Abstract
The normal human brain is characterized by a pattern of gross anatomical asymmetry. This pattern, known as the “torque”, is associated with a sexual dimorphism: The male brain tends to be more asymmetric than that of the female. This fact, along with well-known sex differences in brain development (faster in females) and onset of psychosis (earlier with worse outcome in males), has led to the theory that schizophrenia is a disorder in which sex-dependent abnormalities in the development of brain torque, the correlate of the capacity for language, cause alterations in interhemispheric connectivity, which are causally related to psychosis (Crow TJ, Paez P, Chance SE. 2007. Callosal misconnectivity and the sex difference in psychosis. Int Rev Psychiatry. 19(4):449–457.). To provide evidence toward this theory, we analyze the geometry of interhemispheric white matter connections in adolescent-onset schizophrenia, with a particular focus on sex, using a recently introduced framework for white matter geometry computation in diffusion tensor imaging data (Savadjiev P, Kindlmann GL, Bouix S, Shenton ME, Westin CF. 2010. Local white geometry from diffusion tensor gradients. Neuroimage. 49(4):3175–3186.). Our results reveal a pattern of sex-dependent white matter geometry abnormalities that conform to the predictions of Crow's torque theory and correlate with the severity of patients' symptoms. To the best of our knowledge, this is the first study to associate geometrical differences in white matter connectivity with torque in schizophrenia.
Keywords: brain asymmetry, psychosis, schizophrenia, sex differences, white matter geometry
Introduction
The normal human brain is characterized by a pattern of gross anatomical asymmetry known as “torque”, a bias usually described as from right frontal to left occipital. It is thought to arise from the lateralization of function, especially in relation to language (e.g., Broca 1877; Geschwind and Levitsky 1968; Toga and Thompson 2003). This asymmetry first appears during fetal development in utero and can be imaged as early as at 20 gestational weeks (Kasprian et al. 2011, Rajagopalan et al. 2011). Individuals with schizophrenia have less structural and functional asymmetry than the general population (e.g., Kircher et al. 2001, 2002; Kasai et al. 2003; Pantelis et al. 2003), a finding that has been interpreted as a language-related predisposant to psychosis (e.g., Crow et al. 2007).
Torque underlies an important sexual dimorphism: The male brain tends to be more asymmetric than the female brain (McGlone 1980; Witelson and Kigar 1988; Barrick et al. 2005). Furthermore, there is a well-known sex difference in psychosis—onset in males occurs earlier in life, with generally worse outcomes than in females (e.g., Taylor 2003). These facts have led to a theory of psychosis (Crow et al. 2007), which links psychosis to abnormalities in the normal sexually dimorphic lateralization of language.
This theory relates the neural basis of reception and generation of language to the 4 quadrants of heteromodal association cortex, in the left and right prefrontal cortex, and in the left and right posterior temporo-parietal cortex. The torque imposes a natural asymmetry on the white matter connections between these cortical areas, an asymmetry that is normally greater in males than in females (McGlone 1980; Barrick et al. 2005). The central tenet of Crow's hypothesis is that extremes of the development of the asymmetry pattern will at a certain stage result in psychosis associated with a reduction or even reversal of normal asymmetry. Furthermore, these changes are expected to be sex dependent, thus providing a possible explanation for the sex difference in psychosis. In particular, the theory predicts that asymmetry will be reduced in male patients, while the changes in females may be in the opposite direction.
To test the predictions of Crow's theory, a focus on the morphology and geometry of white matter connections is required. Existing methods, however, are not necessarily appropriate to this task. Studies reviewed by Crow (2004) have focused either on volume measures (e.g., Bilder et al. 1994; Chance et al. 2005) or on cortical surface complexity measures based on the sulco-gyral pattern (e.g., Kikinis et al. 1994; Narr et al. 2001, 2004). The findings have been inconsistent. For example, Meisenzahl et al. (2002) showed that whether or not a left–right asymmetry is revealed depends on the exact definition of the planum temporale. A possible explanation is that asymmetrical change may affect shape rather than volume (Harasty et al. 2003).
While torque has been studied in terms of volumes and cortical morphology, little is known about its macrostructural correlates in the white matter. Most neuroimaging investigations of the white matter have focused on diffusion tensor imaging (DTI) measures such as fractional anisotropy (FA), mean, and radial and axial diffusivities. These measures are thought to reflect local (at the level of a single voxel) tissue properties such as axonal organization or white matter integrity, and it is difficult to relate them to cerebral asymmetry as a global property of the brain.
In the present work, we shift our focus from the more conventional DTI measures of diffusivity and anisotropy, to a novel framework of macrostructural measures of white matter architecture and geometry introduced by Savadjiev et al. (2010). This method is specifically designed to measure the geometry of the white matter, and it allows to directly test the central prediction of Crow's theory of sexually dimorphic changes in interhemispheric white matter connections.
Materials and Methods
Measuring White Matter Geometry with DTI
A majority of methods for assessing the geometry of white matter in diffusion magnetic resonance imaging (MRI) first compute fiber trajectories with a tractography algorithm and then compute the geometry of these trajectories. However, all methods based on fiber tractography are inherently limited in stability and reproducibility. To counter this limitation, Savadjiev et al. (2010) introduced a method for computing white matter geometrical indices directly from diffusion imaging data without the requirement for prior tractography.
The method isolates geometrical information from the gradient of the diffusion tensor field, within the framework of local variation in tensor orientation. Since these indices are defined in terms of tensor field derivatives, they integrate information from a neighborhood of voxels. They are thus philosophically consistent with an idea that was proposed early in the history of DTI (but not later developed) that the information found in “patterns” of tensors and their differential structure may (in addition measures of individual diffusion tensor properties, such as FA) provide further insights into tissue organization, structure, and function (Basser 1997). Of course, as also the case with other in vivo neuroimaging techniques, this method provides only an indirect measurement of the “true” white matter geometry.
The Dispersion Index
The “shape normalized dispersion” (SHD), or dispersion index (Savadjiev et al. 2010), is a quantitative measure computed at each voxel. It measures the extent to which white matter fibers deviate from a parallel configuration within a local 3-dimensional neighborhood. In other words, it calculates a measure of “spread” in white matter fiber layout, which can then be related to brain torque. SHD has previously been used in a study to detect significant differences in schizophrenia via region-of-interest (ROI) based analyses (Whitford et al. 2011). However, this study averaged SHD over large ROIs, an approach that does not permit change to be localized, and furthermore, it did not investigate the effect of sex. Here, we use a tract-based analysis to find locations along major white matter tracts where SHD changes are statistically significant. We also relate these locations to Crow et al.'s predictions relating to sex differences in asymmetry and brain torque.
While the computation of SHD is completely independent of tractography, for visualization it is helpful to compute tractography and to color the resulting fiber tracts with the precomputed SHD values. By way of illustration, we created Figure 1 by computing fiber tractography in a small white matter region just beneath the cortex and by color-coding the resulting fiber tracts with the SHD values. The color-coding follows a rainbow colormap, in which blue denotes high index values, and orange/yellow denotes low values of SHD.
Figure 1.
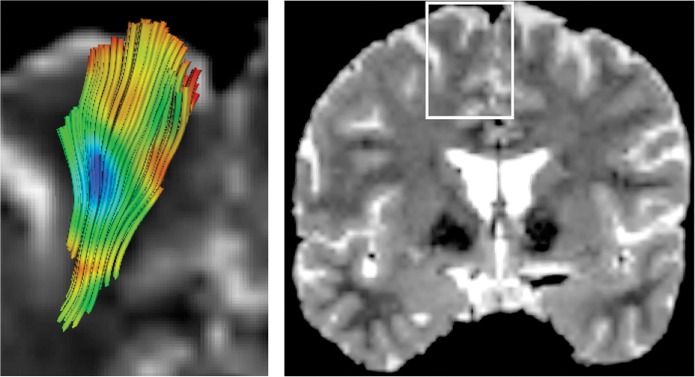
(left) Visual presentation of the dispersion index (SHD) mapped onto a set of fiber tracts that fan towards the para-sagittal cortex, in a region indicated with a white rectangle on a T2-weighted image (right). The color-coding follows the rainbow colormap: Blue tones indicate high SHD values; green, intermediate values; and red, low values.
We give a second illustration in Figure 2, where a tractography of the genu fibers is colored by SHD.
Figure 2.
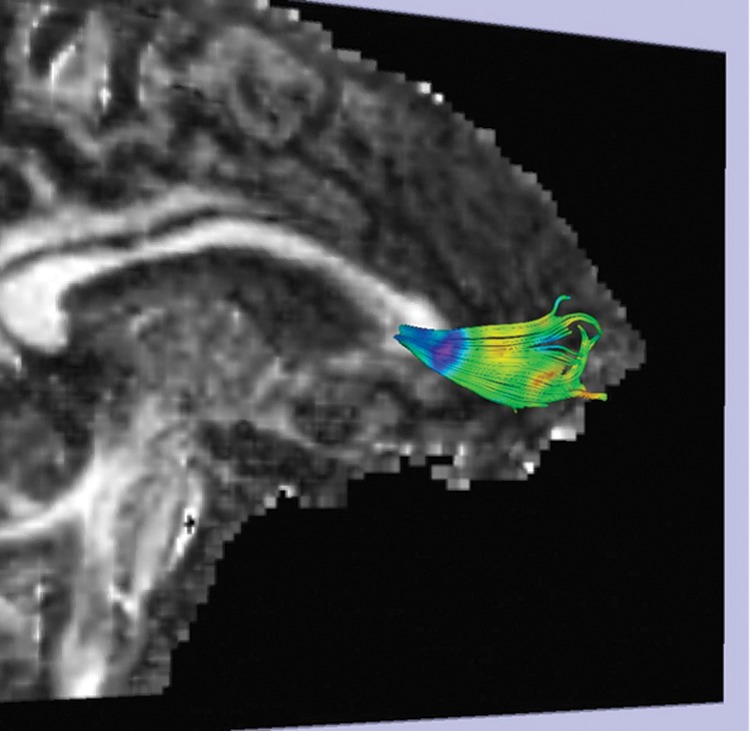
Fiber tractography of the genu of the corpus callosum colored with SHD, with the same rainbow colormap as in Figure 1. The viewpoint is oblique, as indicated by the midsagittal slice of the FA volume shown as background.
Participants
Thirty-nine patients (22 males and 17 females) were recruited from regional adolescent units in the Oxford, UK, area, and the diagnosis of schizophrenia (DSM-IV-TR) was confirmed using the Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al. 1997). Symptom severity was assessed using the Positive and Negative Symptom Scale (PANSS; Kay et al. 1987). Thirty-three healthy participants (16 males and 17 females) were recruited as controls through their general practitioners and were screened using the K-SADS-PL for any history of emotional, behavioral, or medical problems.
The participants' age ranged from 13.55 to 17.88 years (mean: 15.88 years, standard deviation: 1.21 years), and they all attended mainstream schools. Exclusion criteria were moderate mental impairment (intelligence quotient [IQ] < 60), a history of pervasive developmental disorder, significant head injury, neurological disorder, or major medical disorder. The study was undertaken in accordance with the guidance of the Oxford Psychiatric Research Ethics Committee, and written consent was obtained from all participants (and their parents if under the age of 16). For additional details on the subject population, please see Tables 1–3.
Table 1.
Patient subject data. Each row corresponds to one subject
| Sex | Hand | Age | AOS | DUP | Meds | Cpzeq | FSIQ | Pos | Neg |
|---|---|---|---|---|---|---|---|---|---|
| M | 0 | 17.61 | 16.5 | 1.1 | Ol | 300 | 78 | 23 | 23 |
| M | −55 | 17.58 | 15.3 | 2.27 | Ol | 200 | N/A | 23 | 15 |
| M | −68 | 16.46 | 15.7 | 0.75 | Ol | 200 | 97 | 27 | 17 |
| M | 90 | 17.68 | 16.8 | 0.87 | Ol | 400 | 68 | 22 | 15 |
| M | 100 | 15.21 | 14.6 | 0.61 | Ol | 300 | 102 | 24 | 13 |
| M | 100 | 16.41 | 15.8 | 0.59 | Ol | 400 | 85 | 23 | 15 |
| M | 100 | 17.05 | 15.7 | 1.34 | Ol | 200 | 104 | 24 | 9 |
| M | 80 | 14.99 | 13.5 | 1.48 | Ol | 400 | 85 | 26 | 18 |
| M | 70 | 15.98 | 14 | 1.96 | Clz | 500 | 72 | 22 | 23 |
| M | 80 | 15.18 | 13 | 2.17 | Que, Ol | 748 | 74 | 22 | 15 |
| M | 50 | 16.76 | 15.25 | 1.5 | Ol | 250 | 84 | 23 | 13 |
| M | 100 | 17.17 | 14.5 | 2.66 | Clz | 600 | 66 | 25 | 20 |
| M | 90 | 16.89 | 14 | 2.88 | Que | 192 | 113 | 17 | 13 |
| M | −25 | 14.37 | 13 | 2.36 | Ol | 200 | 66 | 21 | 17 |
| M | 100 | 16.47 | 14.47 | 2 | Ris | 150 | 73 | 24 | 11 |
| M | 100 | 16.83 | 16 | 0.83 | Ol, Ris | 250 | 108 | 22 | 17 |
| M | 100 | 14.95 | 14 | 0.95 | Arp, Ris | 300 | 85 | 19 | 14 |
| M | −60 | 16.98 | 15.9 | 1.08 | Ol | 200 | 73 | 21 | 17 |
| M | 100 | 16.88 | 11 | 5.88 | Arp, Ris | 550 | N/A | 26 | 22 |
| M | 100 | 16.28 | 15.6 | 0.65 | Flo, Ol | 250 | 71 | 21 | 15 |
| M | 100 | 17.69 | 16.7 | 1 | Ris | 200 | 117 | 23 | 16 |
| M | 80 | 16.65 | 16 | 0.6 | Ol | 150 | 102 | 22 | 14 |
| F | 100 | 17.01 | 15 | 2 | Ol | 400 | N/A | 27 | 17 |
| F | 100 | 15.7 | 14.9 | 0.79 | Ol | 300 | 101 | 26 | 15 |
| F | 100 | 16.99 | 15.9 | 1.08 | Clz | 350 | 86 | 20 | 16 |
| F | −70 | 15.89 | 14.75 | 1.13 | Ol, Que, Clz | 730 | 73 | 21 | 13 |
| F | 100 | 16.38 | 12 | 4.36 | Sul | 67 | 120 | 20 | 16 |
| F | 80 | 16.09 | 15.3 | 0.79 | Clz, Ol | 1000 | 80 | 18 | 16 |
| F | 100 | 14.14 | 12 | 2.4 | Clz | 900 | 62 | 23 | 18 |
| F | 100 | 17.38 | 12 | 5.8 | Clz | 750 | 100 | 24 | 12 |
| F | 54 | 15.56 | 14.6 | 0.95 | Ol | 200 | 117 | 17 | 12 |
| F | 95 | 17.08 | 16 | 1.08 | Que, Al | 384 | 84 | 20 | 15 |
| F | 100 | 16.77 | 13 | 3.77 | Que, Al | 668 | 88 | 24 | 15 |
| F | 0 | 16.73 | 11 | 5.73 | Ris, Al | 300 | 81 | 24 | 13 |
| F | 100 | 16.78 | 12.7 | 4.08 | Que | 448 | 107 | 24 | 19 |
| F | 100 | 16.19 | 15 | 1.19 | Ris | 200 | 87 | 22 | 19 |
| F | −80 | 14.42 | 13.6 | 0.84 | Ris | 150 | 85 | 29 | 18 |
| F | −100 | 15.08 | 14 | 1 | Ris | 150 | 75 | 18 | 12 |
| F | 100 | 14.4 | 13.5 | 0.9 | Arp | 100 | 96 | 21 | 14 |
Hand = handedness score (Edinburgh handedness inventory); Age = age at scan (years); AOS = age at the onset of symptoms (years); DUP = duration of untreated psychosis; Cpzeq = dosage in chlorpromazine equivalent (mg per day); FSIQ = full-scale intelligence quotient; Pos = positive PANSS symptom score; Neg = negative PANSS symptom score; Meds = medication, where Ol = olanzapine; Que = quetiapine; Ris = risperidone; Clz = clozapine; Arp = aripiprazole; Flo = fluoxetine; Al = sodium valproate; Sul = sulpiride; N/A = not available.
Table 2.
Control subject data. Column headings as in Table 1
| Sex | Hand | Age | FSIQ |
|---|---|---|---|
| M | −20 | 13.55 | 104 |
| M | −100 | 15.55 | 87 |
| M | 80 | 17.73 | 125 |
| M | 80 | 16.2 | 94 |
| M | 60 | 17.88 | 122 |
| M | 100 | 17.61 | 121 |
| M | 80 | 15.21 | 83 |
| M | 100 | 17.33 | 64 |
| M | 100 | 17.35 | 100 |
| M | 90 | 15.24 | 112 |
| M | 92 | 14.94 | 119 |
| M | 100 | 14.81 | 126 |
| M | 100 | 14.36 | 117 |
| M | 71 | 14.11 | 100 |
| M | 50 | 14.38 | 109 |
| M | −100 | 14.15 | 109 |
| F | −80 | 14.43 | 110 |
| F | 80 | 13.58 | 102 |
| F | 70 | 15.87 | 127 |
| F | 100 | 15.25 | 108 |
| F | 60 | 13.8 | 72 |
| F | 80 | 16.18 | 107 |
| F | 90 | 16.15 | 116 |
| F | 90 | 14.94 | 95 |
| F | 75 | 14.71 | 106 |
| F | 95 | 15.39 | 93 |
| F | 80 | 16.93 | 115 |
| F | 80 | 17.14 | 118 |
| F | 85 | 14.56 | 107 |
| F | 90 | 14.7 | 80 |
| F | 80 | 14.78 | 123 |
| F | 95 | 16.35 | 106 |
| F | 50 | 13.7 | 117 |
Table 3.
| Male controls (n = 16) | Female controls (n = 17) | Male patients (n = 22) | Female patients (n = 17) | |
|---|---|---|---|---|
| Age | 15.7 (1.5) | 15.2 (1.1) | 16.5 (0.97) | 16.0 (1.0) |
| Hand | 55.2 (67.6) | 71.8 (41.1) | 60.5 (59.4) | 57.6 (72.1) |
| FSIQ | 105.8 (17.2) | 106.0 (14.4) | 86.2, (16.6) | 90.1 (15.8) |
| AOS | N/A | N/A | 14.9 (1.4) | 13.8 (1.5) |
| DUP | N/A | N/A | 1.6 (1.2) | 2.2 (1.8) |
| Cpzeq | N/A | N/A | 315.4 (160.7) | 417.5 (289.3) |
| Pos | N/A | N/A | 22.7 (2.3) | 22.2 (3.3) |
| Neg | N/A | N/A | 16.0 (3.6) | 15.3 (2.4) |
Note: The row headings correspond to the column headings in Table 1. Each entry shows the mean, with the standard deviation in parentheses.
n = number of subjects; N/A = not applicable.
Image Acquisition
All participants underwent an imaging protocol with diffusion-weighted scanning on a 1.5-T Sonata MR scanner (Siemens, Erlangen, Germany). Data from this population have already been analyzed in a previous study (Douaud et al. 2007), we refer the reader to this work for details on the image acquisition protocol. Briefly, diffusion-weighted images were obtained using echo-planar imaging (SE-EPI, 60 axial slices, voxel size 2.5 × 2.5 × 2.5 mm3) with 60 isotropically distributed orientations for the diffusion-sensitizing gradients at a b-value of 1000 s/mm2 and 5 b = 0 s/mm2 images. All scans were corrected for head motion and eddy currents. Diffusion tensors were then computed from the corrected diffusion-weighted images using weighted least-squares estimation.
Diffusion Image Processing
In our experiments, we compare white matter geometry of male and female brains, patients, and controls. It is known that male brains are on average bigger than female brains (Im et al. 2006). It is also possible that an overall brain size difference exists between controls and patients. To ensure that observed differences in white matter geometry are not driven by differences in overall brain size, we performed an uniform scaling of all DTI volumes, such that the resulting volumes have the same overall size. This is achieved via affine (rigid-body) registration, with no deformations other than uniform scaling.
Following this, the white matter dispersion index SHD was computed as described in Savadjiev et al. (2010). In addition, we also computed FA since our tools (see below) require it. We note, however, that FA differences in the same population have already been reported (Douaud et al. 2007), thus we do not recalculate them here.
Tract-Based Analysis
To test Crow's theory, we determined the white matter locations where the dispersion index SHD differed across diagnosis and sex. While the theory makes predictions only for white matter tracts that are part of the language network, we decided to analyze SHD over all major white matter fasciculi. White matter geometry has not been studied extensively in previous work, it is thus not known whether changes in white matter geometry are limited to language-specific tracts, or whether they occur in other white matter connections as well.
Our analysis is based on the Tract-Based Spatial Statistics (TBSS) method (Smith et al. 2006), where white matter tracts are abstracted as a set of locations of locally maximal FA that are then connected to form a white matter skeleton. TBSS proceeds by first aligning all subjects' FA and SHD images to a common space using the nonlinear registration tool FNIRT (FMRIB Centre, University of Oxford; www.fmrib.ox.ac.uk/analysis/techrep). This nonrigid step refines the initial affine registration with uniform scaling described above. The aligned FA images are then averaged to create a mean FA image, which is then thinned to create the mean FA skeleton (thresholded at an FA value of 0.35). The skeleton represents the centers of all white matter tracts common to all subjects, and each subject's SHD data are projected onto it. To achieve this, for each skeleton voxel in each subject, a search is made for the maximum FA value in the direction perpendicular to the local skeleton structure. This location defines the local tract center and is recorded. Each subject's (aligned) SHD data at these locations were then projected onto the skeleton, and the resulting data were used to perform statistics between subjects.
Statistics
The projected SHD data from all subjects on the white matter skeleton were subjected to statistical analysis via permutation testing, using the randomized tool, part of FSL software library. For details on permutation testing in neuroimaging, see Nichols and Holmes (2002). The ‘randomize’ tool implements permutation testing within a standard generalized linear model (GLM) framework.
Clusters in the data are enhanced via the Threshold-Free Cluster Enhancement method (Smith and Nichols 2009), which allows the use of spatial neighborhood information to increase confidence in statistical differences over groups of neighboring voxels, without requiring an a priori threshold for cluster definition. The P-value for each cluster is then corrected by controlling for the family-wise error rate. Multiple comparison correction is thus performed by accounting for the entire extent of the white matter skeleton.
We computed a GLM for the interaction between diagnosis and sex, incorporating subject IQ, age, and handedness coefficient as confound regressors in the GLM design matrix. We performed testing using 5000 random permutations, which sets the confidence limits for an estimated P-value of 0.05 to ±0.0062 of the true P-value, 95% of the time.
Results
Diagnosis by Sex Interaction
We used TBSS as described above to test for a significant Diagnosis by Sex interaction in the SHD data. Following correction for multiple comparisons, we detected one cluster of voxels where the interaction is significant with P < 0.05. With the acronyms MC, FC, MP, and FP denoting the groups of male controls, female controls, male patients, and female patients, respectively, the interaction is such that MP and FC (as a merged group) have a higher dispersion than MC and FP (as a merged group). This cluster occurs in the left anterior corpus callosum and is shown in Figure 3, where the mean FA image is also shown to provide spatial context.
Figure 3.
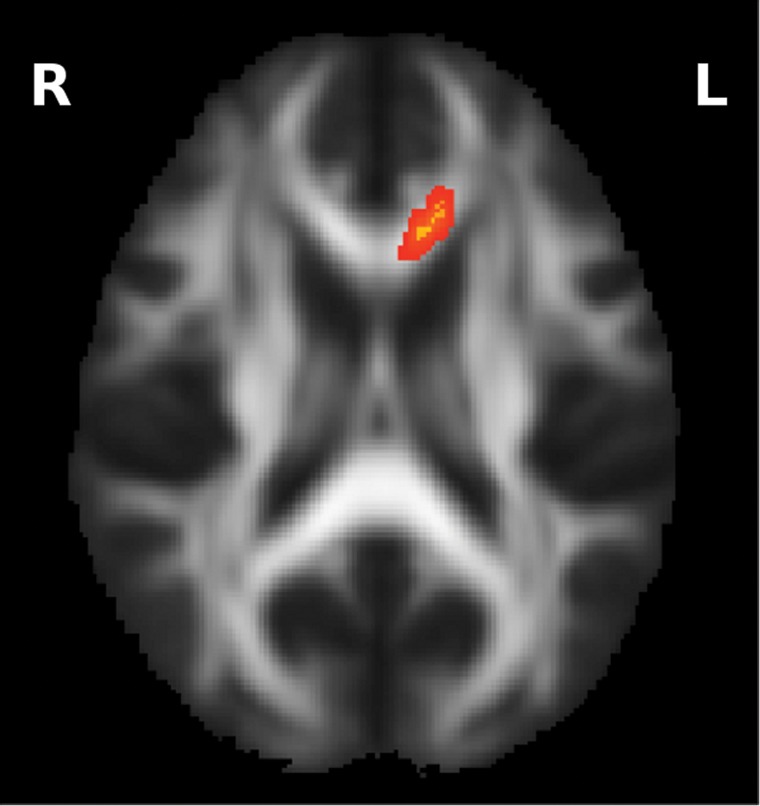
Diagnosis by Sex interaction, such that (MP, FC) > (MC, FP). The cluster with P < 0.05 is shown.
We carried out further analysis in this area of significant interaction by computing the mean SHD value over this cluster in each participant. Here, we remind the reader that TBSS registers the images from each subject to the same canonical space as a preprocessing step. The cluster of significant interaction detected in the canonical space corresponds therefore to the same set of voxels in each subject's image, thus defining a correspondence across subjects.
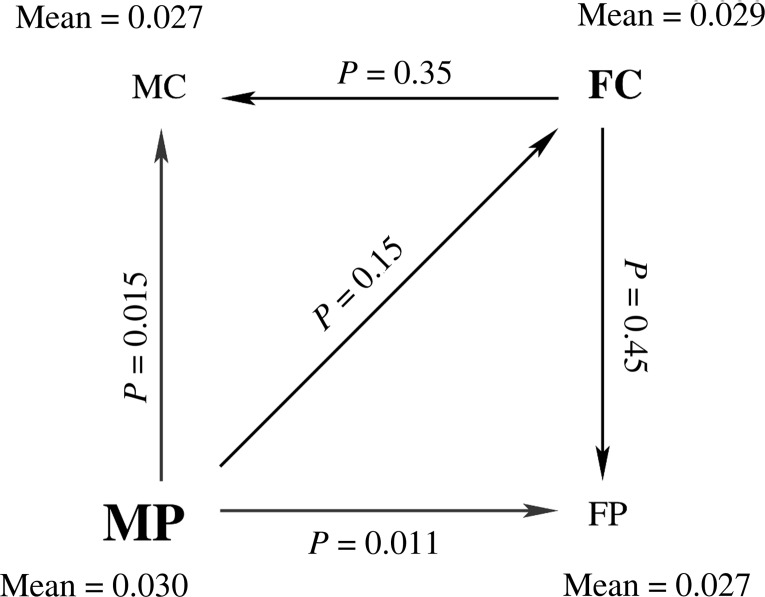
We then computed the mean SHD over each group and observed that the ordering of groups in terms of mean SHD is MP > FC > MC ≈ FP, consistent with the significant interaction in that region. We also observe that SHD is significantly greater in MP compared with MC and FP. These results are summarized in Figure 4.
Figure 4.
A graphic illustration of the relationship between the 4 groups (MC, FC, MP, and FP) in terms of the mean SHD value in the region of significant interaction shown in Figure 3. The mean SHD value for each group is indicated next to its name. The prominence of the font is a visual indication of the magnitude of mean SHD in that group. The arrows denote comparisons between groups, such that the arrow points in the direction of decreasing mean SHD. The P-values for each comparison are indicated next to each arrow.
As presented earlier in the Materials and Methods section, the age of the participants in our study ranges from 13.55 to 17.88 years. Since adolescence is a period during which important changes can be observed in the white matter, we remind the reader that, in our TBSS experiments, we correct for age, in addition to correcting for handedness and IQ. Furthermore, there is a highly nonsignificant Diagnosis by Sex interaction for age (F = 0, P = 1.0) in our subject population, and there are no significant age differences between males and females in either the control or the patient group (t-test, uncorrected P-value: 0.3 in the control group and 0.2 in the patient group). Furthermore, within the patient group, no significant between gender differences exist in either duration of illness (P = 0.2), or exposure to medications (P = 0.2). We measure neuroleptic medication dosage with chlorpromazine equivalents (Stoll 2001). Full details on medication type and dosage, as well as on other characteristics of our sample such as age, handedness, IQ, positive and negative symptom scores, can be found in Tables 1 and 2, with a summary provided in Table 3. There was no history of alcohol, tobacco, or drug use in the month prior to scanning for any of the subjects, and all urine drug tests were negative.
Taken together, these results suggest that the observed Diagnosis by Sex interaction in SHD is not likely to arise due to different stages of maturation of the white matter, or to be due to medication effects. Rather, they reveal changes that occur in white matter geometry in adolescent-onset schizophrenia, as well as a sexual dimorphism in white matter geometry in the normal brain.
Correlation with Negative Symptom Scores
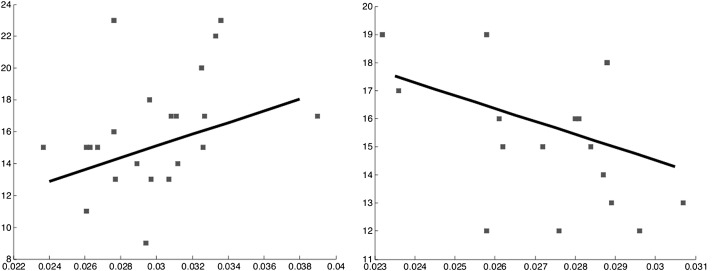
We computed Pearson's correlation between the mean SHD values in the above region of significant interaction and the negative scale of the PANSS, for both male and female patients separately. We focused specifically on the negative scale, as negative symptoms are commoner with earlier, particularly adolescent, onsets. Furthermore, Crow's theory implicates negative symptoms as consequences of deviations in normal sexual dimorphic development in interhemispheric connectivity (Crow et al. 2007). We observed significant P-values for the correlation between the dispersion index and negative PANSS symptom scores for both male and female patients, as shown in Figure 5, with the sign of the correlation reversed: Positive for males, but negative for females.
Figure 5.
Correlation between dispersion (SHD) on the horizontal axis, and negative PANSS symptom scores on the vertical axis, for the male patients group in our study (left) and the female patients group (right). The Pearson correlation coefficient is 0.370 for male and −0.459 for female patients.
The reversal of the correlation sign for females with respect to males is important, as it is predicted by Crow's theory. This is discussed further in the next section. A negative correlation for women (P = 0.032) and a positive correlation for men (P = 0.045) were found. One-tail significance was used to compute these P-values, as the direction of the correlation is predicted by the theory. In addition, 2-tail significance for partial correlations with correction for age and chlorpromazine equivalents was also achieved for both males (P = 0.013) and females (P = 0.022).
Discussion
To our knowledge, this is the first study to examine the geometry of white matter in the context of brain torque in schizophrenia. It provides a novel insight not only into the manner in which the geometry of white matter connections is affected in schizophrenia, but also into the normal sexual dimorphism of these connections.
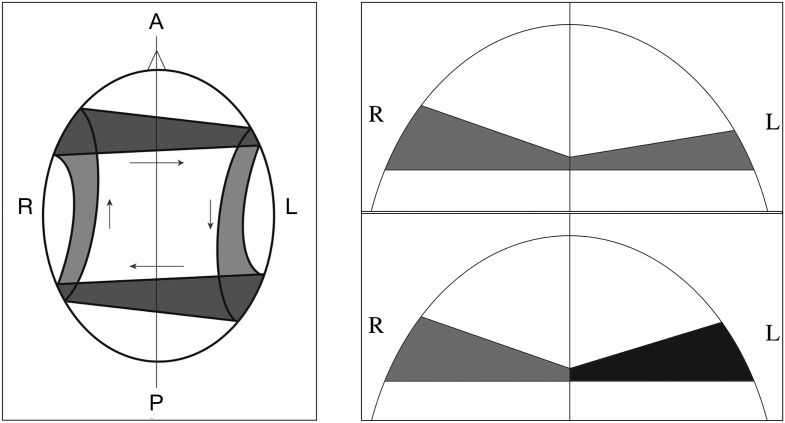
In a schematic interpretation, the left panel of Figure 6 represents Crow's model of the asymmetry of interhemispheric connections involved in the language network (Crow et al. 2007). Figure 6 (right) modifies the anterior part of this schematic to reflect the fact that the midsagittal cross-section of the corpus callosum genu acts as a bottleneck for the fibers, which disperse on either side toward the cortex. The asymmetry inherent in the model of Figure 6 (left) is reflected in the top panel of Figure 6 (right) in that normal cases of asymmetry (in males) have less white matter dispersion toward the cortex on the left relative to the right. Crow's theory predicts that male patients have a reduction of this asymmetry, while the changes in females should be reversed, with “more” asymmetry in patients than controls. Our result of a significant Diagnosis by Sex interaction in the anterior left part of the corpus callosum supports this view. It reflects an increase of dispersion toward the anterior left in the corpus callosum in male patients and female controls, relative to male controls and female patients. This increase is illustrated in the bottom panel of Figure 6 (right) and has the effect of reducing connection asymmetry. Note that a similar change in fiber dispersion in the corona radiata has been hypothesized by Buchsbaum et al. (2006), based on fiber tractography results.
Figure 6.
(left) The normal asymmetry of the “language circuit” (adapted from Crow et al. 2007). (right, top panel) A modification of the anterior part of the schematic in left, which reflects the fact that the midsagittal cross-section of the corpus callosum genu acts as a bottleneck for the fibers that disperse on either side toward the cortex. Male controls and female patients are thus expected to be asymmetric. (right, bottom panel) Increased white matter dispersion from the genu toward the cortex in the left hemisphere (darker shade of gray) can underlie a reduction of connection asymmetry.
In addition to detecting these sex-specific changes in white matter dispersion, we showed that they correlate with patient symptoms, in a manner predicted by Crow's theory. Specifically, we find that, in male patients, negative symptom strength increases with increasing abnormal dispersion toward the left, that is, with a “reduction” in connection asymmetry. At the same time, the trend is reversed for female patients: Stronger negative symptoms are correlated with a decreased dispersion toward the left (and hence an “increase” in asymmetry).
Core negative symptoms such as poverty of speech and affective flattening constitute abnormalities of speech production and inflexion that may be assumed to be interactive (e.g., syntactic) functions of heteromodal cortex in the 2 frontal lobes (Crow 2010). The fact that our dispersion measure in anterior interhemispheric connections correlated with negative symptoms indicates a structural connection between sex, psychosis, and the development of these interhemispheric connections, as argued by Crow et al. (2007). Furthermore, negative symptoms are commoner with earlier, particularly adolescent, onsets. The fact that we observe in adolescent subjects alterations in anterior interhemispheric connections, but not in posterior ones, combined with the fact that these alterations correlate with negative symptoms, indicates a possible implication of a developmental process that progresses in the anterior-to-posterior direction and has not reached yet full maturation at adolescence. In future work, we intend to perform the same analysis on adult subjects to provide further detail on the progression of white matter geometry changes over age.
Other structural studies have also shown opposing correlations between males and females. For example, a reversal of normal sex differences in brain surface asymmetry was observed in schizophrenia patients in a postmortem study (Highley et al. 1998). Other examples of relationships between sex and diagnosis of schizophrenia have been seen in MRI studies of ventricular size and shape (Narr et al. 2001, 2004) and cortical gyrification (Narr et al. 2004).
The cohort of adolescent-onset schizophrenia subjects examined here has been analyzed in a previous study (Douaud et al. 2007), in which FA differences between patients and controls were demonstrated over widespread white matter locations including the corpus callosum. Our present study of white matter geometry advances previous work in 2 main ways.
First, our approach is based on a measure that is specific to the geometry of white matter tracts. In contrast, the interpretation of FA changes and their relationship to biological microstructure remains unclear, as FA values reflect a variety of local tissue properties, such as myelination, axonal packing, glial cells and oligodendrocytes, and general fiber organization (e.g., Gilmore et al. 2007). Thus, our technique allows the separation of geometrical information from other tissue characteristics and the interpretation of the results in the context of the torque-based theory of Crow et al. (2007).
Secondly, the FA reductions in adolescent-onset schizophrenia (Douaud et al. 2007) are widespread throughout the white matter and indicate potential tissue changes of the various types discussed above. In contrast, the significant Diagnosis by Sex differences in geometry we have identified are confined to a single location. Furthermore, our measure is computed on the basis of neighborhoods of voxels. This means that it integrates information at a larger spatial scale than FA, a measure that reflects tissue properties only at the level of the single voxel. Thus, we observe differences in geometry that are localized on the one hand (in the sense that only a single location in the brain exhibit them), but on the other hand present coherence over several neighboring voxels. We consider it unlikely that this type of difference arises from neurodegeneration, disease progression, or medication effects. Rather, we hypothesize that the geometrical differences are due to a development abnormality that alters the topography of white matter connections. If this is indeed the case, these geometrical changes would probably occur earlier than other changes in the white matter, such as the FA abnormalities observed by Douaud et al. (2007) for example. Of course, a longitudinal study is needed in order to verify the timing of these events.
It is important to note that the use of TBSS implies no a priori hypothesis for the location of the differences, as TBSS tests the data and corrects for multiple comparisons over the entire white matter skeleton. It is therefore noteworthy that our observed differences are confined to an area relevant to the brain torque theory.
The classical definition of brain torque is based on regional volume differences between the cerebral hemispheres. However, gross volumetric change is likely to also involve more intricate changes in the local morphological structure of both gray matter and white matter. In this work, our findings are indirectly associated with the classical definition of torque through its effect on white matter geometry. Our results are important because they associate this little known aspect of brain torque to sex differences and symptom strength. The exact underlying mechanism by which these 3 elements are related is still unclear. However, our results do follow the pattern predicted by Crow's theory (Crow et al. 2007), which provides an outline on how deviations from the normal sexually dimorphic asymmetry of interhemispheric white matter connections may affect the normal processing of language and result in psychotic symptoms.
As suggested above, the abnormal patterns of white matter geometry observed in the present study may have a neurodevelopmental origin. To test further this hypothesis, one should also take into account the morphology of the associated gray matter. This view is motivated by works such as Rakic (1988), Metin et al. (2008), and McIntosh et al. (2008), which describe the importance of neuronal migration and axonal outgrowth in the development of the brain, and how genetic and environmental factors disrupting these processes may result in neurological disease. For instance, histological analyses following neuronal enucleation in newborn ferrets show a relation between abnormalities in the layout of the growing axons and abnormal cortical surface area in the affected gray matter regions (Bock et al. 2010). Furthermore, gyrification abnormalities have been associated with functional disruption in schizophrenia, and there is emerging evidence for a link between gyrification and neural connectivity (White and Hilgetag 2011). In future work, we intend to perform a joint analysis of white and gray matter morphology, in order to bring these 2 components of brain morphology together and connect them to the standard volumetric definition of brain torque.
Our findings point to a sexually dimorphic anomaly of white matter in adolescent-onset schizophrenia. The functional significance of this structural abnormality is currently unclear and needs to be examined in future work. One possible avenue of future research relates to the Protocadherin11X/Y sapiens-specific gene pair located in the Xq21.3 region of the X chromosome and the X-transposed region (“XTR”) of the Y chromosome. This gene pair is suggested as critical to the evolution of sexually dimorphic brain asymmetry, and a putative neural correlate of language (Williams et al. 2006; Priddle and Crow 2012). An interesting next step in future work would be thus to connect the structural findings in the present study to the expression of this gene pair.
In conclusion, our findings add emphasis to the view that an interaction between sex and asymmetry of fiber geometry is relevant to the pathophysiology of schizophrenia. They are consistent with a role for a sexual dimorphism in the origins of psychosis and the evolution of language (Crow 2000, 2008).
Notes
This work was supported by the National Institutes of Health [grant numbers R01MH082918, R01MH092862, R01MH074794, R01MH050740, P41RR013218, P41EB015902, P50MH080272, and U54EB005149]; the Department of Veteran Affairs Merit Award (to M.E.S.) and Schizophrenia Center Grant (to MES); the National Health and Medical Research Council of Australia, an overseas-based biomedical training fellowship [NHMRC #520627, to TJW]; the National Alliance for Research on Schizophrenia and Depression (NARSAD) Brain and Behavior Research Fund, Barbara and John Streicker Young Investigator Award (17537, to T.J.W.); the Medical Research Council (G0500092, to A.J.); the Oxfordshire Health Services Research Committee (to A.J.). Conflict of Interest: None declared.
References
- Barrick TR, Mackay CE, Prima S, Vandermeulen D, Crow TJ, Roberts N. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage. 2005;24(3):678–691. doi: 10.1016/j.neuroimage.2004.09.003. doi:10.1016/j.neuroimage.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Basser PJ. New histological and physiological stains derived from diffusion-tensor MR images. Ann NY Acad Sci. 1997;820:123–138. doi: 10.1111/j.1749-6632.1997.tb46192.x. doi:10.1111/j.1749-6632.1997.tb46192.x. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JMJ, Snyder PJ, Lieberman JA. Absence of regional hemispheric volume asymmetries in first episode schizophrenia. Am J Psychiatry. 1994;151:1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- Bock AS, Olavarria JF, Leigland LA, Taber EN, Jespersen SN, Kroenke CD. Diffusion tensor imaging detects early cerebral cortex abnormalities in neuronal architecture induced by bilateral neonatal enucleation: an experimental model in the ferret. Front Syst Neurosci. 2010;4:149. doi: 10.3389/fnsys.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P. Rapport sur un mémoire de M. Armand de Fleury intitulé: de l'inégalité dynamique des deux hémisphères cérébraux. Bull Acad Med. 1877;6:508–539. [Google Scholar]
- Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark R, King-Wai C, Mitelman S, Brickman AM, Shihabuddin L, Haznedar MM, Hazlett EA, et al. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Ann Gen Psychiatry. 2006;5:19. doi: 10.1186/1744-859X-5-19. doi:10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA, Esiri MM, Crow TJ. Macroscopic brain asymmetry is changed along the antero-posterior axis in schizophrenia. Schiz Res. 2005;74(2–3):163–170. doi: 10.1016/j.schres.2004.09.001. doi:10.1016/j.schres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The “big bang” theory of the origin of psychosis and the faculty of language. Schiz Res. 2008;102:31–52. doi: 10.1016/j.schres.2008.03.010. doi:10.1016/j.schres.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The nuclear symptoms of schizophrenia reveal the four quadrant structure of language and its deictic frame. J Neurolinguistics. 2010;23:1–9. doi:10.1016/j.jneuroling.2009.08.005. [Google Scholar]
- Crow TJ. Schizophrenia as the price that Homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Rev. 2000;31:118–129. doi: 10.1016/s0165-0173(99)00029-6. doi:10.1016/S0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Cerebral asymmetry and the lateralization of language: core deficits in schizophrenia as pointers to the gene. Curr Opin Psychiatry. 2004;17:97–106. doi:10.1080/09540260701486282. [Google Scholar]
- Crow TJ, Paez P, Chance SE. Callosal misconnectivity and the sex difference in psychosis. Int Rev Psychiatry. 2007;19(4):449–457. doi: 10.1080/09540260701486282. doi:10.1080/09540260701486282. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith SM, Jenkinson M, Behrens TEJ, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. doi:10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161(3837):186–187. doi: 10.1126/science.161.3837.186. doi:10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Corouge I, Vetsa YSK, Smith JK, Kang C, Gu H, Hamer RM, Lieberman JA, Gerig G. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. Am J Neuroradiol. 2007;28:1789–1795. doi: 10.3174/ajnr.A0751. doi:10.3174/ajnr.A0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasty J, Seldon HL, Chan P, Halliday G, Harding A. The left human speech-processing cortex is thinner but longer than the right. Laterality. 2003;8(3):247–260. doi: 10.1080/13576500244000175. [DOI] [PubMed] [Google Scholar]
- Highley JR, Esiri MM, Cortina-Borja M, McDonald B, Cooper SJ, Herron BM, Crow TJ. Anomalies of cerebral asymmetry in schizophrenia interact with gender and age of onset: a post mortem study. Schizopr Res. 1998;34:13–25. doi: 10.1016/s0920-9964(98)00077-2. doi:10.1016/S0920-9964(98)00077-2. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, Kim SI. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31(1):31–38. doi: 10.1016/j.neuroimage.2005.11.042. doi:10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160(1):156–164. doi: 10.1176/appi.ajp.160.1.156. doi:10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprian G, Langs G, Brugger PC, Bittner M, Weber M, Arantes M, Prayer D. The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cereb Cortex. 2011;21(5):1076–1083. doi: 10.1093/cercor/bhq179. doi:10.1093/cercor/bhq179. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. doi:10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. doi:10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kikinis R, Shenton ME, Gerig G, Hokama H, Haimson J, O'Donnell BF, Wible CG, McCarley RW, Jolesz FA. Temporal lobe sulcogyral pattern anomalies in schizophrenia: an in vivo MR three-dimensional surface rendering study. Neurosci Lett. 1994;182:7–12. doi: 10.1016/0304-3940(94)90192-9. doi:10.1016/0304-3940(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK. Neural correlates of formal thought disorder in schizophrenia: preliminary findings from a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(8):769–774. doi: 10.1001/archpsyc.58.8.769. doi:10.1001/archpsyc.58.8.769. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK. Reversed lateralization of temporal activation during speech production in thought disordered patients with schizophrenia. Psychol Med. 2002;32(3):439–449. doi: 10.1017/s0033291702005287. [DOI] [PubMed] [Google Scholar]
- McGlone J. Sex differences in human brain asymmetry: a critical survey. Behav Brain Sci. 1980;3:215–263. doi:10.1017/S0140525X00004398. [Google Scholar]
- McIntosh A, Moorhead T, Job D, Lymer G, Muñoz Maniega S, McKirdy J, Sussmann JE, Baig BJ, Bastin ME, Porteous D, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Mol Psychiatry. 2008;13:1054–1059. doi: 10.1038/sj.mp.4002103. doi:10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Zetzsche T, Preuss U, Frodl T, Leinsinger G, Möller HJ. Does the definition of borders of the planum temporale influence the results in schizophrenia? Am J Psychiatry. 2002;159(7):1198–1200. doi: 10.1176/appi.ajp.159.7.1198. doi:10.1176/appi.ajp.159.7.1198. [DOI] [PubMed] [Google Scholar]
- Métin C, Vallee R, Rakic P, Bhide P. Modes and mishaps of neuronal migration in the mammalian brain. J Neurosci. 2008;28:11746–11752. doi: 10.1523/JNEUROSCI.3860-08.2008. doi:10.1523/JNEUROSCI.3860-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Kim S, Thompson PM, Szeszko P, Robinson D, Luders E, Toga AW. Abnormal gyral complexity in first-episode schizophrenia. Biol Psychiatry. 2004;55:859–867. doi: 10.1016/j.biopsych.2003.12.027. doi:10.1016/j.biopsych.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Sharma T, Moussai J, Zoumalan C, Rayman J, Toga AW. Three-dimensional mapping of gyral shape and cortical surface asymmetries in schizophrenia: gender effects. Am J Psychiatry. 2001;158:244–255. doi: 10.1176/appi.ajp.158.2.244. doi:10.1176/appi.ajp.158.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. doi:10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. doi:10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Priddle TH, Crow TJ. Protocadherin 11X/Y a human-specific gene pair: an immunohistochemical survey of fetal and adult brains. Cerebral Cortex. 2012;23(8):1933–1941. doi: 10.1093/cercor/bhs181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan V, Scott J, Habas PA, Kim K, Corbett-Detig J, Rousseau F, Barkovich AJ, Glenn OA, Studholme C. Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero. J Neurosci. 2011;31(8):2878–2887. doi: 10.1523/JNEUROSCI.5458-10.2011. doi:10.1523/JNEUROSCI.5458-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. doi:10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Savadjiev P, Kindlmann GL, Bouix S, Shenton ME, Westin CF. Local white geometry from diffusion tensor gradients. Neuroimage. 2010;49(4):3175–3186. doi: 10.1016/j.neuroimage.2009.10.073. doi:10.1016/j.neuroimage.2009.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. doi:10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. doi:10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Stoll AL. The psychopharmacology reference card: antipsychotic treatment guide. Belmont (MA): McLean Hospital; 2001. [Google Scholar]
- Taylor DC. Schizophrenias and epilepsies: why? when? how? Epilepsy Behav. 2003;4:474–482. doi: 10.1016/s1525-5050(03)00160-4. doi:10.1016/S1525-5050(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. doi:10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23:339–352. doi: 10.1017/S0954579410000842. doi:10.1017/S0954579410000842. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Savadjiev P, Kubicki M, O'Donnell LJ, Terry DP, Bouix S, Westin CF, Schneiderman JS, Bobrow L, Rausch AC, et al. Fiber geometry in the corpus callosum in schizophrenia: evidence for transcallosal misconnection. Schiz Res. 2011;132:69–74. doi: 10.1016/j.schres.2011.07.010. doi:10.1016/j.schres.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NA, Close JP, Giouzeli M, Crow TJ. Accelerated evolution of Protocadherin11X/Y: a candidate gene-pair for cerebral asymmetry and language. Am J Med Genet (Part B) 2006;141B:623–633. doi: 10.1002/ajmg.b.30357. doi:10.1002/ajmg.b.30357. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL. Anatomical development of the corpus callosum in humans: a review with reference to sex and cognition. In: Molfese DL, Segalowitz SJ, editors. Brain lateralisation in children, developmental implications. New York: The Guilford Press; 1988. pp. 35–57. [Google Scholar]