Abstract
Personal attitude toward ambiguity contributes to individual differences in decision making in uncertain situations. Operationally, these attitudes reflect the various coping strategies elected to overcome the limited information. A key brain region involved in cognitive control for performance adjustments is the dorsal anterior cingulate cortex (dACC). To test how dACC functional network connectivity would be modulated by uncertainty and differ between individuals, 24 healthy participants underwent functional MRI in 3 sequential runs: 1 resting-state and 2 decision-making task runs. Individuals with lower nonplanning impulsiveness made greater use of a Pass option and avoided uncertain ambiguous situations. Seed-based functional connectivity analysis during the task runs revealed that stronger activation synchrony between the left dACC and the right anterior insula correlated with greater use of a Pass response option. During the resting-state, stronger resting-state functional connectivity between the left dACC and the ventral striatum predicted the adoption of Pass as a behavioral strategy and correlated with stronger task-activated synchrony between the dACC and the right anterior insula. Our findings indicate that that the synchrony between the dACC and insula-striatal circuitry was greater in individuals with low compared with high nonplanning impulsiveness and contributed to adopting Pass as a useful behavioral strategy.
Keywords: ambiguity, anterior cingulate cortex, functional connectivity, impulsivity, uncertainty
Introduction
Decision making requires the ability to adopt behavioral strategies to accommodate to specific contexts and to weigh the number and nature of available options to enhance chances of making a desirable or advantageous decision. Many decisions, however, must be made with limited information to inform or predict outcomes, situations resulting in risky choices because of uncertainty (Huettel et al. 2006). Individuals differ widely in risk perception and in willingness to engage in uncertain situations, which affects coping strategies to overcome uncertainty and biases recruitment of neural networks engaged (Critchley et al. 2001; Hsu et al. 2005).
Converging evidence indicates that processing uncertainty is associated with 2 distinct neural circuits: decisions involving risk, that is, uncertainty with known probabilities, are associated with the orbitofrontal cortex and the rostral extent of the anterior cingulate cortex (rACC), whereas decisions involving ambiguity, that is, uncertainty with unknown probabilities (Huettel et al. 2006), are associated with the dorsolateral prefrontal cortex and the dorsal extent of the anterior cingulate cortex (dACC) (Krain et al. 2006). Although risk can be computed by the expected probabilities of different options, the cognitive processes that contribute to ambiguity are still under debate (Huettel et al. 2006). Based on the notable overlaps between the neural networks associated with uncertainty and networks subserving cognitive control (Mushtaq et al. 2011), the cognitive control of adaptive behavior in uncertain situations has emerged as a process requiring a neurally based mechanism (Behrens et al. 2007; Rushworth and Behrens 2008).
The dACC has been considered to be a principal locus of conflict monitoring (Carter et al. 1998). Its function has been implicated in various uncertainty-related paradigms, including hypothesis testing (Elliott and Dolan 1998), anticipatory arousal (Critchley et al. 2001), prediction difficulty (Schubotz and von Cramon 2002), volatility in information (Behrens et al. 2007), and belief updating (Stern et al. 2010). Recently, the dACC was implicated in context monitoring and executive control signaling, which functions are purported to adjust behavior and guide performance in line with other prefrontal cortical activities (Ridderinkhof et al. 2004; Walton et al. 2004; Venkatraman, Rosati, et al. 2009). Imaging studies have demonstrated that functional connectivity between the dACC and the anterior insula predicted loss-minimizing choices, whereas the functional connectivity between the dACC and the dorsolateral prefrontal cortex predicted strategies attending to overall probability of winning (Venkatraman, Payne, et al. 2009). Although these results demonstrate that the dACC shapes behavioral choices through changes in its functional connectivity, it remains unclear whether the dACC is part of a cognitive control network engaged when adopting a new strategy to optimize responses in ambiguous situations, or, alternatively, is part of an intrinsic functional network marking an intrinsic preference (e.g., harm avoidance) predisposing adoption of a particular response strategy.
We developed a novel decision-making task for use with functional imaging that provided “Pass” as an alternative response option to avert ambiguous situations. We considered the use of Pass as a behavioral measure of ambiguity aversion and its related cognitive processes. The dACC is typically engaged in multiple-choice situations that involve uncertainty (Venkatraman, Payne, et al. 2009) and in risk taking (Rao et al. 2008). Thus, we tested the hypothesis that patterns of functional connectivity of the dACC with the anterior insula and the dorsolateral prefrontal cortex would be modulated in situations that require behavioral adaption to uncertainty (Sheth et al. 2012). The dACC and the anterior insula are further proposed to shape human behavior in uncertain situations (Critchley et al. 2001; Krain et al. 2006; Watson 2008; Mohr et al. 2010). Accordingly, we examined whether an intrinsic functional connectivity pattern between the dACC and the anterior insula could predict the behavioral responses related to adopting Pass as a behavioral strategy.
Operationally, the ability to deal with ambiguity evokes various coping strategies to account for the limited information; however, the component processes that contribute to ambiguity aversion are incompletely understood (Huettel et al. 2006; Bach et al. 2009). In this study, we examined how individual differences in impulsivity affect the neural and behavioral responses to uncertain ambiguous stimuli. Previous studies proposed that impulsive individuals fail to consider multiple contexts in uncertain situations and reported that impulsivity was associated with weaker prefrontal activations in ambiguous decision making (Huettel et al. 2006). We addressed this issue by hypothesizing that individuals with lower impulsivity would be less willing, or more hesitant, to engage in uncertain ambiguous situations (ambiguity aversive) and would make more use of the “Pass” response option. Accordingly, we examined whether resting-state connectivity strength, as a marker of intrinsic functional connectivity, between the dACC and the anterior insula can predict behavioral responses related to adopting Pass as a behavioral strategy.
Materials and Methods
Participants
Participants were 24 adults (16 men, 8 women; mean age: 47.0 years, SD = 11.6; Supplementary Table S1). All were recruited from the local community through flyers, announcements, or word of mouth and provided written informed consent to participate, which was approved by the Institutional Review Boards of Stanford University School of Medicine and SRI International. All participants were administered the Structured Clinical Interview for DSM-IV-TR (First et al. 1997) by a clinical research psychologist or research nurse to exclude those who met criteria for a life-time Axis I psychiatric diagnosis.
Procedure and Stimuli
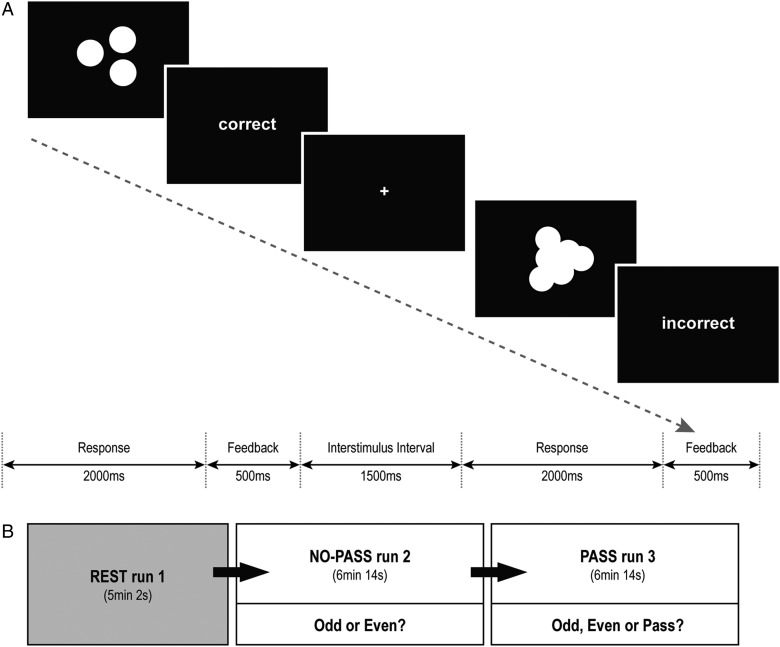
Task stimuli consisted of sets of white solid-colored circles on a black background, emulating scattered coins (Fig. 1A). Each stimulus was presented for 2000 ms; the participants were asked to estimate quickly whether the total number of coins was odd or even. The task consisted of 2 conditions: 1) a certain condition, in which the participants could easily estimate the correct answer; and 2) an uncertain condition, in which the coins were overlapped and the borders were blurred, so the participants could only make a guess. Each response was immediately followed by feedback, indicating correct (gain 1 point) or incorrect (lose 1 point). Each participant underwent 3 sequential runs (Fig. 1B): one resting-state run (REST run 1) and 2 task runs, which were designed in the same manner with the exception that “NO-PASS run 2” allowed only 2 response options (press 1 if Odd or press 2 if Even), whereas “PASS run 3” provided 3 response options (press 1 if Odd, press 2 if Even, press 3 if Pass). If the participant chose Pass, the task moved to the next trial without any gain or loss. The participants were informed that the usage of the Pass option was their choice, and no further explicit instructions about how to use the Pass option was given. The uncertain trials were designed to elicit a prediction error because the percent of correct feedback was fixed at 22.2%, regardless of the participant's responses. In this way, the task was designed into 2 sequential run: NO-PASS run 2and PASS run 3, in which the participant had to decide whether to keep guessing between ODD and EVEN or to make ambiguous-aversive choices by pressing the Pass option. Stimuli were presented via E-prime software (Psychology Software Tools, Inc.), and the participants responded through a keypad connected to the laptop running E-prime. Each task run was composed of 4 certain blocks and 4 uncertain blocks, which were presented in a pseudorandomized order with 12-s interval between the blocks. Each block consisted of 9 trials. Accuracy and reaction times (RT) were recorded. For data analysis, the subjects were divided into 2 groups—high-Pass (HP) group (N = 12) and low-Pass (LP) group (N = 12)—according to the percentage of Pass responses made.
Figure 1.
Odd-Even-Pass Task. (A) The task consisted of 2 conditions: a certain condition, in which the participants could easily estimate the correct answer; and an uncertain condition, in which the coins were overlapped and the borders were blurred so the participants could only make a guess. (B) Each participant underwent 3 serial runs: REST run 1, NO-PASS run 2, and PASS run 3. The task runs were repeated in the same manner with the exception that NO-PASS run2 allowed only 2 response options (Odd or Even), whereas PASS run 3 provided 3 response options (Odd, Even, or Pass).
Prior to the scanning session, participants repeated a practice run until accuracy rate exceeded 90% for the certain trials. The practice run was first rehearsed without a Pass option and then with a Pass option. After the scanning session, the participants completed a debriefing questionnaire evaluating their attribution about the task difficulty on a 9-point Likert scale, that is, “Why do you think the uncertain trials were difficult? (1 = totally due to me, 9 = totally due to the task).” In addition, impulsivity was measured through the self-report, the Barratt Impulsiveness Scale (BIS; Patton et al. 1995), which comprises 3 subscales: 1) Motor Impulsiveness, characterized by acting on the spur of the moment and an inconsistent lifestyle; 2) Cognitive (Attention) Impulsiveness, reflecting difficulty in focusing on the task at hand and involving “thought insertions and racing thoughts;” and 3) Nonplanning Impulsiveness, characterized by lack of planning and cognitive complexity.
Image Acquisition
MR imaging was conducted on a GE (General Electric Medical Systems, Signa, Waukesha, WI, USA) 3T whole body MRI scanner equipped with an 8-channel head coil. A dual fast spin-echo anatomical scan (axial acquisition; time echo [TE] = 17/98 ms; time repetition [TR] = 5000 ms; field of view [FOV] = 24 cm; 256 × 256 matrix; NEX = 1.0; slice thickness = 5 mm; 36 slices) was acquired together with the fMRI data. A field map was generated from a gradient recalled echo (GRE) sequence pair (TE = 3/5 ms, TR = 460 ms, slice thickness = 2.5 mm, 62 slices; (Pfeuffer et al. 2002). High-order shimming was performed before the functional scans (Kim et al. 2002). Whole-brain fMRI data were acquired with a gradient echo planar pulse sequence (axial, mode = 2D, scan timing: TE = 30 ms, TR = 2200 ms, flip angle = 90, matrix = 64 × 64, slice thickness = 5 mm, 36 slices). Two task runs of 6:14 min each, synchronized with the beginning of fMRI volume acquisitions, were acquired. Before the task runs, a resting-state run of 5:02 min was acquired, during which subjects were instructed to lie still with eyes opened, relaxed, and not to fall asleep.
Image preprocessing and fMRI Contrast Analysis
Spatial preprocessing and statistical analysis of functional images were performed using SPM8 (Wellcome Department of Cognitive Neurology). Motion artifacts were assessed in individual subjects by visual inspection of realignment parameter estimations to confirm that the maximum head motion in each axis was <2 mm and absent any abrupt head motion. One participant was excluded from the resting-state functional connectivity analysis due to excessive movement during the scan (>4 mm). Functional images were realigned and unwarped (correction for field distortions) using the gradient echo field maps (constructed from the complex difference image between 2 echoes [3 and 5 ms] of the GREs series). Unwarped functional images were registered to structural images for each subject. The anatomical volume was then segmented into gray matter, white matter, and cerebrospinal fluid. The gray matter image was used for determining the parameters of normalization onto the standard Montreal Neurological Institute gray matter template. The spatial parameters were then applied to the realigned and unwarped functional volumes that were finally resampled to voxels of 2 × 2 × 2 mm and smoothed with an 8-mm full-width at half-maximum kernel.
Individual statistics were computed using a general linear model approach (Friston et al. 1995) as implemented in SPM8. Statistical preprocessing consisted of high-pass filtering at 128 s, low-pass filtering through convolution with SPM8 canonical hemodynamic response function and global scaling. A random effect analysis was conducted for group averaging and population interference, where 1 image per contrast was computed for each subject, and these images were subjected to t-test, which produced a statistical image for the contrast Uncertain > Certain (UNC > CER) in each subject. The contrast UNC > CER of each subject entered in 1-sample t-test (N = 24) with a familywise error (FWE) corrected P-value height threshold of 0.05 and k = 10 as extent threshold for multiple comparison for the whole brain. A 2-sample t-test compared the HP group and the LP group; an uncorrected P-value height threshold of 0.001 and k = 20 as extent threshold for the whole brain was used.
Functional Connectivity Analyses
For functional connectivity analysis, the dACC seed (x = −4, y = 32, z = 32) was derived from the significant cluster in the t-contrast UNC > CER (Table 1). Seed-to-voxel connectivity was measured by correlating the time course of the BOLD activity of each task and resting-state run. Before averaging individual voxel data, the waveform of each brain voxel was filtered using a bandpass filter (0.0083/s < f < Inf for task runs; 0.0083/s < f < 0.15/s for resting-state run) to reduce the effect of low-frequency drift and high-frequency noise. Signals from the ventricular regions and signal from the white matter were removed from the data through linear regression. Connectivity analysis was conducted with the “conn” toolbox, implemented in the SPM8 (http://www.fil.ion.ucl.ac.uk/spm/ext/). To extract the brain regions that had significant functional connectivity with the dACC seed, we created exclusive masks for each group with SPM maps with a threshold at P < 0.05. Statistical inferences were thresholded using an uncorrected P-value height threshold of 0.001 for the whole-brain volume with a minimum cluster extent of 30 contiguous voxels.
Table 1.
Brain regions exhibiting significant activations to contrast Uncertain > Certain (N = 24)
| Region | BA | NO-PASS run 2a |
PASS run 3b |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| kE | Tmax | x | y | z | kE | Tmax | x | y | z | ||
| Anterior cingulate cortex | |||||||||||
| Left | 32 | 37 | 5.89 | −4 | 32 | 32 | 10 | 5.32 | 2 | 36 | 30 |
| Superior frontal gyrus | |||||||||||
| Right | 8 | 91 | 6.08 | 8 | 20 | 50 | |||||
| Anterior insula | |||||||||||
| Right | 289 | 6.43 | 52 | 20 | −2 | ||||||
| Left | 61 | 5.69 | −32 | 20 | −10 | ||||||
| Inferior frontal gyrus | |||||||||||
| Right | 44 | 81 | 5.49 | 48 | 14 | 26 | |||||
| Precentral gyrus | |||||||||||
| Right | 44 | 16 | 5.38 | 44 | 8 | 34 | |||||
| Inferior parietal lobule | |||||||||||
| Right | 40 | 14 | 5.81 | 56 | −44 | 50 | 125 | 6.34 | 62 | −46 | 42 |
| Right | 39 | 21 | 5.55 | 50 | −56 | 42 | |||||
| Inferior parietal sulcus | |||||||||||
| Left | 7 | 16 | 5.40 | −28 | −58 | 44 | |||||
| Right | 7 | 27 | 6.12 | 16 | −72 | 52 | |||||
| Fusiform gyrus | |||||||||||
| Right | 37 | 21 | 5.35 | 38 | −58 | −24 | |||||
| Cerebellum, VIIb | |||||||||||
| Right | 23 | 5.44 | 24 | −74 | −48 | ||||||
| Cerebellum, Crus II | |||||||||||
| Left | 45 | 5.85 | −30 | −74 | −44 | 272 | 6.04 | −32 | −76 | −42 | |
| Cerebellum, Crus I/II | |||||||||||
| Left | 32 | 5.74 | −16 | −82 | −32 | 51 | 5.65 | −14 | −84 | −22 | |
Threshold was FWE-corrected P < 0.05 (T = 5.04), kE > 10 voxels for the whole brain.
aWhen Pass was not provided as an alternative response option.
bWhen Pass was provided as an alternative response option.
Statistical Analysis
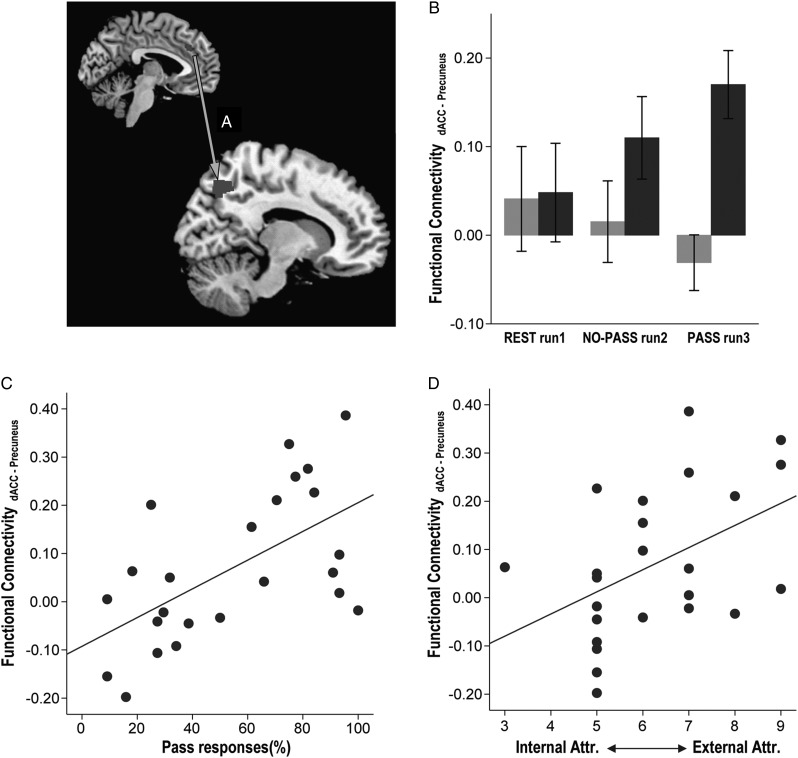
To examine brain-behavior relationships, we extracted the Fisher-transformed Z-value measures of functional connectivity between the dACC seed and the target regions of interest (ROIs) that were identified clusters through functional connectivity analysis of the task runs. Based on our hypotheses, the target ROIs for the connectivity analysis were the right anterior insula (NO-PASS run 2 [x = 38, y = 12, z = 0]; PASS run 3 [x = 28, y = 22, z = 8]) and right dorsolateral prefrontal cortex (NO-PASS run 2 [x = 46, y = 40, z = 12]; PASS run 3 [x = 32, y = 30, z = 28]). In addition, the right precuneus (PASS run 3 [z = 12, y = −68, z = 44]) was selected as a ROI to examine the recruitment of the default mode network (Fransson and Marrelec 2008) relative to recruitment of a task-activated network during external agency (Ruby and Decety 2001). We further selected the ventral striatum as an ROI (REST run 1 [x = 16, y = 6, z = −12], [x = −6, y = 2, z = 0]), because it is part of the reward circuit (Redgrave et al. 2011) and structurally interconnected with anterior insula (Chikama et al. 1997; Fudge et al. 2005). Recent studies have reported that resting-state ventral striatal activity predict anterior insular activity (Cauda et al. 2011).
To compare behavioral performance (accuracy rate, RT) and the functional seed-target connectivity across runs, we conducted a repeated measures analysis of variance (ANOVA) with 2-tailed P < 0.05. Pearson correlations tested relations between the functional seed–target connectivity and mean RTs. Spearman correlation analyses tested relations between the functional seed–target connectivity and 1) the percentage of Pass responses and 2) the subjective attribution of uncertainty. To correct for multiple correlation analyses, we applied a false discovery rate corrected threshold (P = 0.010) that was calculated according to the procedure of Benjamini and Hochberg (1995). Discriminant function analysis determined whether connectivity could predict a subject would be a HP or a LP. Statistical analyses were conducted with SPSS (Chicago, IL, USA).
Results
Use of Pass Responses
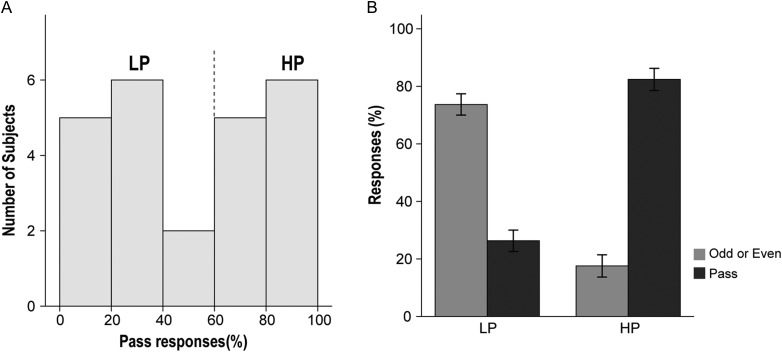
Participants underwent 3 sequential runs: REST run 1, NO-PASS run 2, and PASS run 3. The percentage of Pass responses, measured only in PASS run 3, was highly variable across subjects (9–100%) and represented a bimodal distribution (Fig. 2A). Subjects who made Pass responses >50%, which was the median, were defined as the HP group (n = 12), whereas subjects who made Pass responses less than 50% of the time were defined as the LP group (n = 12). The mean percentage of Pass response of the HP group (82.4%, SD = 12.5) was significantly higher than that of the LP group (26.3%, SD = 11.0; t = 11.199, P < 0.001) (Fig. 2B). There was no significant difference between the HP group and LP group in age, sex, education years, or estimated IQ (Supplementary Table S1).
Figure 2.
Percentage of Pass responses. (A) The distribution of percentage of Pass responses demonstrated a bimodal distribution, which divided the participants into 2 groups: the HP group (n = 12) and the LP group (n = 12). (B) The mean percentage of Pass responses of the HP group was significantly higher than that of the LP group (t = 11.199, P < 0.001). (Sidebars represent ±1 standard error).
Accuracy Rate and Reaction Time
In certain trials, the mean percentage of correct responses exceeded 97.0% in both the HP group and the LP group. ANOVA indicated that there was no significant effect of group (F1,22 = 0.597, P = 0.449) or run (F1,22 = 0.242, P = 0.628) on the accuracy rate. In uncertain trials, the percentage of correct feedback was predetermined at 22.2% and was unrelated to the subjects’ responses. However, whenever a subject failed to respond within 2000 ms (missing response), a negative feedback (no response was detected) was presented and was counted as an incorrect response. ANOVA revealed significant effects of group (F1,22 = 24.646, P < 0.001) and run (F1,22 = 210.408, P < 0.001) on the accuracy rate (Supplementary Table S2).
ANOVA indicated significant effects of group (F1,22 = 7.607, P = 0.011), run (F1,22 = 19.858, P < 0.001), and condition (uncertain vs. certain, F1,22 = 142.486, P < 0.001) on the mean RT (Supplementary Fig. S1). The group-by-condition interaction was also significant (F1,22 = 6.063, P = 0.022) with the HP group (certain = 821.5 ms, SD = 116.6 ms; uncertain = 1184.4 ms, SD = 272.1 ms) demonstrating faster RT in uncertain trials relative to the LP group (certain = 906.5 ms, SD = 192.3 ms; uncertain = 1458.0, SD = 191.1 ms).
Correlation Between Impulsiveness and Behavioral Performance
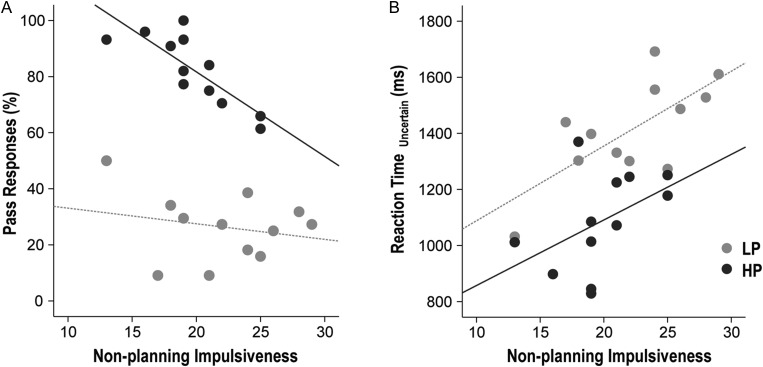
During PASS run 3, the percentage of Pass responses demonstrated a negative correlation trend with nonplanning impulsiveness (ρ = −0.456, P = 0.025) but not with cognitive impulsiveness (ρ = 0.146, P = 0.496) or motor impulsiveness (ρ = −0.387, P = 0.061). The negative correlation between Pass responses and nonplanning impulsiveness was driven mainly by the HP group (r = −0.819, P = 0.001) and not the LP group (r = −0.221, P = 0.490; Fig. 3A).
Figure 3.
Correlation between impulsivity and task performance. During PASS run 3, the percentage of Pass responses demonstrated a negative correlation with nonplanning impulsiveness within the HP group (r = −0.819, P = 0.001), but not within the LP group (r = −0.221, P = 0.490; Fig. 3A). During Pass run 3, slower RTs in uncertain trials correlated with nonplanning impulsiveness within the LP group (r = 0.709, P = 0.010) but not within the HP group (r = 460, P = 0.132; Fig. 3B).
During PASS run 3, slower RTs in uncertain trials correlated with nonplanning impulsiveness (ρ = 0.533, P = 0.007), but not with cognitive impulsiveness (ρ = 0.093, P = 0.665) or motor impulsiveness (ρ = 0.356, P = 0.088). This positive correlation between slower RTs and nonplanning impulsiveness was significant within the LP group (r = 0.709, P = 0.010) but not within the HP group (r = 460. P = 0.132; Fig. 3B). The correlation between RTs in certain trials and impulsiveness was not significant.
Subjective Attribution
The HP group had a stronger attribution to external factors (i.e., “The task was too difficult”) than internal factors (i.e., “I was not good”) in comparison with the LP group. The mean score of the HP group (6.9, SD = 1.6) in response to “Why do you think the uncertain trials were difficult?” was significantly higher than that of the LP group (5.6, SD = 1.3; t = 2.263, P = 0.034).
Brain Activations Associated With Uncertainty
In NO-PASS run 2, when Pass was not a response option, the contrast between uncertain and certain trials (UNC > CER) was associated with significant BOLD responses in the left dorsal anterior cingulate cortex (dACC), right superior and inferior frontal gyrus, right and left anterior insula, right inferior parietal lobule, left inferior parietal sulcus (IPS), right fusiform gyrus, and the cerebellum (7b and Crus II). In PASS run 3, when Pass was as an alternative option, uncertain trials in contrast to certain trials (UNC > CER) was associated with significant BOLD activity in the dACC, precentral gyrus, right inferior parietal lobule, right IPS, and left cerebellum (Crus I and II) (Table 1).
Between-group differences were observed in the cerebellum, which demonstrated stronger BOLD responses in the LP group relative to the HP group (Supplementary Table S5). By contrast, the difference between certain trials to uncertain trials (CER > UNC) showed no significant brain activations either in NO-PASS run 2 or PASS run 3.
Functional Connectivity of the dACC in Task Runs
In NO-PASS run 2, left dACC activity synchronized with activations in the left dorsolateral prefrontal cortex, bilateral anterior insula, bilateral precentral gyrus, and bilateral IPS in the HP group but not the LP group. In PASS run 3, left dACC activity synchronized with activations of the bilateral frontal pole, ventromedial prefrontal cortex (vmPFC), bilateral anterior insula, right precentral gyrus, left ventral striatum, bilateral left ventral pallidum, posterior cingulate cortex (PCC), right precuneus and the bilateral calcarine gyrus in the HP group but not the LP group (Supplementary Table S6).
Functional Connectivity of the dACC in Resting-State Run
The left dACC showed synchronous activity with the right frontal pole, left dorsolateral prefrontal cortex, ACC, right and left ventral striatum, and dorsomedial thalamus in the HP but not the LP group. In contrast, the left dACC showed synchronous activity with the right dorsolateral prefrontal cortex, bilateral precentral gyrus, superior frontal gyrus, PCC, and the right cerebellum in the LP but not the HP group (Supplementary Table S7).
Correlation Between Task-Activated Functional Connectivity and Behavioral Response
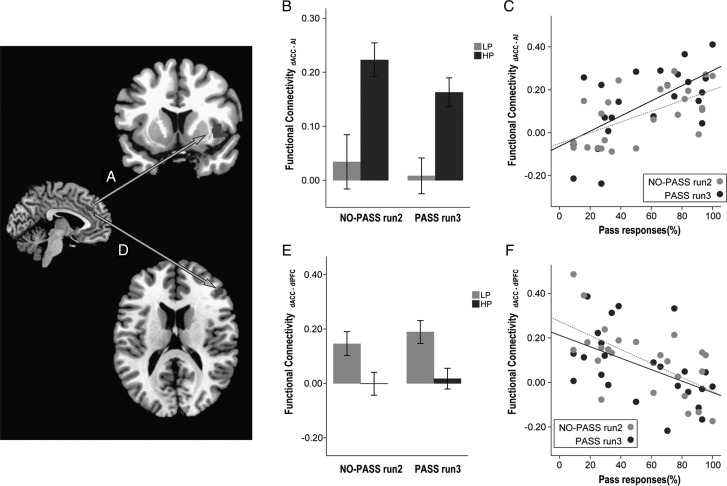
Greater use of the Pass option correlated with stronger synchronous activity between left dACC and right anterior insula (Fig. 4A), both in NO-PASS run 2 (ρ = 0.581, P = 0.003) and PASS run 3 (ρ = 0.574, P = 0.003; Fig. 4C). By contrast, less use of Pass correlated with stronger synchronous activity between the left dACC and the right dorsolateral prefrontal cortex, both in NO-PASS run 2 (ρ = −0.559, P = 0.005) and PASS run 3 (ρ = −0.594, P = 0.002; Fig. 4F). Discriminant function analysis demonstrated that the dACC-right anterior insula synchrony and dACC-right dorsolateral prefrontal cortex synchrony of run 2 could predict whether the individual would be a HP or a LP in the following run3 with 79.2% accuracy (Wilk's λ = 0.593, χ2 = 10.962, df = 2,P = 0.004).
Figure 4.
Correlation between task-activated functional connectivity and task performance. Synchronous activity between dACC and the right anterior insula (AI, A) was stronger in the HP group compared to the LP group (B; F1,22 = 23.908, P = 0.001). Stronger synchronous activity between dACC and the right AI, both in NO-PASS run 2 and PASS run 3, predicted greater use of Pass in PASS run 3 (C). By contrast, synchronous activity between dACC and the dorsolateral prefrontal cortex (DLPFC, D) was stronger in the LP group compared to the HP group (E; F1,22 = 17.436, P = 0.001) and stronger synchronous activity between dACC and the right DLPFC, both in NO-PASS run 2 and PASS run 3, predicted less use of Pass in PASS run 3 (F). (Sidebars represent ±1 standard error).
Greater use of Pass correlated with stronger synchronous activity between left dACC and right precuneus in PASS run 3 (ρ = 0.559, P = 0.004; Fig. 5C), but not in NO-PASS run 2 (ρ = 0.238, P = 0.262). In addition, stronger synchronous activity between left dACC and right precuneus showed a correlation trend with higher external attribution scores (ρ = 470. P = 0.021; Fig. 5D).
Figure 5.
Correlation between task-activated functional connectivity, task performance, and subject attribution. Synchronous activity between the dACC and the right precuneus (A) was stronger in the HP group compared to the LP group (F1,21 = 6.534, P = 0.018; B). Stronger dACC-precuneus synchrony correlated with higher use of Pass (C) and higher attribution to external factors of uncertainty (D). (Sidebars represent ±1 standard error).
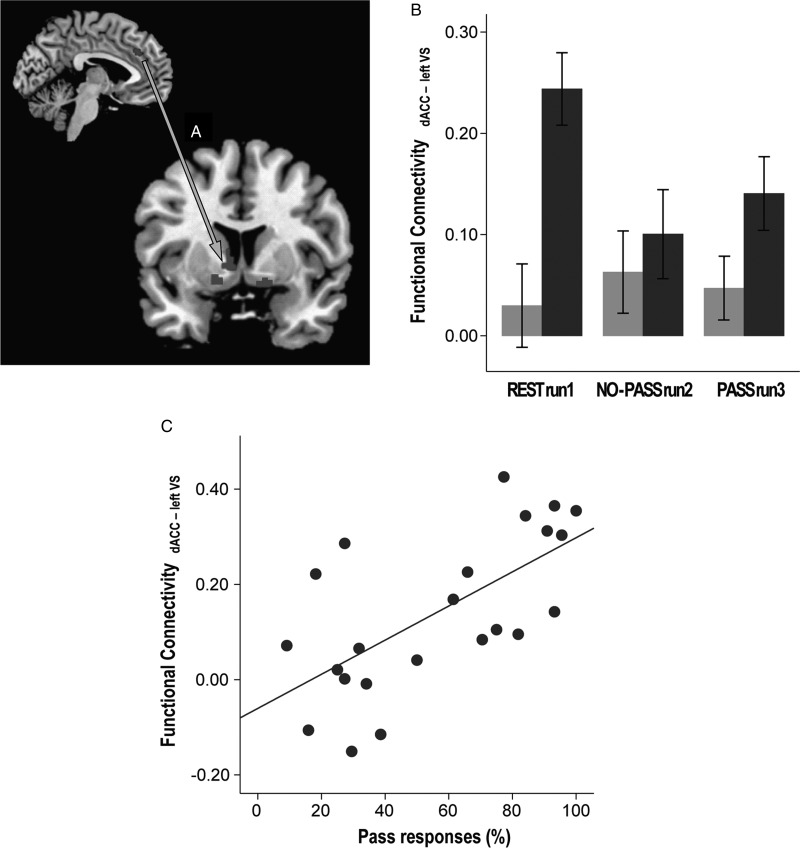
Correlation Between Resting-State Functional Connectivity and Behavioral Response
Greater use of Pass demonstrated correlation with resting-state left dACC-left ventral striatum synchrony (ρ = 0.658, P = 0.001; Fig. 6C). Discriminant function analysis demonstrated that the resting-state dACC-left ventral striatum and dACC-right ventral striatum synchrony could predict whether the individual would be a HP or a LP in the following run3 with 87.0% accuracy (Wilk's λ = 0.534, χ2 = 12.543, df = 2, P = 0.002).
Figure 6.
Correlation between resting-state functional connectivity, task performance, and impulsivity. The synchronous activity between dACC and the left ventral striatum (VS; A) was stronger in the HP group compared to the LP group (F1,21 = 7.739, P = 0.011; B). Stronger resting-state dACC-VS synchrony correlated with greater use of Pass in PASS run 3 (C). (Sidebars represent ±1 standard error).
Correlation Between Resting-State and Task-Activated Functional Connectivity
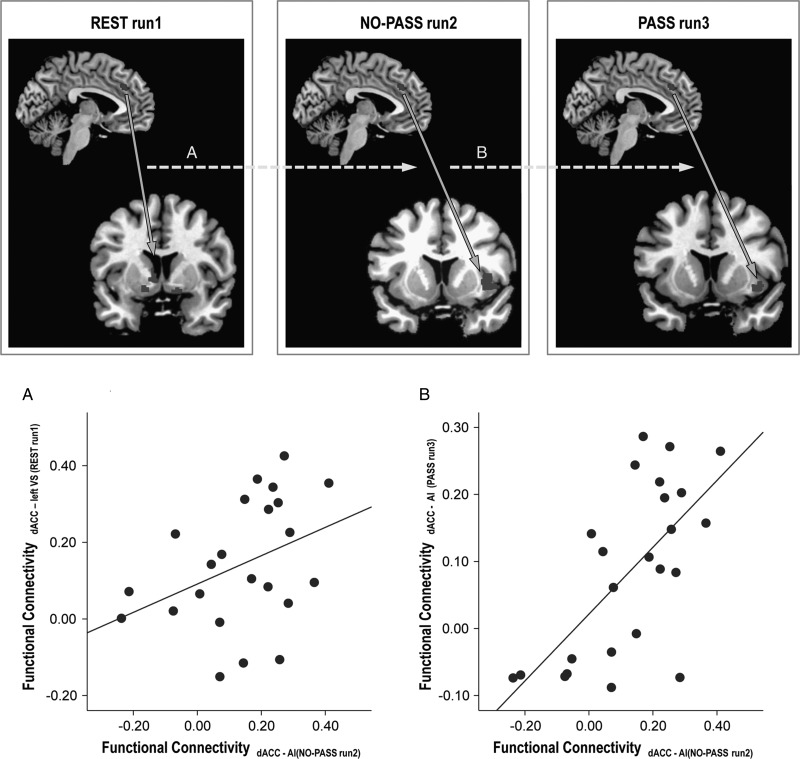
The resting-state left dACC-left ventral striatum synchrony demonstrated a correlation with the task-activated left dACC—right anterior insula synchrony during NO-PASS run 2 (ρ = 0.436, P = 0.038; Fig. 7A). Similarly, the task-activated left dACC—right anterior insula synchrony of NO-PASS run 2 correlated with the left dACC -right anterior insula synchrony of PASS run 3 (ρ = 0.659, P < 0.001; Fig. 7B).
Figure 7.
Correlation between resting-state and task-activated functional connectivity. Stronger resting-state functional connectivity between the dACC and the left ventral striatum (VS) predicted stronger dACC-AI synchrony in NO-PASS run 2 (A). In the same way, stronger dACC-AI synchrony in NO-PASS run 2 predicted stronger dACC-AI synchrony in PASS run 3 (B).
Discussion
Our results reveal 3 novel findings regarding neural networks implicated in uncertainty and the cognitive control of adaptive behavior. First, task-activated functional connectivity between the left dACC and the right anterior insula correlated with greater use of the Pass option, whereas functional connectivity measured between the left dACC and the right dorsolateral prefrontal cortex correlated with less use of the Pass option. Second, adopting the Pass option as a behavioral strategy correlated with lower nonplanning impulsiveness, which likely contributed to the aversive attitude toward ambiguity. Third, stronger resting-state functional connectivity between the left dACC and the ventral striatum predicted the adoption of Pas as a behavioral strategy and correlated with stronger task-activated synchrony between the dACC and the right anterior insula.
Uncertainty-related Brain Activity
The contrast between uncertainty and certainty revealed significant BOLD responses in the dorsolateral prefrontal cortex, parietal cortex, and cerebellum, which imply that that the executive control network (Habas et al. 2009) was the primary brain system activated in response to the uncertain trials. Previous studies have shown that the brain responses to uncertainty largely depend on the demands of the experimental task (Huettel et al. 2005), and that there is notable overlap between the neural networks associated with uncertainty and networks subserving cognitive control (Mushtaq et al. 2011). The executive control network is likely linked to the cognitive processes implicated in the primary response selection between numerical estimates of Odd or Even. Comparing the 2 groups, it is noteworthy that the LP group demonstrated stronger cerebellar activity in response to uncertain conditions. The cerebellum has been reported to be involved in the habituation of exploratory behavior to external stimuli, while cerebellar lesions resulted in active or passive avoidant behavior (Caston et al. 1998; Bauer et al. 2011). We might speculate that the stronger activation of the cerebellum reflects the stronger exploratory motivation in the LP group, whereas the HP group recruited other networks to avoid the ambiguous conditions, which is discussed in the following sections.
Task Activated Synchronous Activity Between dACC and Right Anterior Insula
During NO-PASS run 2, the HP group demonstrated synchronous activity between the left dACC and right anterior insula, which was not observed in the LP group. This relation is consistent with previous observations of stronger anterior insula activations correlating with greater harm avoidance and neuroticism (Paulus et al. 2003). The dACC and right anterior insula comprise the anchor of the emotional salience network, and its functional connectivity was reported to correlate with subject ratings of prescan anxiety (Seeley et al. 2007). Supporting evidence for the link between dACC and anterior insula in ambiguity-aversion derives from our correlation analysis. Stronger synchronous activity between left dACC and right anterior insula correlated with not only in PASS run 3 but also in NO-PASS run 2, when Pass was not even provided as a response option. These findings suggest that the synchrony between the dACC and the anterior insula is associated with ambiguity-aversive bias, which predisposes and shapes the cognitive processes responsible for response selection toward adopting Pass as a behavioral strategy.
Although the dACC is considered the key region of cognitive control for performance adjustments (Ridderinkhof et al. 2004) and strategy preference (Venkatraman, Payne, et al. 2009), the role of anterior insula in decision making has usually been linked to the interoceptive representation of the physical conditions and subjective feelings from the body, such as pain and disgust (Critchley et al. 2001). Accruing evidence suggests more comprehensive role of the anterior insula for integrating current and predictive feeling states, which are modulated by individual preferences such as risk aversion and contextual appraisal (Singer et al. 2009). The anterior insula encodes both risk prediction and “risk prediction error,” in analogy with the dopamine reward system, which encodes both reward prediction and reward prediction errors (Preuschoff et al. 2008). In our study, activation of the bilateral anterior insula in response to uncertainty occurred in NO-PASS run 2 but not in PASS run 3. This difference in local neural recruitment might be related to the risk prediction error between the predicted risk (50% chance) and the realized risk (predetermined correct rate of 22.2%), which was experienced in NO-PASS run 2 but was avoidable in PASS run 3.
In addition to the anterior insula, the ventral striatum was activated in the HP group in contrast to the LP group, which implies that avoiding the aversive ambiguous state, on the other hand, was perceived as a reward or relief (Andreatta et al. 2012). Decision under uncertainty must consider both the magnitude of each outcome and the probability of its occurrence (Berns and Bell 2012). While various regions—anterior insula and ACC—have been reported to encode information related to probability (Hsu et al. 2005; Huettel et al. 2006), the ventral striatum has been consistently linked with processing information related to magnitude (Knutson et al. 2001). Diverse integration processes of magnitude and probability information should contribute to the individual differences in decision under uncertainty (Berns and Bell 2012).
Nonplanning Impulsiveness and Subjective Attribution
As hypothesized, the number of Pass responses correlated with lower nonplanning impulsiveness. In addition, subjects with lower nonplanning impulsiveness responded faster to uncertain trials. Although the attitude toward ambiguity cannot be linked to a single component, these findings indicate that individuals with lower nonplanning impulsiveness had a tendency to avoid uncertain trials. Traditionally, nonplanning impulsiveness has been assessed by the delay-discounting task, which focuses on the inability of planning for the future. We can assume that individuals with higher nonplanning impulsiveness were more “present-oriented” and did not think in advance about the following PASS run 3 during NO-PASS run2. On the other hand, these findings might be related to another characteristic of nonplanning impulsiveness, which is lower cognitive complexity, that is, less willingness to do puzzles or thought experiments (Patton et al. 1995). As a result, individuals with high nonplanning impulsiveness are prone to avoid complex mental activities that require evaluating and choosing between multiple response options (Kam et al. 2012), which, in our study, may have contributed to keeping choices simple between Odd and Even and to avoiding a third response option (Pass). The 3 sub-traits of impulsivity are proposed to be dissociable and related to different cognitive processes subserving executive functions (Kam et al. 2012). Although our findings provide evidence that nonplanning impulsiveness is inversely related to the ability to adopt behavioral strategies, this effect is not dissociated from other forms of impulsivity such as cognitive and motor impulsiveness.
The dACC-dorsolateral prefrontal cortex functional connectivity correlated with the number of nonpass responses, that is, Odd and Even. The dorsolateral prefrontal cortex is known to be a key node of the executive control network (Seeley et al. 2007) and decision making under ambiguous conditions (Krain et al. 2006). In line with our findings, the dorsolateral prefrontal cortex is involved in willed action when making a choice from several response options without an explicit risk (Hyder et al. 1997). The activation of the dACC and the dorsolateral prefrontal cortex in decisions involving ambiguity has been proposed to represent cognitive “cool” executive functions in comparison to affectively laden “hot” executive functions of the orbitofrontal cortex and rACC in decision involving risk (Krain et al. 2006).
When participants made use of the Pass response, the functional connectivity pattern of the dACC changed and exhibited synchronous activity with the PCC and precuneus, which are key nodes of the default mode network (Raichle et al. 2001; Greicius et al. 2003; Fransson and Marrelec 2008). Our data provide evidence that the exploratory usage of Pass, which enabled the subjects to avoid ambiguous conditions and reduce the need of high-order cognitive control (Pearson et al. 2009; 2011), was accompanied with the recruitment of the default mode network. Our findings comport with previous studies that suggested the dACC plays a critical role in switching between the activation and deactivation of executive control network and the default mode network (Sridharan et al. 2008; Menon and Uddin 2010).
The precuneus plays a central role in self-referential processing and the experience of agency, that is, the feeling that the self is the cause of action (Cavanna and Trimble 2006; Spreng et al. 2009), and consistent with findings reporting right parietal cortex involvement in agency processing (Yomogida et al. 2010). A recent meta-analysis suggests that neural recruitment patterns differ depending on the sense of agency, with precuneus, temporo-parietal junction, pre-SMA, and dorsomedial prefrontal cortex recruitment during external agency; and insula recruitment during self-agency. In our study, external agency (reflected in high external attribution scores) was related to synchronous activity in the right precuneus and dACC. In other words, individuals who attributed uncertainty to external factors beyond their own control engaged a precuneus-dACC network more than those with an internal attribution, that is, the feeling that uncertainty is due to lack of information that could be overcome with effort (Kahneman and Tversky 1982). Correspondingly, precuneus activations have been proposed as a neural measure of lessened cognitive effort and behavioral disengagement (Zhang and Li 2010) and dACC activations as neural correlate of decision-making and behavioral adaption to uncertainty (Sheth et al. 2012). Thus, our data suggest that external agency contributed to the use of the Pass option, as a parallel cognitive process mediated through the precuneus (Cavanna and Trimble 2006), reflecting decreased cognitive effort or an effort-avoidance attitude in individuals who “opted-out” of ambiguity by choosing the Pass option.
Resting-state Synchronous Activity Between dACC and Ventral Striatum
Expanding our functional connectivity findings to the resting-state run, we found stronger intrinsic functional connectivity between the left dACC and the left ventral striatum in the HP group, which correlated with task-activated functional connectivity between the left dACC and the right anterior insula. The anterior insula and ventral striatum are structurally interconnected (Chikama et al. 1997; Fudge et al. 2005) and implicated in the categorization of uncertain stimuli (Grinband et al. 2006). A recent combined functional connectivity and structure-based meta-analysis has reported that resting-state spontaneous activity in ventral striatum predicts activity in the insula (Cauda et al. 2011). Furthermore, research in adolescents suggests that risky behaviors are associated with an imbalance between the regulatory circuit (dACC) and the reward circuit (ventral striatum; Van Leijenhorst et al. 2010). These relations are consistent with our current findings by showing that resting-state dACC-ventral striatum synchrony correlated not only with dACC-anterior insula synchrony in the following task runs and also with behavioral strategy, that is, the percentage of Pass responses.
Conclusion
We investigated the functional network interacting with the dACC and its pattern in individuals who had a strategic preference in a decision-making context of ambiguity aversion. Our findings demonstrated that the functional synchrony between the left dACC and the right anterior insula were associated with ambiguity aversion, which correlated with lower nonplanning impulsiveness. In addition, the resting-state intrinsic functional connectivity between the left dACC and the ventral striatum correlated with the task-activated functional connectivity between the left dACC and the right anterior insula and predicted the adoption of Pass as a behavioral strategy.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institute of Health grants (AA010723, AA012388, AA017168, AA017923, and AG017919).
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Andreatta M, Fendt M, Mühlberger A, Wieser MJ, Imobersteg S, Yarali A, Gerber B, Pauli P. Onset and offset of aversive events establish distinct memories requiring fear and reward networks. Learn Mem. 2012;19:518–526. doi: 10.1101/lm.026864.112. [DOI] [PubMed] [Google Scholar]
- Bach DR, Seymour B, Dolan RJ. Neural activity associated with the passive prediction of ambiguity and risk for aversive events. J Neurosci. 2009;29:1646–1656. doi: 10.1523/JNEUROSCI.4578-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DJ, Kerr AL, Swain RA. Cerebellar dentate nuclei lesions reduce motivation in appetitive operant conditioning and open field exploration. Neurobiol Learn Mem. 2011;95:166–175. doi: 10.1016/j.nlm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Berns GS, Bell E. Striatal topography of probability and magnitude information for decisions under uncertainty. Neuroimage. 2012;59:3166–3172. doi: 10.1016/j.neuroimage.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Caston J, Chianale C, Delhaye-Bouchaud N, Mariani J. Role of the cerebellum in exploration behavior. Brain Res. 1998;808:232–237. doi: 10.1016/s0006-8993(98)00847-6. [DOI] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D'Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioral correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. Activation of different anterior cingulate foci in association with hypothesis testing and response selection. Neuroimage. 1998;8:17–29. doi: 10.1006/nimg.1998.0344. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW National Institute of Mental Health (U.S.) User's guide for the structured clinical interview for DSM-IV axis I disorders: SCID-I clinician version. Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490:101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–763. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci. 2005;25:3304–3311. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Hyder F, Phelps EA, Wiggings CJ, LaBar KS, Blamire AM, Shulman RG. “Willed action”: a functional MRI study of the human prefrontal cortex during a sensorymotor task. Proc Natl Acad Sci USA. 1997;94:6989–6994. doi: 10.1073/pnas.94.13.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Variants of uncertainty. Cognition. 1982;11:143–157. doi: 10.1016/0010-0277(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Kam JW, Dominelli R, Carlson SR. Differential relationships between sub-traits of BIS-11 impulsivity and executive processes: an ERP study. Int J Psychophysiol. 2012;85:174–187. doi: 10.1016/j.ijpsycho.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med. 2002;48:715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanous FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PN, Biele G, Heekeren HR. Neural processing of risk. J Neurosci. 2010;30:6613–9. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq F, Bland AR, Schaefer A. Uncertainty and cognitive control. Front Psychol. 2011;2:249. doi: 10.3389/fpsyg.2011.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr Biol. 2009;19:1532–1537. doi: 10.1016/j.cub.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. 2011;15:143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer J, Van de Moortele PF, Ugurbil K, Hu X, Glover GH. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magn Reson Med. 2002;47:344–353. doi: 10.1002/mrm.10065. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Vautrelle N, Reynolds JN. Functional properties of the basal ganglia's re-entrant loop architecture: selection and reinforcement. Neuroscience. 2011;198:138–151. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. A blueprint for target motion: fMRI reveals perceived sequential complexity to modulate premotor cortex. Neuroimage. 2002;16:920–935. doi: 10.1006/nimg.2002.1183. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patal SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioral adaptation. Nature. 2012;488:218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Gonzalez R, Welsh RC, Taylor SF. Updating beliefs for a decision: neural correlates of uncertainty and underconfidence. J Neurosci. 2010;30:8032–8041. doi: 10.1523/JNEUROSCI.4729-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron. 2009;62:593–602. doi: 10.1016/j.neuron.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Rosati AG, Taren AA, Huettel SA. Resolving response, decision, and strategic control: evidence for a functional topography in dorsomedial prefrontal cortex. J Neurosci. 2009;29:13158–13164. doi: 10.1523/JNEUROSCI.2708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Watson KK. Evolution, risk and neural representation. Ann N Y Acad Sci. 2008;1128:8–12. doi: 10.1196/annals.1399.002. [DOI] [PubMed] [Google Scholar]
- Yomogida Y, Sugiura M, Sassa Y, Wakusawa K, Sekiguchi A, Fukushima A, Takeuchi H, Horie K, Sato S, Kawashima R. The neural basis of agency: an fMRI study. Neuroimage. 2010;50:198–207. doi: 10.1016/j.neuroimage.2009.12.054. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CS. A neural measure of behavioral engagement: task-residual low-frequency blood oxygenation level-dependent activity in the precuneus. Neuroimage. 2010;49:1911–1918. doi: 10.1016/j.neuroimage.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.