance (F1,24 = 45.67, P < 0.01), but a main effect of reward/punishment (F2,48 = 2.65, P = 0.08) and interaction between the 2 factors (F2,48 = 0.24, P = 0.79) was not significant.
fMRI Results
Confirming our first prediction, activations in the right VTA/SN and right NA were greater in the Reward and Punishment conditions than in the Control condition (Fig. 3). Significant activations in these regions were confirmed by the SVC procedures. As shown in Table 2, other regions showing greater activation during the predictions of both reward and punishment were also identified in the left inferior frontal, precentral, and middle temporal, globus pallidus regions and the right inferior occipital gyrus. In addition, the right insular cortex was identified as a punishment-selective region, where the activation increased as a linear function of punishment (Fig. 3). This activation was still significant after applying the SVC procedure. As shown in Table 3, reward-selective regions were also found in several regions, including the anterior cingulate gyrus, supplementary motor area, cuneus, claustrum, fornix, and cerebellum. In addition, using 1-way repeated-measures ANOVAs, we examined whether the right VTA/SN, right NA, and right insular activations identified in this analysis were also affected by a factor of successful encoding (IWS, IO, and ME). In this analysis, these regions showed no significant effect of the successful encoding factor (right VTA/SN: F2,24 = 0.59, P = 0.56; right NA: F2,24 = 2.01, P = 0.16; and right insular cortex: F2,24 = 0.78, P = 0.47).
Figure 3.
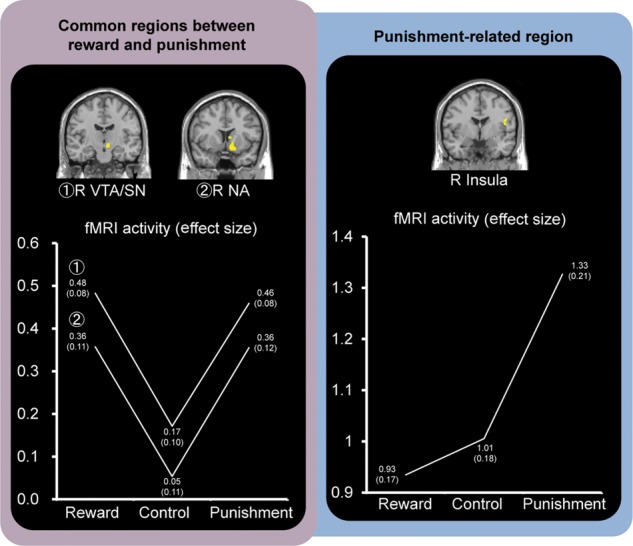
Activation images and activation profiles in reward-/punishment-related regions. Regions associated with both rewards and punishments were identified in the right VTA/SN and right NA regions. The right insular activation reflected a linearly increasing function of punishment. The numbers of each point show the mean effect size (SE) in each condition.
Table 2.
Regions associated with both reward and punishment
| Regions | L/R | BA | Z-score | Coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Nucleus accumbens | R | 3.45 | 11 | 8 | −15 | |
| Ventral tegmental area/substantia nigra | R | 3.38 | 8 | −11 | −8 | |
| Inferior frontal gyrus | L | 47 | 3.57 | −30 | 34 | 0 |
| Inferior frontal gyrus | L | 44 | 2.87 | −56 | 8 | 19 |
| Precentral gyrus | L | 6 | 3.31 | −15 | −11 | 68 |
| Middle temporal gyrus | L | 20 | 3.75 | −64 | −41 | −11 |
| Middle temporal gyrus | L | 39 | 2.98 | −41 | −64 | 23 |
| Inferior occipital gyrus | R | 18 | 3.40 | 30 | −90 | −8 |
| Globus pallidus | L | 3.50 | −15 | −4 | 4 | |
| Globus pallidus | L | 3.65 | −15 | −15 | −8 | |
BA, Brodmann area; L, left; R, right.
Table 3.
Reward-selective and punishment-selective regions
| Regions | L/R | BA | Z-score | Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Reward-selective regions | ||||||
| Anterior cingulate gyrus | R | 11 | 3.40 | 11 | 41 | 4 |
| Supplementary motor area | R | 6 | 3.11 | 15 | −23 | 53 |
| Cuneus | R | 17 | 3.02 | 15 | −105 | 0 |
| Claustrum | L | 3.15 | −26 | −8 | −15 | |
| Fornix | LR | 3.63 | −4 | 0 | 15 | |
| Cerebellar vermis | R | 3.19 | 4 | −34 | −11 | |
| Cerebellar hemisphere | L | 2.77 | −8 | −60 | −34 | |
| Punishment-selective regions | ||||||
| Insula | R | 48 | 3.28 | 45 | 0 | 19 |
BA, Brodmann area; L, left; R, right.
Confirming our second prediction, activation in the right hippocampus and left PHC during encoding predicted the subsequent retrieval success of source memories (Fig. 4). These activations were still significant after the SVC procedures. However, the right PHC activation was not significant under the SVC procedures. As shown in Table 4, other regions reflecting a quasiexponential function for the subsequent retrieval success of source memories were identified in the lateral and medial prefrontal regions, lateral temporal, parietal, and occipital regions, basal ganglia, and cerebellum. In addition, we investigated whether activations in the right hippocampus and left PHC identified in this analysis were modulated by a factor of reward/punishment (Reward, Control, and Punishment) by 1-way repeated-measures ANOVAs. In these analyses, the right hippocampus demonstrated a significant effect of the reward/punishment factor, where there were significant differences in activations between Reward and Control (P < 0.05), and Punishment and Control (P < 0.05; F2,24= 3.44, P < 0.05, Fig. 5). The left PHC showed no significant effect of the reward/punishment factor (F2,24 = 3.04, P = 0.07).
Figure 4.
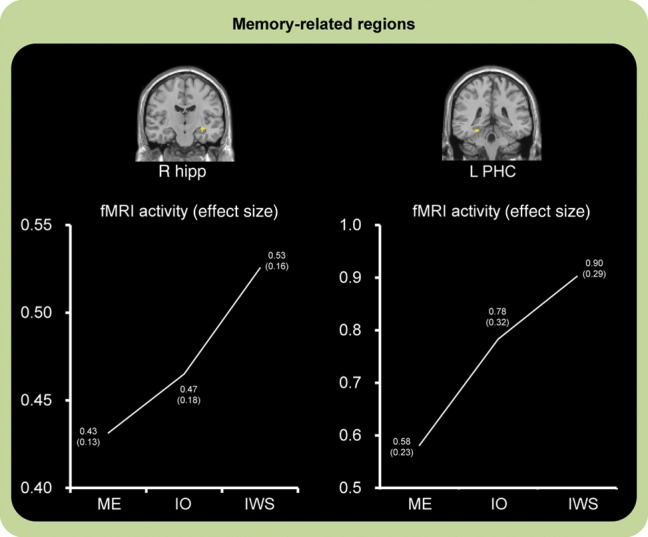
Activation images and activation profiles in encoding-related regions predicting successful retrieval of IWS memories. The right hippocampus and left PHC regions showed increasing activation associated with the subsequently successful retrieval of IWS memories. IWS: successful encoding of item with source memories, IO: successful encoding of IO memories, ME: missed encoding of memories. The numbers of each point show the mean effect size (SE) in each condition.
Table 4.
Regions showing a parametric effect of subsequent memory
| Regions | L/R | BA | Z-score | Coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Memory-related regions (predicted regions) | ||||||
| Hippocampus | R | 3.30 | 30 | −15 | −11 | |
| Parahippocampal cortex | R | 3.21 | 26 | −26 | −26 | |
| Parahippocampal cortex | L | 3.11 | −26 | −38 | −15 | |
| Memory-related regions (other regions) | ||||||
| Hippocampus | R | 3.30 | 30 | −15 | −11 | |
| Parahippocampal cortex | L | 3.27 | −19 | −41 | −15 | |
| Parahippocampal cortex | R | 3.14 | 19 | −8 | −30 | |
| Middle frontal gyrus | L | 46 | 2.87 | −38 | 56 | 11 |
| Inferior frontal gyrus | R | 45 | 3.79 | 53 | 26 | 19 |
| Inferior frontal gyrus | L | 45 | 5.50 | −56 | 23 | 26 |
| Precentral gyrus | L | 6 | 3.45 | −23 | −23 | 60 |
| Supplementary motor area | R | 6 | 2.97 | 19 | −19 | 53 |
| Medial orbitofrontal cortex | R | 11 | 2.77 | 15 | 26 | −11 |
| Paracentral lobules | L | 4 | 3.17 | −15 | −38 | 64 |
| Supplementary motor area | L | 6 | 3.67 | −4 | 15 | 53 |
| Supplementary motor area | L | 6 | 3.06 | −4 | −4 | 53 |
| Cingulate gyrus (middle part) | R | 24 | 3.74 | 8 | 4 | 30 |
| Temporal pole | R | 38 | 3.65 | 49 | 11 | −26 |
| Middle temporal gyrus | L | 21 | 2.66 | −60 | −23 | −8 |
| Middle temporal gyrus | R | 21 | 2.77 | 53 | −26 | −4 |
| Middle temporal gyrus | R | 21 | 2.79 | 41 | −68 | 15 |
| Middle temporal gyrus | L | 21 | 3.39 | −41 | −49 | 11 |
| Inferior temporal gyrus | L | 20 | 4.83 | −60 | −38 | −15 |
| Inferior parietal lobule | L | 40 | 4.23 | −38 | −38 | 41 |
| Middle occipital gyrus | L | 19 | 5.15 | −34 | −79 | 34 |
| Caudate nucleus | R | 3.48 | 23 | −4 | 19 | |
| Putamen | R | 3.41 | 23 | 15 | 11 | |
| Cerebellar hemisphere | L | 3.26 | −26 | −34 | −30 | |
BA, Brodmann area; L, left; R, right.
Figure 5.
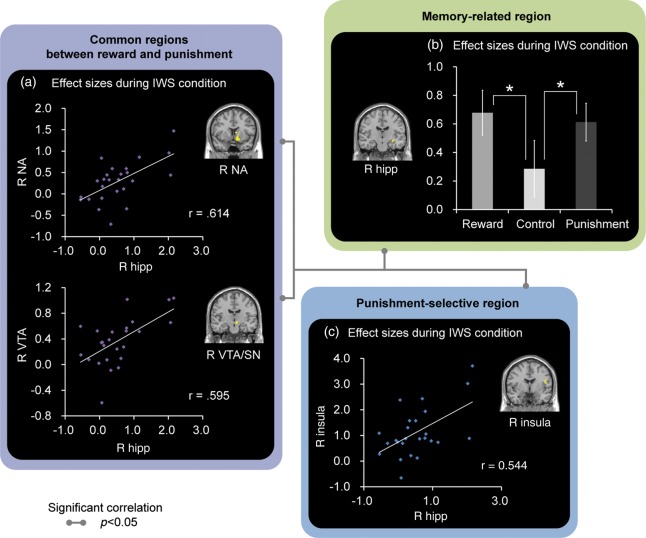
Correlation patterns between activations in the reward-/punishment-related and memory-related hippocampal regions and activation pattern in the hippocampal region. (a) Correlations between the reward-/punishment-related VTA and NA regions and the memory-related hippocampal region. (b) Correlation between the punishment-related insular and the memory-related hippocampal regions. Gray lines represent significant correlations between reward-/punishment-related and memory-related hippocampal regions (P < 0.05). (c) Right hippocampal activation, which reflected the successful encoding of IWS memories, was also affected by a reward/punishment factor, where the activation was greater during the successful encoding of IWS memories under the Reward and Punishment conditions than the Control condition. Error bars represent standard errors. *P < 0.05.
Confirming our third prediction, memory-related hippocampal activation was significantly correlated with activation in reward-related and/or punishment-related regions across participants (Fig. 5). Activation in the right hippocampus as IWS memory-related region showed significant correlations with that in the right VTA/SN and right NA as common regions between reward and punishment (right VTA/SN: r = 0.60, P < 0.05 and right NA: r = 0.61, P < 0.05), and that in the right insula as punishment-selective region (r = 0. 54, P < 0.05) in the IWS condition. The left PHC identified as an IWS memory-related region showed significant correlations with the right NA as common regions between reward and punishment (right NA: r = 0.60, P < 0.05) and with the right insula as a punishment-selective region (r = 0.49, P < 0.05) in the IWS condition. However, correlation patterns observed in the left PHC region were affected by one potential outlier. After removing the data of this outlier, no significant correlation was found between these regions (right NA: r = 0.36, P > 0.1 and right insula: r = 0.04, P > 0.1). The correlation coefficient between the left PHC and right VTA/SN was not significant (P > 0.1) in the IWS condition.
To investigate further whether correlations between memory-related hippocampus and reward-/punishment-related regions were modulated by the effects of rewards and/or punishments during the successful encoding of IWS memories, we performed additional correlation analyses separately in each condition of Reward, Punishment, and Control during IWS across participants. In all conditions of Reward, Punishment, and Control, activations in the right hippocampus were significantly correlated with those in the right NA (Reward: r = 0.74, P < 0.05; Punishment: r = 0.44, P < 0.05; and Control: r = 0.70, P < 0.05) and with those in the right insula (Reward: r = 0.50, P < 0.05; Punishment: r = 0.41, P < 0.05; and Control: r = 0.53, P < 0.05). Correlation coefficients between the right hippocampus and right VTA/SN were significant in Reward (r = 0.67, P < 0.05) and Control (Control: r = 0.76, P < 0.05), but not in Punishment (r = 0.33, P = 0.11). These findings suggest that the correlation patterns identified in the original analyses could be almost replicated even when conditions of Reward, Punishment, and Control were separately analyzed.
Additionally, correlation analyses based on individual trial activations demonstrated that the hippocampus showed a higher correlation with either reward-related or punishment-related regions than the PHC during the successful encoding of IWS memories. However, these patterns of correlations were not significant during the unsuccessful encoding of IWS memories (i.e., IO memories). As shown in Table 5, a main effect of memory-related region in a 2-way repeated-measures ANOVA for correlations during IWS showed that individual trial activations in the VTA/SN, NA, and insular cortex were more highly correlated with hippocampal activations than with PHC activations. We confirmed that the correlation patterns were identified in the left NA (−4, 8 −15), right VTA (8, −8, −8), and right insula (38, −4, 23) after applying the SVC methods. However, we did not find significant activations of any regions in a main effect of reward/punishment, and an interaction between the 2 factors of memory-related region and reward/punishment. To further investigate whether the correlation patterns identified in a main effect of memory-related region (hippocampus and PHC) were observed in the unsuccessful encoding of IWS memories (i.e., IO memories), we compared hippocampal-related correlations with PHC-related correlations in the same ROIs during IO. This analysis showed no significant correlation in any ROIs after the SVC methods. These results also confirmed the correlation patterns identified in the original correlation analysis. Results of trial-by-trial correlation analyses are summarized in Table 5.
Table 5.
Regions identified in the functional connectivity analysis
| Regions | L/R | Z-score | Coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| ROIs defined in our results | |||||
| Main effect of region | |||||
| Nucleus accumbens | L | 2.90 | −4 | 8 | −15 |
| Ventral tegmental area | R | 2.26 | 19 | −23 | −8 |
| Ventral tegmental area | R | 2.70 | 8 | −8 | −8 |
| Ventral tegmental areaa | R | 2.41 | 4 | −8 | 8 |
| Insula | R | 1.84 | 38 | −4 | 23 |
| Main effect of reward/punishment | |||||
| No significant activation | |||||
| Interaction between region and reward/punishment | |||||
| No significant activation | |||||
| ROIs defined in previous studies | |||||
| Main effect of region | |||||
| Nucleus accumbensb | L | 3.26 | −11 | 4 | 15 |
| Nucleus accumbens | L | 2.90 | −4 | 8 | −15 |
| Nucleus accumbens | R | 2.66 | 0 | 8 | −11 |
| Ventral tegmental area | R | 1.96 | 15 | −19 | −8 |
| Ventral tegmental area | R | 2.70 | 8 | −8 | −8 |
| Ventral tegmental area | R | 2.24 | 0 | −11 | 8 |
| Insula | L | 2.60 | −41 | 8 | 15 |
| Insula | R | 1.84 | 38 | −4 | 23 |
| Main effect of reward/punishment | |||||
| No significant activation | |||||
| Interaction between region and reward/punishment | |||||
| No significant activation | |||||
Note. Regions labeled with (a) or (b) included a part of the thalamus (a) and the caudate nucleus (b). However, these regions were not survived after the SVC method.
L, left; R, right.
Discussion
Three main findings emerged from the current study. First, activation in the VTA/SN and NA was greater for both conditions of Reward and Punishment than for Control, and activation in the insula selectively increased as a linear function of punishment. Second, activation in the hippocampus and PHC during encoding predicted the subsequent retrieval success of IWS memories. Third, correlations between activations in the hippocampus as an IWS memory-related and reward/punishment-related regions, including VTA/SN, NA, and insula, were significant during the successful encoding of IWS memories, and the hippocampal activation, which reflected the subsequent recollection process, was affected by a factor of reward/punishment during encoding. However, the IWS memory-related PHC activation, where a significant effect of the reward/punishment factor was not identified by ANOVA, showed no significant correlation with any reward/punishment-related regions. These 3 findings are discussed separately below.
Reward-/Punishment-Related Regions
The first main finding of our study was that the VTA/SN and NA regions showed increasing activation for predicting both rewards and punishments, and that activation in the insular cortex reflected a linearly increasing function for predicting punishments only. These findings suggest that the VTA/SN and NA regions could contribute to the prediction of both monetary rewards and punishments during memory encoding, and that the insular cortex could be involved in the prediction of monetary punishments during memory encoding.
The involvement of VTA and NA in the prediction of monetary rewards is consistent with neuroscientific evidence that reward-related information is processed in the dopaminergic pathway, where the dopaminergic neurons in the VTA regions are projected to the NA and mOFC regions (Olds and Milner 1954; Haber and Knutson 2010). Previous neurophysiological studies suggest that the dopaminergic neurons could code the prediction and prediction errors of rewards, and that the prediction and prediction errors of rewards could reinforce the approaching behaviors to rewards (Schultz et al. 1997; Schultz 1998). The VTA/SN and NA activations identified in the present study could reflect the prediction of reward-related information and could contribute to the generation of approaching behaviors to rewards.
One possible explanation for VTA/SN and NA activations in our study is the difference between primary and secondary rewards. For example, one fMRI study reported that NA activations were associated only with the processing of primary rewards (Beck et al. 2010), whereas another fMRI study showed significant NA activations during the processing of secondary rewards (Valentin and O'Doherty 2009). There is also fMRI evidence linking NA activations to coding the values of both primary and secondary rewards (Sescousse et al. 2010). These findings suggest that NA responses could not be modulated by the difference of primary and secondary rewards. A number of functional neuroimaging studies have demonstrated that the VTA/SN and NA codes the prediction and prediction errors of monetary rewards (Knutson, Adams, et al. 2001; Knutson, Fong, et al. 2001; Dreher et al. 2006; D'Ardenne et al. 2008; Staudinger et al. 2009; Valentin and O'Doherty 2009; Sescousse et al. 2010), which are consistent with our findings. Although further investigations are required to confirm whether the primary or secondary reward is more important in VTA/SN and NA activations, it is possible that VTA/SN and NA regions could be modulated by secondary rewards, or money.
In the present study, the mOFC region showed no increasing activation associated with the prediction of rewards. The absence of mOFC activation is inconsistent with previous functional neuroimaging studies linking this region to the prediction of rewards (O'Doherty, Critchley, et al. 2003; Cox et al. 2005; Hare et al. 2008; Bray et al. 2010). The difference in mOFC activation may be explained by low magnitude of rewards and greater demands of memory. Previous studies have reported increasing mOFC activation associated with magnitude of rewards and subjective pleasantness of rewards, suggesting that the mOFC region could code subjective reward values (O'Doherty et al. 2001; Kringelbach et al. 2003; O'Doherty, Winston, et al. 2003; Galvan et al. 2005). In our previous study (Shigemune et al. 2010), using almost the same paradigm as that used in the present study, we investigated the effects of monetary rewards on encoding-related activations and found greater mOFC activations during the encoding of pictures with high rewards than with low rewards. However, the magnitude of monetary rewards employed in our previous study was greater than that in the current study, and the memory demands were lower than those in the current study. Thus, the absence of mOFC activation in the current study could be caused by the attenuation of subjective rewarding values due to greater memory demands.
VTA/SN and NA also showed greater activation during the prediction of monetary punishments. This result is consistent with functional neuroimaging evidence, which demonstrated significant activations in the VTA/SN and NA regions during predictions of both rewards and punishments (Knutson et al. 2000; Samanez-Larkin et al. 2007; Wrase et al. 2007; Carter et al. 2009; Delgado et al. 2011). The responses of VTA/SN and NA regions to the prediction of punishments may be explained by the concept that avoidance of punishments may be processed as the obtainment of rewards (Kim et al. 2006). For example, one fMRI study demonstrated that VTA/SN and NA regions showed significant activations during the anticipation of punishments, but not during the acceptance of punishments (Bjork et al. 2010). This result could reflect worry by participants when they anticipate subsequent punishments about accepting those punishments as well an expectation of being able to avoid punishments.
Another fMRI study reported that NA activation was identified in the uncertain situation, where it is possible to avoid punishments, but activation in this region was not found in the situation in which there is no possibility of avoiding punishments (Cooper and Knutson 2008). In addition, there is functional neuroimaging evidence that the ventral striatum region showed decreasing activations in the processing of loss outcome (Delgado et al. 2000), and that the VTA/SN and NA regions were not involved in the processing of negative-reward information (Liu et al. 2007). Given that participants in the present study could avoid monetary punishments if they successfully remembered item and source memories encoded in the Punishment condition, and that the motivation for avoiding subsequent punishments could enhance the encoding process of IWS memories, the present findings of VTA/SN and NA activations could reflect the prediction of the possible avoidance of punishments rather than the acceptance of punishment outcomes. The prediction of avoiding possible punishments, which leads to total gain, may be processed as the prediction of rewards. However, further investigations would be required to clarify whether VTA/SN and NA activations are associated either with the prediction of avoiding punishments or with that of accepting punishments.
There are some electrophysiological studies in which responses in dopaminergic neurons are enhanced only by rewards, not by punishments (Ljungberg et al. 1992; Schultz 1998; Guarraci and Kapp 1999; Coizet et al. 2006), whereas other studies have reported that VTA includes 2 types of neurons, one of which is inhibited by punishments and the other is excited by punishments (Ungless et al. 2004; Matsumoto and Hikosaka 2009). The inconsistent results could possibly be explained by dissociable roles between excitable dorsolateral and inhibitable ventromedial VTAs (Matsumoto and Hikosaka 2009). In the present study, we found ventromedial VTA/SN activations associated with the motivation of both receiving rewards and avoiding punishments. These findings also support the interpretation that the present findings of VTA/SN and NA activations could reflect the possible avoidance of punishments, which are processed as the prediction of receiving rewards, rather than the general process of punishments, which could be involved in the dorsolateral VTA regions.
In the present study, we did not find amygdala activations associated with the prediction of both rewards and punishments. This finding is inconsistent with previous cognitive neuroscience studies involving experimental animals and human subjects, where the amygdala showed increasing activations in fear conditioning, aversive learning, appetitive learning, and memory motivated by punishments (Dolan 2000; Everitt et al. 2000; Gallagher 2000; LeDoux 2000; Murty et al. 2012). The lack of amygdala activations in our study could have 3 possible explanations. The first possible explanation is that the arousal of monetary rewards and punishments in our study may not be enough to give rise to significant activations in the amygdala. Functional neuroimaging studies have shown that the amygdala is involved in coding emotional arousal rather than emotional valence, and that amygdala activation is greater in the processing of high arousal stimuli than that of low arousal stimuli (Anderson et al. 2003; Small et al. 2003; Kensinger 2004; Lewis et al. 2007). In the present study, the outcome, that is, whether participants received rewards or avoided punishments in the subsequent retrieval phase, was uncertain in the encoding phase. This uncertainty may be the reason why there is no significant activation in the amygdala. The second possible explanation is the difference in incentive types between primary and secondary rewards/punishments. For example, one fMRI study reported that the amygdala showed significant activations associated with aversive conditioning by primary punishments such as electrical shock, but not by secondary punishments such as monetary loss (Delgado et al. 2011). Another fMRI study demonstrated that amygdala activation provoked by electrical shock was associated with the memory enhancement by punishments (Murty et al. 2012). However, there are other neuroimaging studies, indicating that activations in the amygdala are enhanced by monetary loss as secondary punishments (Breiter et al. 2001; Yacubian et al. 2006; Smith et al. 2009). Thus, further investigations would be required to clarify whether activations in the amygdala are affected by the difference between primary and secondary rewards/punishments. The third possible explanation is that the amygdala is involved in reward-motivated or punishment-motivated learning, but not in the episodic memory processes motivated by rewards or punishments. Functional neuroimaging studies have consistently shown no amygdala activations associated with the episodic memory processes motivated by rewards (Wittmann et al. 2005; Adcock et al. 2006; Tsukiura and Cabeza 2008; Wittmann et al. 2008; Shigemune et al. 2010; Tsukiura and Cabeza 2011). However, this account is not a sufficient basis on which to interpret the absence of amygdala activation in our study, because memory enhancement for emotional stimuli is modulated by amygdala–hippocampus interactions (Hamann et al. 1999; Dolcos et al. 2004), because amygdala activations aroused by positive emotion have a beneficial effect on memory enhancement by rewards (Wittmann et al. 2008), and amygdala activations aroused by negative emotion also contributes to memory enhancement by punishments using electrical shocks (Murty et al. 2012). Thus, the lack of amygdala activations in our study cannot simply be explained by in terms of a learning-episodic memory distinction.
Activation in the insular cortex reflected a linearly increasing function for punishment. This finding of insular activations is consistent with functional neuroimaging evidence linking this region to the processing of pain (Coghill et al. 1994; Ploghaus et al. 1999) or disgust (Phillips et al. 1997; Wicker et al. 2003; Nitschke et al. 2006). Roles of the insular cortex have also been identified in the processing of subsequent avoidance behaviors (Knutson and Greer 2008). The present finding of insular activation associated with the motivation of avoiding punishments could reflect the prediction of receiving punishments, which could make us avoid punishments. Therefore, the VTA/SN and NA regions could predict avoiding punishments as though it was the same as getting rewards, whereas the insular cortex as a punishment-selective region could contribute to the prediction of aversion to the perceived punishments and of behavior to be avoided.
Memory-Related Regions
The second main finding of our study was that activation in the hippocampus and PHC during encoding reflected a function of the subsequent retrieval success of item and source memories. The finding that the hippocampus and PHC showed the highest activation in IWS suggests that these regions could predict the subsequent recollection or retrieval of memory details including item and contextual information of an event.
The importance of the hippocampus and PHC in the successful encoding of item and source memories is consistent with functional neuroimaging evidence that activation in these regions during encoding reflected either the subsequent recollection of memories or the subsequent retrieval of IWS memories (Davachi et al. 2003; Ranganath et al. 2004; Weis et al. 2004; Daselaar et al. 2006; Kensinger and Schacter 2006; Montaldi et al. 2006; Uncapher et al. 2006; Wais 2008). Given that the human memory system could be divided into 2 independent processes, recollection and familiarity (Jacoby 1991; Yonelinas 2002), the finding of hippocampal and PHC activation could contribute to the subsequent retrieval of memory details, but not to the retrieval of memory items only. In addition, recent review studies have implied that the hippocampus could be involved in the association processes between item and source information, whereas the PHC region could contribute to the processing of source or contextual information (Davachi 2006; Diana et al. 2007; Eichenbaum et al. 2007; Mitchell and Johnson 2009). Activation in the hippocampus and PHC identified in the present study could reflect these dissociable processes between association and source information.
Interactions Between Reward-/Punishment-Related and Memory-Related Regions
The third main finding of our study was that activation in the hippocampus as a memory-related region showed significant correlations with that in reward/punishment-related regions including the VTA/SN, NA, and insula, and that the hippocampal activation during the successful encoding of item and source memories was also greater in the Reward and Punishment conditions than in the Control condition. This correlation pattern was identified in both levels of inter- and intraparticipants. However, such correlation patterns were not identified in the PHC as another memory-related region, and activations in the VTA/SN, NA, and insula were not affected by a successful encoding factor. These findings suggest that activation in reward/punishment-related regions could modulate source memory-related hippocampal activation, but not PHC activation.
The finding of significant correlations between the hippocampus and reward/punishment-related regions during the successful encoding of IWS memories is consistent with previous findings, where the hippocampus showed both anatomical and functional connections with regions including the reward circuit (Lisman and Grace 2005; Haber and Knutson 2010) and insular region (Pribram and Maclean 1953; Rutecki et al. 1989). For example, there is functional neuroimaging evidence that the enhancing effect of rewards on memory is greater in the recollection process than in the familiarity process, and this memory enhancement is modulated by interactions between reward-related regions, including VTA/SN, NA, and mOFC, and a recollection-related region including the hippocampus (Adcock et al. 2006; Tsukiura and Cabeza 2008, 2011). Other fMRI studies demonstrated that activations in the insula and hippocampus reflected the subsequent successful retrieval of aversive events and faces with bad impressions (Rasch et al. 2009; Tsukiura et al. 2012). Given that the hippocampus is involved in the processing of associations between item and source information, but not in the processing of source information itself (Davachi 2006; Diana et al. 2007; Eichenbaum et al. 2007; Mitchell and Johnson 2009), the present patterns of correlation identified in the hippocampus extend previous findings by showing that the monetary rewards and/or punishments processed in the reward/punishment-related circuits affected the association processes involved in the hippocampus. However, we did not find that correlations between reward-/punishment-related and memory-related regions were varied by the effects of a reward/punishment factor. This negative result may be due to lower statistical powers by a limited number of trials or participants. If correlations between reward-/punishment-related and memory-related regions are modulated by a factor of reward/punishment, the current study could be far stronger in association with our behavioral results. Further investigations would be required in future.
However, such correlation patterns were not found between the PHC and reward-/punishment-related regions. The findings are consistent with functional neuroimaging evidence that smiling facial expressions as a social reward enhanced activation in both the reward-related mOFC and memory-related hippocampal and parahippocampal regions, whereas the correlation enhancement by social rewards were identified only between mOFC and the hippocampus, and not between mOFC and PHC (Tsukiura and Cabeza 2008). The dissociable pattern of correlations within the hippocampal and parahippocampal regions suggests that the effects of reward-/punishment-related activations on memory-related activations could contribute to activations in the hippocampus, but not to those in the PHC. The association process involved in the hippocampus could be modulated by the motivation of receiving rewards or avoiding punishments, and the processing of contextual information involved in the PHC region could be modulated by the association-related hippocampal activity.
Conclusions
Using event-related fMRI, we investigated the effect of monetary reward and punishment on brain activation during the successful encoding of IWS memories. Activation in the VTA/SN and NA regions reflected the predictions of both monetary rewards and punishments, whereas activation in the insular region increased as a function of monetary punishments. Activation in the hippocampus and PHC during encoding predicted the subsequent retrieval success of item and source memories. Finally, correlation coefficients between activations in the hippocampus and reward/punishment-related regions during the successful encoding of item and source memories were significant, but the PHC region showed no significant interaction with any reward-/punishment-related regions. Taken together with our behavioral results, the enhancing effect of rewards/punishments on IWS memories could be modulated by interactions between reward/punishment-related and memory-related hippocampal regions.
Funding
This study was funded by “Grant-in-Aid for Young Scientists (B) (24730617)” to Y.S. from Japan Society for the Promotion of Science (JSPS) and “Funding Program for Next Generation World-Leading Researchers (LZ001)” to T.T. from the Cabinet Office, Government of Japan. Funding to pay the Open Access publication charges for this article was provided by Funding Program for Next Generation World-Leading Researchers (LZ001).
Notes
Conflict of Interest: None declared.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol. 1980;189:573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Beck SM, Locke HS, Savine AC, Jimura K, Braver TS. Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS One. 2010;5:e9251. doi: 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Shimojo S, O'Doherty JP. Human medial orbitofrontal cortex is recruited during experience of imagined and real rewards. J Neurophysiol. 2010;103:2506–2512. doi: 10.1152/jn.01030.2009. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Carter DA, Fibiger HC. Ascending projections of presumed dopamine-containing neurons in the ventral tegmentum of the rat as demonstrated by horseradish peroxidase. Neuroscience. 1977;2:569–576. doi: 10.1016/0306-4522(77)90052-5. [DOI] [PubMed] [Google Scholar]
- Carter RM, Macinnes JJ, Huettel SA, Adcock RA. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Front Behav Neurosci. 2009;3:21. doi: 10.3389/neuro.08.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SM, Andrade A, Johnsrude IS. Learning to like: a role for human orbitofrontal cortex in conditioned reward. J Neurosci. 2005;25:2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Phelps EA. Neural systems underlying aversive conditioning in humans with primary and secondary reinforcers. Front Neurosci. 2011;5:71. doi: 10.3389/fnins.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Functional neuroimaging of the amygdala during emotional processing and learning. In: Aggleton JP, editor. The amygdala: a functional analysis. 2nd ed. UK: Oxford University Press; 2000. pp. 631–653. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Berman KF. Neural coding of distinct statistical properties of reward information in humans. Cereb Cortex. 2006;16:561–573. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW. Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: Aggleton JP, editor. The amygdala: a functional analysis. 2nd ed. UK: Oxford University Press; 2000. pp. 353–390. [Google Scholar]
- Friedman DP, Murray EA, O'Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol. 1986;252:323–347. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Gallagher M. The amygdala and associative learning. In: Aggleton JP, editor. The amygdala: a functional analysis. 2nd ed. UK: Oxford University Press; 2000. pp. 311–329. [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. J Neurosci. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarri A, Campana E, Pacitti C, Hajdu F, Tombol T. Organization of the projections from the ventral tegmental area of Tsai to the hippocampal formation in the rat. J Hirnforsch. 1991;32:429–437. [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Innocenzi R, Pacitti C, Brioni JD. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–1044. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668:71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res. 1999;99:169–179. doi: 10.1016/s0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsukushima Y, Ishihara O, Nagata Y, Koike Y. Research of two-Chinese character word attributes: imagery, concreteness, and ease of learning. Psychol Res Nihon Univ. 1991;12:1–19. [Google Scholar]
- Jacoby LL. A process dissociation framework: separating automatic from intentional uses of memory. J Mem Lang. 1991;30:513–541. [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cereb Cortex. 2007;17:2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering emotional experiences: the contribution of valence and arousal. Rev Neurosci. 2004;15:241–251. doi: 10.1515/revneuro.2004.15.4.241. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J Neurosci. 2007;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol. 2006;4:e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. fMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala and emotion: a view through fear. In: Aggleton JP, editor. The amygdala: a functional analysis. 2nd ed. UK: Oxford University Press; 2000. pp. 289–310. [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cereb Cortex. 2007;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Martig AK, Mizumori SJ. Ventral tegmental area disruption selectively affects CA1/CA2 but not CA3 place fields during a differential reward working memory task. Hippocampus. 2011;21:172–184. doi: 10.1002/hipo.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Murty VP, Labar KS, Adcock RA. Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. J Neurosci. 2012;32:8969–8976. doi: 10.1523/JNEUROSCI.0094-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari-Serenjeh F, Rezayof A, Zarrindast MR. Functional correlation between GABAergic and dopaminergic systems of dorsal hippocampus and ventral tegmental area in passive avoidance learning in rats. Neuroscience. 2011;196:104–114. doi: 10.1016/j.neuroscience.2011.08.073. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- O'Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RG. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learn Mem. 2006;13:760–769. doi: 10.1101/lm.321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Otten LJ. Fragments of a larger whole: retrieval cues constrain observed neural correlates of memory encoding. Cereb Cortex. 2007;17:2030–2038. doi: 10.1093/cercor/bhl111. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Peters J, Suchan B, Koster O, Daum I. Domain-specific retrieval of source information in the medial temporal lobe. Eur J Neurosci. 2007;26:1333–1343. doi: 10.1111/j.1460-9568.2007.05752.x. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Pribram KH, Maclean PD. Neuronographic analysis of medial and basal cerebral cortex. II. Monkey. J Neurophysiol. 1953;16:324–340. doi: 10.1152/jn.1953.16.3.324. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci USA. 2009;106:19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Cabeza R. Role of amygdala connectivity in the persistence of emotional memories over time: an event-related FMRI investigation. Cereb Cortex. 2008;18:2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Memory, attention, and decision-making: a unifying computational neuroscience approach. 1st ed. UK: Oxford University Press; 2007. Reward- and punishment-related learning; emotion and motivation; pp. 113–261. [Google Scholar]
- Rutecki PA, Grossman RG, Armstrong D, Irish-Loewen S. Electrophysiological connections between the hippocampus and entorhinal cortex in patients with complex partial seizures. J Neurosurg. 1989;70:667–675. doi: 10.3171/jns.1989.70.5.0667. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson Y, Wu JJ, Friedman AH, Davis JN. Catecholaminergic innervation of the hippocampus in the cynomolgus monkey. J Comp Neurol. 1990;298:250–263. doi: 10.1002/cne.902980209. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Redoute J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30:13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Singer T, Dolan R. The neurobiology of punishment. Nat Rev Neurosci. 2007;8:300–311. doi: 10.1038/nrn2119. [DOI] [PubMed] [Google Scholar]
- Shigemune Y, Abe N, Suzuki M, Ueno A, Mori E, Tashiro M, Itoh M, Fujii T. Effects of emotion and reward motivation on neural correlates of episodic memory encoding: a PET study. Neurosci Res. 2010;67:72–79. doi: 10.1016/j.neures.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Smith BW, Mitchell DG, Hardin MG, Jazbec S, Fridberg D, Blair RJ, Ernst M. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;44:600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Rose M, Glascher J, Wolbers T, Buchel C. Dissociable contributions within the medial temporal lobe to encoding of object-location associations. Learn Mem. 2005;12:343–351. doi: 10.1101/lm.90405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol Sci. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Abler B, Walter H. Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. Neuroimage. 2009;47:713–721. doi: 10.1016/j.neuroimage.2009.04.095. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R. Orbitofrontal and hippocampal contributions to memory for face-name associations: the rewarding power of a smile. Neuropsychologia. 2008;46:2310–2319. doi: 10.1016/j.neuropsychologia.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R. Remembering beauty: roles of orbitofrontal and hippocampal regions in successful memory encoding of attractive faces. Neuroimage. 2011;54:653–660. doi: 10.1016/j.neuroimage.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Shigemune Y, Nouchi R, Kambara T, Kawashima R. Insular and hippocampal contributions to remembering people with an impression of bad personality. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss025. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Encoding and the durability of episodic memory: a functional magnetic resonance imaging study. J Neurosci. 2005;25:7260–7267. doi: 10.1523/JNEUROSCI.1641-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Valentin VV, O'Doherty JP. Overlapping prediction errors in dorsal striatum during instrumental learning with juice and money reward in the human brain. J Neurophysiol. 2009;102:3384–3391. doi: 10.1152/jn.91195.2008. [DOI] [PubMed] [Google Scholar]
- Wais PE. fMRI signals associated with memory strength in the medial temporal lobes: a meta-analysis. Neuropsychologia. 2008;46:3185–3196. doi: 10.1016/j.neuropsychologia.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Weis S, Specht K, Klaver P, Tendolkar I, Willmes K, Ruhlmann J, Elger CE, Fernandez G. Process dissociation between contextual retrieval and item recognition. Neuroreport. 2004;15:2729–2733. [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schiltz K, Boehler CN, Duzel E. Mesolimbic interaction of emotional valence and reward improves memory formation. Neuropsychologia. 2008;46:1000–1008. doi: 10.1016/j.neuropsychologia.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related fMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, Heinz A. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007;36:1253–1262. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yefet K, Merhav M, Kuulmann-Vander S, Elkobi A, Belelovsky K, Jacobson-Pick S, Meiri N, Rosenblum K. Different signal transduction cascades are activated simultaneously in the rat insular cortex and hippocampus following novel taste learning. Eur J Neurosci. 2006;24:1434–1442. doi: 10.1111/j.1460-9568.2006.05009.x. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]