Abstract
Introduction:
Advancing the understanding of smoking cessation requires a complex and nuanced understanding of behavior change. To this end, ecological momentary assessments (EMA) are now being collected extensively. The time-varying effect model (TVEM) is a statistical technique ideally suited to model processes that unfold as behavior and nicotine dependence change. Coefficients are expressed dynamically over time and are represented as smooth functions of time.
Methods:
The TVEM approach is demonstrated using data from a smoking-cessation trial. Time-varying effects of baseline nicotine dependence (a time-invariant covariate) and negative affect (a time-varying covariate) on urge to smoke during a quit attempt were estimated for monotherapy, combination therapy, and placebo groups. SAS syntax for conducting TVEM is provided so that readers can adapt it for their research.
Results:
During the first 2 days after quitting, the association between negative affect and craving was significantly stronger among individuals in the placebo group, suggesting an early positive impact of treatment. For the monotherapy and combination therapy groups, during the second week of the quit attempt, baseline dependence was less strongly related to craving compared with the placebo group, indicating a different positive impact of treatments later in the quit attempt.
Conclusions:
The results reveal information about the underlying dynamics that unfold during a quit attempt and how monotherapy and combination therapy impact those processes. This suggests possible mechanisms to target in an intervention and indicates timepoints that hold the greatest promise for effective treatment. TVEM is a straightforward approach to examining time-varying processes embedded in EMA.
INTRODUCTION
The harmful effects of smoking are well understood; this behavior is the leading preventable cause of disease, disability, and death in the United States (Centers for Disease Control and Prevention [CDC], 2010). More than one-fifth of American adults smoke cigarettes, and although approximately 15 million smokers attempt to quit every year (CDC, 2002, 2004), fewer than 5% achieve long-term abstinence (CDC, 2002, 2010). Most smokers eventually relapse, despite an awareness of the consequences of smoking, motivation to quit, prior success in resisting smoking, and success in the initial withdrawal period. Even when using effective smoking-cessation aids, less than 30% achieve long-term abstinence (Fiore, Bailey, & Cohen, 2000). While much is known about the ultimate success or failure of quit attempts, far less is known about the dynamic process of quitting. Conceptualizing smoking cessation, and the role of treatments for improving chances of success, as a dynamic process rather than a specific goal (e.g., 7-day point-prevalence abstinence at 6 months postquit; Piasecki, 2006; Shiffman, Scharf et al., 2006) is relatively new. Understanding cessation as a process that unfolds over time suggests that cessation treatment may have an effect that varies over time during a cessation attempt. It is important to understand such nuances so that improved treatments, including those that adapt with time, may be developed.
Recently, there has been a strong push to use new methodological approaches to unpack the complex relations between processes leading to poor health and the impact of intervention on these processes (Institute of Medicine [IOM], 2010). Such approaches, paired with intensive longitudinal measurement of craving, lapses, mood, and contextual influences during smoking-cessation attempts, could hold the key to understanding the dynamics that unfold during a quit attempt. For instance, craving is one key component that has been shown to vary over time during a smoking-cessation attempt and be highly related to treatment efficacy and cessation success (Bolt, Piper, Theobald, & Baker,2012; Piasecki et al., 2000; Piper et al., 2011; Zhou et al., 2009; Shiffman, Ferguson, Gwaltney, Balabanais, & Shadel, 2006). Craving during a quit attempt may be related to time-invariant variables (e.g., treatment group) or time-varying variables (e.g., negative affect assessed repeatedly throughout the day). In traditional statistical models, the association between a craving and another predictor (regardless of whether it is itself time invariant or time varying) are typically assumed to be constant (i.e., a time-invariant effect). However, it may be that, in fact, the relations between craving and predictor variables do, indeed, vary over the course of a quit attempt (i.e., a time-varying effect). Understanding the relation of craving to time-varying and time-invariant variables and how these relations stay the same or vary over time could provide important insight into how best to ameliorate craving, a primary predictor of relapse.
With the advent of ecological momentary assessments (EMA; Stone & Shiffman, 1994), tobacco researchers are able to assess withdrawal symptoms throughout the day in a more reliable manner than could be obtained with periodic paper–pencil surveys, which often result in recall bias (Hughes, 2007; Shiffman, Ferguson et al., 2006; Stone & Shiffman, 2002). EMA also allows researchers to capture the immediate context surrounding momentary events such as smoking relapse. EMA data, collected over numerous days prior to and after quit date, also provide the information necessary to examine shifts in the dynamic processes that occur upon quitting (i.e., allows for reliable assessment of prequit and postquit withdrawal symptoms; McCarthy, Piasecki, Fiore, & Baker, 2006; Piper et al., 2008). Further, in the context of a smoking-cessation treatment program, EMA can provide new information about the extent to which the effects of various treatments on outcomes, such as withdrawal or smoking event, change with time during quit attempts. However, despite the increasing number of EMA studies in tobacco research, the wealth of information embedded in such datasets remains largely untapped. New statistical techniques recently have become available to analyze EMA data, offering the potential to address more complex questions about the dynamics of the smoking-cessation process.
Time-Varying Effect Model
A classic way to analyze EMA is to use a multilevel model to separate within-subject and between-subject variability in the repeatedly measured outcome and in the influence of time-varying covariates on that outcome. The between-subject effects are assumed to be constant over time and are useful to address questions such as over the course of the study, how is an individual’s mean level of negative affect associated with his or her mean nicotine craving? The within-subject effects reflect the association between an individual’s deviation from their personal mean and their level on the outcome; a corresponding question is over the course of the study, how is an individual’s momentary level of negative affect associated with his or her momentary level of craving?
Yet important predictors of nicotine withdrawal, including negative affect and baseline nicotine dependence, may exert effects that vary over time during a quit attempt. Exploring these time-varying associations, as well as how smoking-cessation treatments impact these associations, could reveal a better understanding of how different treatments work—and ultimately provide insight into how to adapt treatments to maximize their effectiveness. New methods now exist to allow smoking researchers to examine time-varying processes such as these. The time-varying effect model (TVEM), introduced in the statistical literature over a decade ago (Hastie & Tibshirani, 1993; Hoover, Rice, Wu, & Yang, 1998), is a flexible approach that allows researchers to answer questions about the dynamic associations that unfold with time. In the area of psychology, Li, Root, and Shiffman (2006) demonstrated a variation of the model with applications to smoking. However, despite the abundance of EMA data being collected in social and behavioral sciences, models with time-varying effects are not regularly used in practice. This is likely due to the fact that user-friendly software was not available until now and that the literature has lacked applications of TVEM to behavioral data. Recently, however, a SAS macro suite, %TVEM (Yang, Tan, Li, & Wagner, 2012), has been developed for fitting models with time-varying effects; an introduction to this technique is presented by Shiyko, Lanza, Tan, Li, and Shiffman (2012).
With TVEM, there is no need to assume that change over time in an outcome occurs as a parametric (i.e., linear, quadratic) function of time or that coefficients corresponding to effects of covariates on the outcome are constant over time. Instead, the direction and strength of coefficients can be estimated as a function of time using EMA from multiple individuals (i.e., regression coefficients can change with time). This approach accommodates variability across individuals in timing and spacing of observations, which is inherent in EMA studies that randomly prompt participants. Let us consider first the basic TVEM for predicting craving from a single time-varying covariate, negative affect (NA). This model can be expressed as
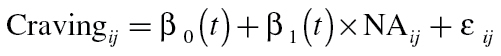 |
(1) |
where Cravingij and NAij are intensively measured longitudinal variables for subject i from assessment j measured at time t ij. In this case, the outcome Cravingij is assumed to be a continuous variable although TVEM can accommodate binary and count outcomes (Yang et al., 2012). β0 and β1 are intercept and slope parameters, respectively, that can vary with time as characterized by flexible, smooth functions of time. Within a particular time t, the typical assumptions of linear regression are made (e.g., relationships between the independent variables and the dependent variable are linear; for a continuous outcome, errors are assumed to be normally distributed). In this example, β0(t) represents mean craving over time for a centered value of negative affect. Similarly, β1(t) is a slope function describing the estimated time-varying association between negative affect and craving for the population. These intercept and slope functions are summarized graphically by plotting their values and corresponding confidence intervals over time. We note that in basic TVEMs (i.e., models with no time-varying coefficients), coefficients based on the entire sample are assumed to change with time in a smooth way. This does not imply, however, that change for an individual must be smooth; change in an individual’s level on the predictor or outcome can be sudden or even discontinuous. Further, to the extent that one can predict an outcome well with time-varying predictors, incorporating such variables can allow coefficient functions based on the entire sample to shift in a discontinuous way.
The random errors εij in Equation (1) are assumed to be continuous, but not necessarily normally distributed. The intraindividual variance structure can be specified in various ways (e.g., autoregressive, unstructured; Raudenbush & Bryk, 2002). TVEM is a nonparametric model, requiring no constraints on shapes of intercept and slope functions. Instead, shapes are estimated from the available data; the only assumption is that changes with time happen in a smooth way. This model relies on a P-spline approach to flexibly estimate the parameter functions. This is done by splitting a complex function into numerous segments, each of which is estimated with a polynomial model. A model can be perfectly fitted if the number of splitting points (referred to as knots) is large enough. However, this may introduce too much variance into the model, and the outcome curves may become wiggly (known as overfitting). On the other hand, if we fit the model with only a few knots, the curves, though smoother, may fail to capture instant changes of the data (known as underfitting). Thus, for each model there is an optimal number of knots. In the %TVEM macro calls that invoke the P-spline method, the number of knots is automatically selected to control model complexity for the coefficient functions by a maximum likelihood approach (Tan, Shiyko, Li, Li, & Dierker, 2012). More technical details about model fitting and estimation can be found in the studies by Shiyko et al. (2012) and Tan et al. (2012).
Current Study
This study demonstrates the use of a new analytic technique, TVEM, to advance understanding of craving during the smoking-cessation process. Such information may improve researchers’ ability to develop and implement effective smoking-cessation interventions. We seek a more nuanced understanding of the complex processes occurring as smokers try to quit and how treatment affects these processes. Results from this study can help to move clinical work on smoking cessation toward individualized (tailored to the person) and adaptive (tailored with time) treatments based on sound, innovative statistical findings.
METHODS
Study and Participants
The data for this study are from a randomized, placebo-controlled clinical trial of five active smoking-cessation therapies. Participants (N = 1,504; 58% female; 83% White) smoked at least 10 cigarettes a day for 6 months and were motivated to quit smoking (≥8 on a 1–10 scale where 10 is highly motivated to quit). They attended three in-person baseline sessions, in which they completed various medical assessments and a questionnaire on demographics, smoking history, and medical history. Tobacco dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND; α = 0.61; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) and the Wisconsin Inventory of Smoking Dependence Motives (Piper et al., 2004). At the third baseline session, participants were randomized to one of six conditions: bupropion slow release (SR; n = 264); nicotine lozenge (n = 260); nicotine patch (n = 262); nicotine patch + nicotine lozenge (n = 267); bupropion SR + nicotine lozenge (n = 262), or placebo (five placebo conditions that matched the five active conditions; total n = 189). To efficiently examine treatment effects, we adopted a paradigm developed by the U.S. Public Health Service Guideline: Treating Tobacco Use and Dependence (Fiore et al., 2000) where the three active monotherapy treatment conditions (bupropion, lozenge, patch) are combined into one treatment group (“Monotherapy”), and the two combination treatment conditions (patch + lozenge and bupropion + lozenge) are combined into another (“Combination Therapy”). Both groups are compared with the placebo group. We note, however, that there is no strict limit to the number of groups that can be accommodated in TVEM. All participants completed assessments at visits on their target quit day (TQD) and 1, 2, 4, and 8 weeks postquit. Participants were also followed up at 12 and 26 weeks postquit. During the period from 2 weeks pre-TQD to 2 weeks post-TQD, participants completed four daily EMA reports (just after waking, prior to bed, and at 2 random times).
Because our goal was to study dynamics related to craving during a quit attempt, our analysis is based on 1,106 participants who succeeded in establishing initial abstinence (defined as having quit for at least 24hr between the TQD and 6 days post-TQD) and did not relapse (smoke on 7 consecutive days) during the first 2 weeks following the TQD. This resulted in a total of 29,497 EMA occasions.
Measures
EMA prompts included an assessment of how participants felt within the last 15min in terms of withdrawal symptoms (e.g., negative affect, craving) using items from the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) but with a 10-point response scale to increase response variability (McCarthy et al., 2006). The longitudinal outcome in the analysis is EMA reports of craving during the first 2 weeks of participants’ quit attempts, measured with the item “Urge to smoke” from the WSWS, rated on an 11-point scale from 0 for disagree and 10 for agree. Predictors are negative affect and baseline nicotine dependence. Negative affect is a time-varying covariate assessed intensively via EMA using two items from the Positive and Negative Affect Scale (Watson, Clark, & Tellegen, 1998): feeling upset and distressed. Dependence was operationalized based on the FTND item assessing how soon after waking an individual smokes a first cigarette. Although the FTND score is not time varying, its effect may vary with time. We also controlled for smoking lapse, which we operationalized as number of cigarettes smoked since last EMA prompt, during this 2-week period by including it as a time-varying covariate. For convenience, all covariates were standardized to facilitate interpretation of results. Table 1 presents descriptive statistics of all variables examined in this study.
Table 1.
Descriptive Statistics of Study Variables for Placebo, Monotherapy, and Combination Therapy Groups
| Placebo, M (SD) | Monotherapy, M (SD) | Combination therapy, M (SD) | |
|---|---|---|---|
| Craving | 4.6 (3.6) | 4.4 (3.5) | 4.0 (3.4) |
| Negative affect | 1.7 (1.7) | 1.5 (1.5) | 1.4 (1.4) |
| Baseline dependence | 5.3 (2.1) | 5.2 (2.2) | 5.4 (2.0) |
| Number of cigarettes | 0.4 (2.0) | 0.2 (1.7) | 0.2 (1.3) |
Note. Craving, negative affect, and number of cigarettes were assessed using EMA data and represent means scores across time and individuals; baseline dependence was measured once at baseline, and it represents the mean across individuals; N = 1,106 individuals.
Analytic Approach
Models were estimated separately for the placebo, monotherapy, and combination therapy groups in order to capture all effects of treatment on the dynamic processes. An alternative (equivalent) parameterization is to include two dummy-coded indicators for treatment group membership and interaction terms for every combination between the group indicators and the other predictors. The choice of parameterization should be driven by ease of interpretation of the results. For each group, the following model was specified for predicting craving from the time-varying covariate negative affect (NA) and baseline dependence (FTND), controlling for any cigarette use (CIGUSE) during the 2-week time period:
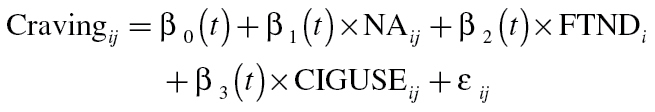 |
(2) |
where Cravingij, NAij, and CIGUSEij are intensively measured longitudinal variables for individual i from assessment j measured at time t ij and FTNDi represents baseline nicotine dependence for individual i. In this model, β0(t) represents mean craving over time for individuals with values of zero on all other predictors. Similarly, β1(t) is a slope function describing the time-varying association between negative affect and craving, β2(t) is a slope function describing the time-varying association between baseline dependence and craving, and β3(t) is a slope function describing the time-varying association between cigarette use and craving.
To prepare data for the analysis, we stacked the data so that each record contained one EMA assessment for an individual (i.e., each individual had j records in the dataset). In addition to the predictor and outcome variables described above, two additional variables were necessary to run the TVEM. First, a time variable was created, representing the time at which a given EMA took place (this is t ij). Given that assessment times were random and differed for each person, the time scale can be considered as nearly continuous. Second, to enable the program to calculate the intercept function (see Supplementary Appendix; Yang et al., 2012), we created a variable that was coded 1 for every record. Readers who wish to study technical details are referred to the study by Tan et al. (2012).
Software
The SAS macro %TVEM_normal was used to estimate the model. This macro is available free for download at methodology.psu.edu. See the Supplementary Appendix for the SAS syntax used to specify the final model for each treatment group.
RESULTS
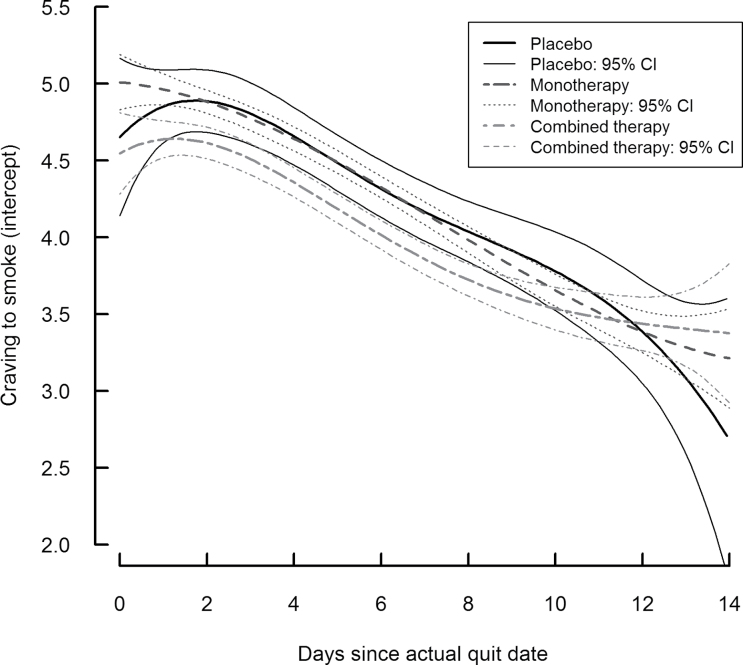
Figure 1 presents the intercept functions separately for the placebo group (solid line) and each treatment group (dashed lines), along with the corresponding 95% pointwise confidence intervals. At any point in time, the level on this curve represents the mean level of craving for nonsmoking individuals in that treatment group with average negative affect and baseline dependence (i.e., for individuals with values of 0 on all covariates). If at a particular time point a confidence interval does not include 0, there is a nonzero mean level of craving, that is, there is a significant urge to smoke. Further, if at a particular time the confidence intervals for two of the groups do not overlap, craving is statistically significant between those groups at that specific time. The TVEM SAS macro does not provide simultaneous confidence intervals, which are appropriate for overall group comparisons across the entire period of time; this is an important topic for future research. The 95% pointwise confidence intervals, however, are appropriate for comparing groups at a particular time. Figure 1 shows that, among nonsmoking individuals with average negative affect and baseline dependence, craving decreased over time for all treatment groups; between Days 5 and 8, those in the combination therapy group had significantly lower mean craving.
Figure 1.
Intercept function (i.e. time-varying mean craving during first 2 weeks of quit attempt) by treatment group.
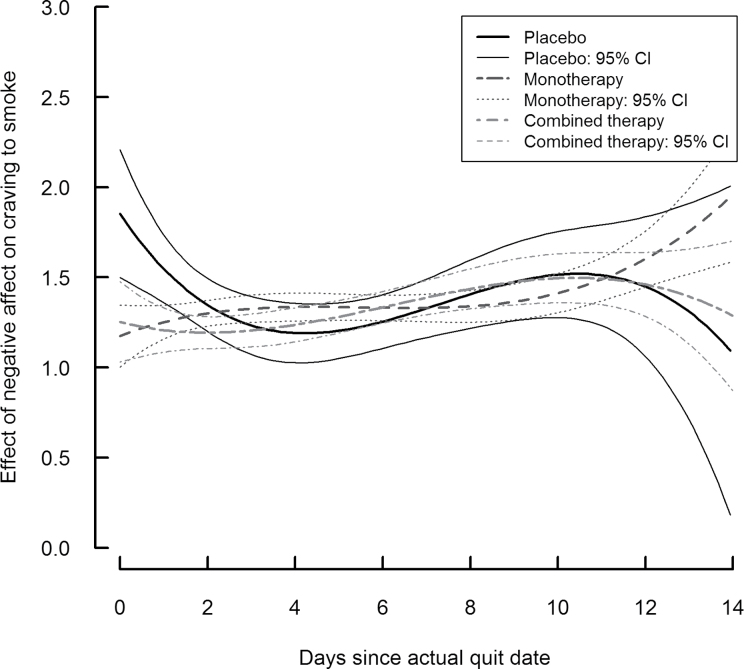
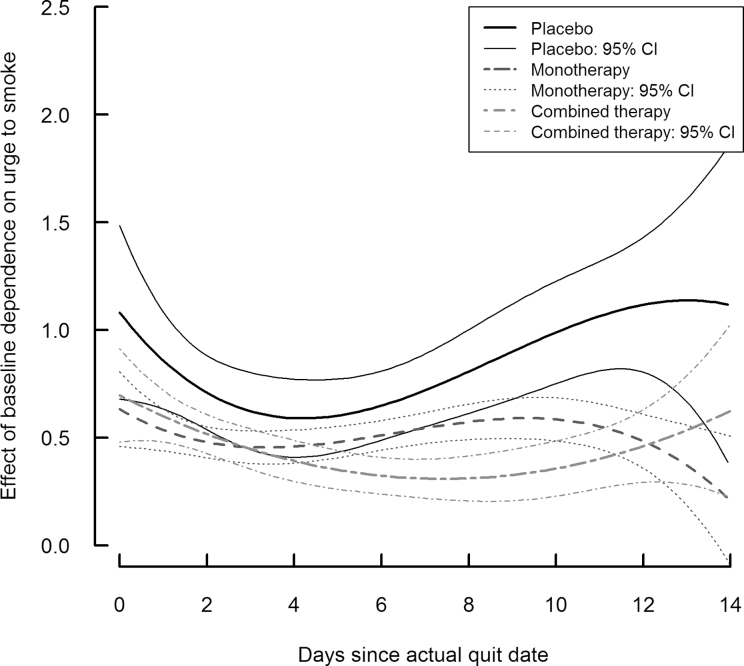
Figures 2 and 3 show the time-varying association between craving and two covariates for the placebo group (solid line) and two treatment groups (dashed lines), along with the corresponding 95% confidence intervals. The time-varying effect of negative affect is depicted in Figure 2, and the time-varying effect of baseline dependence is depicted in Figure 3. At any point in time, the level on a curve represents that time-specific association between the covariate and craving. If at a particular time point a confidence interval does not include 0, there is significant effect of the covariate on craving. Further, if at a particular time the confidence intervals for two of the groups do not overlap, the effect of the covariate on craving is significantly different between those groups.
Figure 2.
Time-varying effect of negative affect on craving by treatment group.
Figure 3.
Time-varying effect of baseline nicotine dependence on craving by treatment group.
Figure 2 shows that immediately upon quitting (Days 0 and 1), the association between negative affect and craving was significantly stronger among individuals in the placebo group, suggesting that monotherapy and combination therapy both had a positive impact early in the quit attempt. However, by Day 2 the association within the placebo group weakened to match that of the treatment group, and from Day 2 to Day 14, there was a significant positive association between negative affect and craving that did not differ between groups. This association increased slightly over the time period although it never reached the original strength observed in the placebo group. To further interpret the slight increase over time in association within the monotherapy and combination therapy groups, an increase of 1 SD on negative affect was associated with about a 1.2-unit increase in craving at the TQD, whereas a 1 SD increase in negative affect was associated with a 1.5-unit increase in craving at Day 12. The association between negative affect and craving increased over the entire time period in a roughly linear manner in the groups receiving treatment, whereas for the placebo group, there was a sharper decline during Days 0–4, followed by a somewhat sharper increase through Day 10.
Figure 3 represents the time-varying association between baseline dependence and craving, showing that they were significantly correlated at all points during the study for all three treatment groups (although this associated approached nonsignificance at Day 14 for the monotherapy group). This association was relatively stable over time for the monotherapy and combination therapy groups, with 1 SD higher baseline dependence associated with approximately a 0.5-unit higher craving. During Days 6–10, combination therapy resulted in a significantly weaker association compared with the monotherapy group. In the placebo group, the association doubled with time between Days 4–14. For these individuals, 1 SD higher baseline dependence was associated with 0.6-unit higher craving at Day 4 and 1.2-unit higher craving at Day 12. The difference between placebo and combination therapy groups was significant from Days 6 to 13, when the association between baseline dependence and craving was stronger for the placebo group. The difference between placebo and monotherapy groups was less pronounced and only present from Days 9 to 13.
DISCUSSION
Implications of Results for Theory and Practice
Consistent with previous literature, we found that overall craving levels decrease over the first 2 weeks postquit (Hughes, 2007). This demonstrates that regardless of treatment condition, craving does decrease over the first 2 weeks of a quit attempt.
It is important to disentangle the finding that negative affect becomes more strongly associated with craving over time, given that both craving and negative affect may be related to relapse risk, with craving perhaps being a more influential predictor (Chandra, Scharf & Shiffman, 2011; Piper et al., 2011; Van Zundert, Ferguson, Shiffman, & Engels, 2012). The mechanisms and direction of this association are unclear. It may be that smokers are initially able to cope with negative affect using limited alternate coping strategies, but over time, the ability to use such strategies, or the effectiveness of such strategies, may diminish, leaving smokers with less and less ability to resist the urge to smoke. Conversely, given that the association between craving and negative affect is likely bidirectional, it may be that smokers are able to tolerate cravings initially, but over time the cravings come to elicit an increasingly stronger negative affective response. This may, in turn, increase the smokers’ urges to smoke in order to alleviate such negative affect. While there was an initial effect of treatment on the association between craving and negative affect in the first few days postquit, the lack of a consistent treatment effect suggests that these treatments may not effectively decouple these constructs. Future research to explore how the association between craving and negative affect influences ability to maintain abstinence over time, and how treatments can influence this risk factor, is key to understanding the clinical relevance of this finding.
The significant association between baseline dependence and craving fits with many theories of addiction that posit that dependence influences withdrawal and craving (Edwards & Gross, 1976; Siegel, 1983; Solomon & Corbit, 1974; Wikler, 1980; although cf. Baker et al., 2012). The impact of treatment on the association between dependence and craving over time is consistent with the literature that shows nicotine replacement and bupropion exert their effects by reducing craving (Durcan et al., 2002; Lerman et al., 2002; Piper et al., 2008; Shiffman, Ferguson et al., 2006). These medications may reduce the impact of nicotine dependence on withdrawal symptoms over time, including craving, with combination pharmacotherapy having a somewhat stronger impact at times than monotherapy. However, without these medications, the underlying dependence may produce increased demand and motivation for nicotine over an extended period of abstinence. There are genetic data that support the notion that effective treatments may mitigate the genetic risk for relapse posed by dependence (Chen et al., 2012).
We note the limitation that findings may only generalize to individuals who were successful in achieving 2-week abstinence. Participants who could not be included in the analysis, primarily because of failure to achieve abstinence, had significantly higher nicotine dependence at baseline and prequit levels of craving and negative affect. In addition, participants were smokers who were motivated to quit and participate in a long-term clinical trial. Thus, these findings may not generalize to all smokers.
Potential of TVEM to Advance Knowledge in Other Areas of Health Research
EMA studies that are designed to help researchers continue to better understand the dynamics of smoking cessation are likely to play an increasing role in the future. Research on smoking cessation is leading the way toward how dynamic systems of behavior and related psychological and social processes can be studied using new methods. In recent decades, the main health risks in the United States have shifted from infectious diseases to chronic illnesses such as arthritis, cancer, depression, and diabetes (IOM, 2012). The dynamics of many of these conditions can now be studied using new technology for data collection such as smart phones and wearable devices such as pedometers. These data will provide new avenues to pursue in improving many aspects of health other than smoking behavior (see Smyth & Stone, 2003 for a summary of early research in behavioral medicine that relied on EMA). TVEM can be used to address questions that investigate changes in health and health behaviors related to such chronic conditions.
One important example is the rapid increase in the number of EMA studies that are being conducted to study aspects of obesity such as physical movement and food intake (Boseck et al., 2007; Engel et al., 2009). TVEM could be brought to bear on data from those studies to answer questions such as, “How does the association between snacking and temptations to eat in the absence of hunger change with time or context?” and “What is the impact of an intervention program on that association, and does the effect of the intervention weaken with time?” Other recent EMA studies have focused on asthma severity and coping strategies (Nazarian, Smyth, & Sliwinski, 2006), comorbid substance use (Piasecki et al., 2011), and the association between mood and craving and use of cocaine and heroin (Epstein et al., 2009). In time, more intensive assessments related to health behaviors such as smoking will become standard practice; by necessity, new methods will follow so that important, new public health questions about behavior can be addressed.
CONCLUSIONS
As data collection methods such as EMA via smart phones becomes common practice, tobacco researchers will be able to investigate more nuanced questions about the underlying dynamics of behavior change and treatment. New, user-friendly statistical approaches such as TVEM will allow those questions to be addressed. These answers will provide insight into possible mechanisms to target in interventions, and help to move clinical work on smoking cessation toward individualized (tailored to the person) and adaptive (tailored with time) treatments.
SUPPLEMENTARY MATERIAL
Supplementary Appendix can be found online at http://www.ntr.oxfordjournals.org.
FUNDING
This work was supported by Award Numbers P50-DA010075-17, R21-DA024260, P50-DA0197 and T32-DA017629 from the National Institute on Drug Abuse ; P50-CA84724 and R01-CA168676 from the National Cancer Institute; and M01-RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute on Drug Abuse, National Cancer Institute, the National Institutes of Health, or the National Center for Research Resources.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
The authors thank Dr. Junyi Lin for organizing the data used in this analysis and Amanda Applegate for feedback on an earlier draft of this manuscript.
REFERENCES
- Baker T. B., Piper M. E., Schlam T. R., Cook J. W., Smith S. S., Loh W. Y., Bolt D. (2012). Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptualizations of dependence. Journal of Abnormal Psychology, 121, 909–921. 10.1037/a0027889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt D. M., Piper M. E., Theobald W. E., Baker T. B. (2012). Why two smoking cessation agents work better than one: role of craving suppression. Journal of Consulting and Clinical Psychology, 80, 54–65 PMCID: PMC3265654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boseck J. J., Engel S. G., Allison K. C., Crosby R. D., Mitchell J. E., de Zwaan M. (2007). The application of ecological momentary assessment to the study of night eating. The International Journal of Eating Disorders, 40, 271–276. 10.1002/eat.20359 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2002). Cigarette smoking among adults: United States, 2000. Morbidity and Mortality Weekly Report, 51, 642–645 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2004). Cigarette smoking among adults: United States, 2002. Morbidity and Mortality Weekly Report, 53, 427–431 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2010). Vital signs: Current cigarette smoking among adults aged ≥18 Years: United States, 2005–2010. Morbidity and Mortality Weekly Report, 60, 1207–1212 [PubMed] [Google Scholar]
- Chandra S., Scharf D., Shiffman S. (2011). Within-day temporal patterns of smoking, withdrawal symptoms, and craving. Drug and Alcohol Dependence, 117, 118–125. 10.1016/j.drugalcdep.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. S., Baker T. B., Piper M. E., Breslau N., Cannon D. S., Doheny K. F. … Bierut L. J. (2012). Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. The American Journal of Psychiatry, 169, 735–742. 10.1176/appi.ajp.2012.11101545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan M. J., Deener G., White J., Johnston J. A., Gonzales D., Niaura R. … Sachs D. P. (2002). The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clinical Therapeutics, 24, 540–551 [DOI] [PubMed] [Google Scholar]
- Edwards G., Gross M. M. (1976). Alcohol dependence: provisional description of a clinical syndrome. British Medical Journal, 1, 1058–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S. G., Kahler K. A., Lystad C. M., Crosby R. D., Simonich H. K., Wonderlich S. A. … Mitchell J. E. (2009). Eating behavior in obese BED, obese non-BED, and non-obese control participants: a naturalistic study. Behaviour Research and Therapy, 47, 897–900. 10.1016/j.brat.2009.06.018 [DOI] [PubMed] [Google Scholar]
- Epstein D. H., Willner-Reid J., Vahabzadeh M., Mezghanni M., Lin J. L., Preston K. L. (2009). Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry, 66, 88–94. 10.1001/archgenpsychiatry.2008.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M. C., Bailey W. C., Cohen S. J. (2000). Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service [Google Scholar]
- Hastie T., Tibshirani R. (1993). Varying-coefficient models. Journal of the Royal Statistical Society, Series B, 55, 757–779 [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hoover D. R., Rice J. A., Wu C. O., Yang L. P. (1998). Nonparametric smoothing estimates of time-varying coefficient models with longitudinal data. Biometrika, 85, 809–822. 10.1093/biomet/85.4.809 [Google Scholar]
- Hughes J. R. (2007). Measurement of the effects of abstinence from tobacco: a qualitative review. Psychology of Addictive Behaviors, 21, 127–137. 10.1037/0893-164X.21.2.127 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2010). For the public’s health: The role of measurement in action and accountability. Washington, DC: The National Academies Press, National Academy of Science; Retrieved from www.iom.edu/reports [Google Scholar]
- Institute of Medicine. (2012). Living well with chronic illness: A call for public health action (Report Brief). The National Academies Press, National Academy of Science; Retrieved from www.iom.edu/reports [Google Scholar]
- Japuntich S. J., Leventhal A. M., Piper M. E., Bolt D. M., Roberts L. J., Fiore M. C., Baker T. B. (2011). Smoker characteristics and smoking-cessation milestones. American Journal of Preventive Medicine, 40, 286–294. 10.1016/j.amepre.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C., Roth D., Kaufmann V., Audrain J., Hawk L., Liu A. … Epstein L. (2002). Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence, 67, 219–223 [DOI] [PubMed] [Google Scholar]
- Li R., Root T., Shiffman S. (2006). A local linear estimation procedure for functional multilevel modeling. In Walls T., Schafer J. (Eds.), Models for intensive longitudinal data (pp. 63–83). New York, NY: Oxford University Press [Google Scholar]
- McCarthy D. E., Piasecki T. M., Fiore M. C., Baker T. B. (2006). Life before and after quitting smoking: an electronic diary study. Journal of Abnormal Psychology, 115, 454–466. 10.1037/0021-843X.115.3.454 [DOI] [PubMed] [Google Scholar]
- Nazarian D., Smyth J. M., Sliwinski M. J. (2006). A naturalistic study of ambulatory asthma severity and reported avoidant coping styles. Chronic Illness, 2, 51–58 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M. (2006). Relapse to smoking. Clinical Psychology Review, 26, 196–215. 10.1016/j.cpr.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Jahng S., Wood P. K., Robertson B. M., Epler A. J., Cronk N. J. … Sher K. J. (2011). The subjective effects of alcohol-tobacco co-use: an ecological momentary assessment investigation. Journal of Abnormal Psychology, 120, 557–571. 10.1037/a0023033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki T. M., Niaura R., Shadel W. G., Abrams D., Goldstein M., Fiore M. C., Baker T. B. (2000). Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology, 109, 74–86 [DOI] [PubMed] [Google Scholar]
- Piper M. E., Piasecki T. M., Federman E. B., Bolt D. M., Smith S. S., Fiore M. C., Baker T. B. (2004). A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). Journal of Consulting and Clinical Psychology, 72, 139–154. 10.1037/0022-006X.72.2.139 [DOI] [PubMed] [Google Scholar]
- Piper M. E., Federmen E. B., McCarthy D. E., Bolt D. M., Smith S. S., Fiore M. C., Baker T. B. (2008). Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology, 117, 94–105. 10.1037/0021-843X.117.1.94 [DOI] [PubMed] [Google Scholar]
- Piper M. E., Schlam T. R., Cook J. W., Sheffer M. A., Smith S. S., Loh W. Y. … Baker T. B. (2011). Tobacco withdrawal components and their relations with cessation success. Psychopharmacology, 216, 569–578. 10.1007/s00213-011-2250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S. W., Bryk A. S. (2002). Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage [Google Scholar]
- Shiffman S., Ferguson S. G., Gwaltney C., Balabanais M., Shadel W. (2006). Reduction of abstinence induced withdrawal and craving using nicotine replacement therapy. Psychopharmacology, 184, 637–644. 10.1007/s00213-005-0184-3 [DOI] [PubMed] [Google Scholar]
- Shiffman S., Scharf D. M., Shadel W. G., Gwaltney C. J., Dang Q., Paton S. M., Clark D. B. (2006). Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology, 74, 276–285. 10.1037/0022-006X.74.2.276 [DOI] [PubMed] [Google Scholar]
- Shiyko M. P., Lanza S. T., Tan X., Li R., Shiffman S. (2012). Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: differences between successful quitters and relapsers. Prevention Science, 13, 288–299. 10.1007/s11121-011-0264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. (1983). Classical conditioning, drug tolerance, and drug dependence. In Smart R. G., Glaser F. B., Israel Y., Kalant R., Popham E., Schmidt W. (Eds.), Research advances in alcohol and drug problems (7th ed.), New York, NY: Plenum; 7, 207–246 [Google Scholar]
- Smyth J. M., Stone A. A. (2003). Ecological assessment research in behavioral medicine. Journal of Happiness Studies, 4, 35–52 [Google Scholar]
- Solomon R. L., Corbit J. D. (1974). An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review, 81, 119–145. 10.1037/h0036128 [DOI] [PubMed] [Google Scholar]
- Stone A. A., Shiffman S. (1994). Ecological momentary assessment (EMA) in behavorial medicine. Annals of Behavioral Medicine, 16, 199–202 [Google Scholar]
- Stone A. A., Shiffman S. (2002). Capturing momentary, self-report data: a proposal for reporting guidelines. Annals of Behavioral Medicine, 24, 236–243. 10.1207/S15324796ABM2403_09 [DOI] [PubMed] [Google Scholar]
- Tan X., Shiyko M., Li R., Li Y., Dierker L. (2012). Intensive longitudinal data and model with varying effects. Psychological Methods, 17, 61–77. 10.1037/a0025814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zundert R. M., Ferguson S. G., Shiffman S., Engels R. (2012). Dynamic effects of craving and negative affect on adolescent smoking relapse. Health Psychology, 31, 226–234. 10.1037/a0025204 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Welsch S. K., Smith S. S., Wetter D. W., Jorenby D. E., Fiore M. C., Baker T. B. (1999). Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology, 7, 354–361. 10.1037/1064-1297.7.4.354 [DOI] [PubMed] [Google Scholar]
- Wikler A. (1980). Opioid dependence. New York, NY: Plenum [Google Scholar]
- Yang J., Tan X., Li R., Wagner A. (2012). TVEM (time-varying effect model) SAS macro suite users’ guide (Version 2.0.0). University Park, PA: The Methodology Center, Penn State; Retrieved from http://methodology.psu.edu [Google Scholar]
- Zhou X., Nonnemaker J., Sherrill B., Gilsenan A. W., Coste F., West R. (2009). Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addictive Behaviors, 34, 365–373 [DOI] [PubMed] [Google Scholar]