Abstract
Introduction:
Researchers have increasingly begun to gather ecological momentary assessment (EMA) data on smoking, but new statistical methods are necessary to fully unlock information from such data. In this paper, we use a new technique, the logistic time-varying effect model (logistic TVEM), to examine the odds of smoking in the 2 weeks after a quit attempt.
Methods:
Data are from a subsample of participants from a randomized, placebo-controlled trial of smoking cessation pharmacotherapies who achieved initial abstinence (N = 1,106, 58% female). Participants completed up to 4 EMA assessments per day during the 2 weeks after their quit day. Predictors include baseline nicotine dependence, EMA measures of craving and negative affect, and whether an individual was assigned to a placebo, monotherapy, or combination therapy condition. Time-varying effects of these predictors were estimated using logistic TVEM.
Results:
Cravings were a significant predictor of smoking throughout the entire 2 weeks postquit, whereas the effect of baseline dependence became nonsignificant by the second week, and the effect of negative affect increased over time. Individuals in the monotherapy and combination therapy conditions had decreased odds of smoking compared with placebo in the first week postquit, but these differences were nonsignificant in the second week.
Conclusions:
Findings suggest that pharmacotherapies are more effective compared with placebo earlier in a quit attempt, when the effect of baseline nicotine dependence on smoking is stronger, whereas the effect of craving and negative affect increased over time. Future cessation therapies may be more successful by providing additional support in the second week after quit attempt.
INTRODUCTION
An increasing number of smoking studies have included collection of ecological momentary assessment (EMA) data to uncover processes associated with addiction, quitting, withdrawal, lapse, and relapse (Colvin & Mermelstein, 2010; Piasecki et al., 2011; Piper et al., 2011; Shiffman et al., 2007). Collecting this kind of data is an important step in understanding addiction processes, as it gives ecologically valid assessments of process-related measures, such as individuals’ current mood and context as well as substance use (Shiffman, 2009). Such data also allow researchers to answer new questions that were could not be answered with traditional sources of data, and new analytic techniques are necessary to fully realize the potential of EMA data. Currently, EMA data are often analyzed with multilevel models (MLMs) that examine how momentary predictors, such as craving or negative affect are associated with a momentary or daily occurrence of an outcome, such as smoking. This method is useful for examining associations between variables and for understanding how the variance in an outcome is attributable to between- and within-person effects. While providing important information about correlates of smoking, most current MLM studies do not take into account the time-varying nature of the EMA data; specifically, researchers have not typically examined how an association between two variables shifts with time or identified windows of time during which an effect is statistically significant. In this study, we provide a demonstration of the logistic time-varying effect model (logistic TVEM), a nonparametric model that allows associations between two variables to be flexibly estimated over time. We apply this method to better understand complex, time-varying processes associated with a dichotomous event—smoking lapses after a quit attempt.
Understanding the process of quitting smoking is an important public health concern, given that smoking is the leading cause of preventable death in the United States (Centers for Disease Control and Prevention [CDC], 2004) and quitting smoking can greatly benefit an individual’s health (Peto et al., 2012; Taylor, Hasselblad, Henley, Thun, & Sloan, 2002; Thun & Heath, 1997). However, successful long-term abstinence is rare; only about 5% are able to quit successfully without cessation therapy (CDC, 2004). Smoking cessation therapies, such as pharmacotherapy or counseling, can more than double abstinence rates, but even with such aids, only 15%–30% of individuals are successful in a given quit attempt (Fiore, Bailey, & Cohen, 2000). Piasecki, Fiore, McCarthy, and Baker (2002) have suggested that this lack of treatment success may result from a poor understanding of addiction processes and that basic research on smoking and cessation dynamics is essential.
Despite this increased attention to the dynamic nature of smoking cessation and attempts to hypothesize time-varying processes involved in smoking cessation (Piasecki et al., 2002), little is known about time-varying associations between smoking outcomes and their potential covariates (Shiyko, Lanza, Tan, Li, & Shiffman, 2012). For example, although smoking urges and negative affect have been shown to predict smoking lapse after a quit attempt (Berkman, Dickenson, Falk, & Lieberman, 2011; Shiffman & Waters, 2004), it is not known whether these factors play a stronger role in smoking lapse during certain periods. Such knowledge could be important in developing more efficacious, targeted cessation therapies, as researchers could identify time periods where particular factors are more likely to lead to lapse, as well as time periods where certain treatments may be strategically used to impact a particular aspect of withdrawal. This could allow for the development of adaptive interventions in which clinicians tailor treatment (e.g., add an additional medication) at a certain point in the cessation attempt if a risk factor emerges as well as the development of new treatments or treatment combinations that address such risk factors.
Researchers have begun to examine these processes with a new statistical technique, the TVEM (Tan, Shiyko, Li, Li, & Dierker, 2012). TVEM arose out of the field of functional data analysis, and it does not assume that changes over time follow any particular shape. Instead, it flexibly estimates trajectories using intensive longitudinal assessments from multiple individuals. A few recent studies have demonstrated the sort of processes related to smoking cessation that can be uncovered using TVEM. For example, one study demonstrated that individuals who eventually relapsed had no association between their self-efficacy and urges to smoke immediately after quitting smoking, whereas successful quitters initially showed a negative association between self-efficacy and smoking urges (Shiyko et al., 2012). This sort of knowledge can help to identify individuals who are at risk of smoking lapse. In addition, TVEM can help to uncover time-varying efficacy of smoking cessation therapies. However, prior studies only examined continuous outcomes and thus examined smoking urges as a proxy for actual smoking behavior. A recent extension to TVEM that accommodates a binary outcome over time (logistic TVEM; Yang, Tan, Li, & Wagner, 2012) is applied in this study to examine time-varying predictors of whether an individual smokes at various times after a quit attempt.
Motivating Example: Predictors of Smoking After Quit Attempt
To better understand time-varying predictors of smoking after a quit attempt, we present a model looking at two different types of predictors. First, we examine withdrawal and baseline dependence to understand the processes involved in smoking after a quit attempt. Prior research has demonstrated that withdrawal is a key factor involved in lapse and relapse (Javitz, Brigham, Lessov-Schlaggar, Krasnow, & Swan, 2009; Piasecki, Jorenby, Smith, Fiore, & Baker, 2003; Piper et al., 2011). Two primary withdrawal factors have been identified, which are strongly related to relapse or later smoking status: smoking urges and negative affect (Piasecki et al., 2000; Piper et al., 2011; Zhou et al., 2009). In addition to predicting longer term relapse, increases in smoking urges or cravings and negative affect are associated with immediate smoking lapses (Berkman et al., 2011; Shiffman & Waters, 2004). Baseline nicotine dependence is associated with smoking relapse, with stronger nicotine addiction predicting relapse (Japuntich et al., 2011; Piasecki et al., 2000; Zhou et al., 2009). However, although research has demonstrated the importance of smoking urges, negative affect, and baseline dependence on lapse and relapse, prior research has not examined time-varying associations: for example, whether particular factors are more strongly associated with smoking early versus later in a quit attempt.
Similarly, although research has demonstrated the efficacy of smoking cessation therapies compared with placebo, little is known about how they may differentially protect against smoking at different points after a quit attempt. Although no smoking cessation treatment produces long-term abstinence rates greater than 50% (Fiore et al., 2000), five nicotine replacement therapies and two non–nicotine replacement pharmacotherapies (sustained-release bupropion [bupropion SR] and varenicline) have been shown to be effective relative to placebo and are recommended for most smokers (Fiore et al., 2008). When these pharmacotherapies were compared in a head to head study, the combination therapy of nicotine patch plus nicotine lozenge was the most efficacious relative to placebo (Piper et al., 2009). However, most studies of these therapies focus on longer term abstinence, and little is known about the process by which they may work to prevent initial lapses or particular periods in which they may be more effective (although cf., Japuntich et al., 2011).
In this study, we demonstrate two different approaches to EMA data that examine how momentary craving and negative affect, baseline dependence, and treatment predict smoking lapse. We first use MLM to provide an example of a traditional, time-invariant approach, followed by a TVEM approach, which extends this analysis to examine time-varying associations. The MLM answered the question “How do craving, negative affect, baseline dependence, and treatment group predict momentary smoking across the 2 weeks postquit?” The TVEM addresses similar associations but focuses on how they vary over time. Specifically:
How do associations between smoking lapse and craving, negative affect, and baseline dependence vary during the first 2 weeks following a smoking quit attempt?
How does the effectiveness of mono and combination smoking cessation therapies vary over the 2 weeks postquit?
METHODS
Participants
Data are from a randomized, placebo-controlled trial of five active smoking cessation pharmacotherapies (see Piper et al., 2009 for further details). Inclusion criteria included motivation to quit smoking and smoking at least 10 cigarettes/day for at least 6 months. Participants (N = 1,504; 58% female; 83% White) were randomized in a double-blind fashion, using a blocking scheme based on gender and race, to one of six conditions: bupropion slow release (SR; n = 264), nicotine lozenge (n = 260), nicotine patch (n = 262), nicotine patch + nicotine lozenge (n = 267), bupropion SR + nicotine lozenge (n = 262), or placebo (five placebo conditions that matched the five active conditions; total n = 189). In this study, we analyzed data from 1,106 participants who were able to establish initial abstinence (quit for at least 24hr within the first week after the target quit day) and provided EMA data.
Procedures
Participants completed a series of baseline assessments before being randomized to one of the six study conditions. All medications were provided for 8 weeks postquit except the nicotine lozenge, which was provided for 12 weeks postquit (consistent with prescribing instructions). Participants completed EMA assessments on palmtop computers in response to prompts 4 times a day (just after waking, prior to going to bed, and at two other random times, with all prompts separated by at least an hour) for up to 2 weeks prior to and 2 weeks after their target quit date (68% of participants completed at least half of the prompts; 47% of all possible random prompts completed across the 2 weeks). Assessments initiated by a prompt and had to be completed within 15min. We include postquit EMA occasions up until the point of relapse (defined as seven consecutive days of smoking), as predictors of smoking behavior may differ after an individual has fully returned to smoking; in that case, we would no longer be studying the relapse process, but rather typical smoking behavior. Only a small percentage (7%) of participants relapsed within this 2-week period. However, there was some attrition over the course of the 2 weeks; on average, 632 people completed at least one measurement occasion each day in Week 2. This study includes 29,484 EMA observations.
Measures
Baseline
Three measures from the baseline assessments are used as predictors in this study. These variables were only measured once, although we examined how their effects on smoking varied over time. Two dichotomous indicators are used to measure treatment status (monotherapy and combination therapy) with placebo as the reference group; these classifications were used because research has found combination therapy to be more effective at reducing craving and preventing relapse than monotherapy (Bolt, Piper, Theobald, & Baker, 2012; Piper et al., 2009; Smith et al., 2009; Sweeney, Fant, Fagerström, McGovern, & Henningfield, 2001) and to simplify analyses for this demonstration. Baseline dependence was measured with one item from the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) that assessed how soon after waking an individual smokes a first cigarette on a 4-point scale from after 60 min to within 5 min. This item has been shown to have the strongest predictive validity (Baker et al., 2007). The baseline dependence measure was standardized (M = 0, SD = 1). In addition, gender was entered as a covariate with a time-invariant effect (0 = male, 1 = female).
Ecological Momentary Assessment
Three constructs from the EMA data are used in this analysis. For our outcome variable, momentary smoking, we dichotomized an item asking the number of cigarettes smoked since the last occasion (0 = no smoking, 1 = one or more cigarettes). The two time-varying predictors from the EMA were craving and negative affect. Craving was assessed with a single item from the Wisconsin Smoking Withdrawal Scale asking how bothered the participant was by a desire to smoke a cigarette, rated on a 10-point scale from strongly disagree to strongly agree (Welsch et al., 1999). Negative affect was assessed by the average of two items from the Positive and Negative Affect Scale asking about how upset or distressed the participant felt on a 5-point scale from slightly or not at all to very much (Watson, Clark, & Tellegen, 1988). Both craving and negative affect scores were standardized (M = 0, SD = 1). A summary of the demographic and smoking dependence distribution of our sample is presented in Table 1.
Table 1.
Descriptive Statistics for Demographic and Study Variables
| Placebo | Monotherapy | Combined therapy | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Demographics | |||
| Age | 43.39 (12.63) | 44.98 (11.02) | 44.95 (10.42) |
| % Female | 56.8 | 58.7 | 59.4 |
| % White | 88.3 | 87.5 | 84.7 |
| Predictors | |||
| Craving | 4.12 (3.54) | 3.87 (3.41) | 3.55 (3.37) |
| Negative affect | 1.69 (1.67) | 1.52 (1.54) | 1.41 (1.44) |
| Baseline dependence | 1.90 (0.90) | 1.92 (0.89) | 2.06 (0.80) |
| Outcome | |||
| % of days smoked | 11.64 | 8.13 | 7.42 |
| % of people smoked | 60.4 | 48.8 | 49.6 |
Analytic Approach
First, we used a two-level MLM to demonstrate a traditional approach to predicting momentary smoking. We used the following equations:
For our primary analysis, we used logistic TVEM (Yang et al., 2012) to model intensively measured smoking lapse behavior during the 2 weeks following a quit attempt as a function of both baseline characteristics and predictors assessed intensively over time. This model was run in SAS using the %TVEM_logistic macro (Yang et al., 2012). The TVEM macro has two different estimation options that involve different steps for model selection. We use the P-spline method, which automatically selects the best-fitting model with an appropriate number of knots (splitting points). A further discussion of the different TVEM options is presented elsewhere (Tan et al., 2012; Yang et al., 2012). The following equation specified our model:
where
In this model, the exponentiated intercept represents the odds of smoking over time when all other predictors are 0 (i.e., male from placebo group with average scores on baseline dependence, craving, and smoking). Exponentiated slopes and represent the time-varying difference in the odds of smoking lapse for individuals in the monotherapy and combination therapy groups, respectively. Next, exponentiated slopes and represent the time-varying association between craving and negative affect and the odds of smoking lapse for the placebo group. Similarly, represents the time-varying association between baseline dependence and the odds of smoking lapse. Finally, β6 represents the time-invariant effect of gender (female = 1), which was entered as a control. In summary, this analysis examines three effects: (a) the time-varying effect of the time-varying predictors craving and negative affect (based on EMA data); (b) the time-varying effect of the time-invariant predictors—treatment (monotherapy, combination therapy) and baseline dependence—measured once at baseline; and (c) the time-invariant effect of the time-invariant predictor gender. Note that we refer to certain variables as predictors in order to designate how they were specified in our model; both smoking lapse and its predictors were measured at the same EMA occasion. A multivariate model is presented, as a comparison with what is typically presented in MLM. However, univariate models were also run, and while the magnitude of coefficients did differ somewhat, the substantive pattern of time-varying results was similar.
As described in further detail in the Results section, time-varying coefficients for the predictors are presented as plots, with time represented in the x-axis. This is necessary because a coefficient for the association between a predictor and outcome is estimated at each point in continuous time. However, we draw attention to findings at illustrative points across each curve. These plots show the strength of associations as a function of time, with dotted lines representing 95% CIs that indicate whether associations at time t are statistically significant (e.g., CI does not contain 1).
RESULTS
The results for the MLM predicting smoking lapse are presented in Table 2. All predictors were significantly associated with lapse; having greater momentary urge to smoke and negative affect and stronger baseline dependence were associated with greater odds of smoking at a given EMA occasion, whereas being in either type of treatment group was associated with lesser odds of smoking relative to the placebo group.
Table 2.
Logistic Multilevel Model Predicting Smoking Lapse in the 2 Weeks Postquit
| Fixed effects | Coefficient | SE | OR |
|---|---|---|---|
| Average odds of smoking β0 | |||
| Intercept (placebo men with average dependence) γ00 | −1.86*** | 0.23 | 0.16 |
| Female γ01 | −0.45** | 0.16 | 0.64 |
| Baseline dependence γ02 | 0.25** | 0.08 | 1.28 |
| Monotherapy γ03 | −0.61* | 0.24 | 0.54 |
| Combination therapy γ04 | −0.96*** | 0.25 | 0.38 |
| Effect of momentary urge to smoke β1 | |||
| Intercept γ10 | 0.51*** | 0.03 | 1.67 |
| Effect of momentary negative affect β2 | |||
| Intercept γ20 | 0.31*** | 0.08 | 1.36 |
| Random effects | |||
| Intercept variance | 4.14 | 0.26 | |
Note. OR = odds ratio.
*p < .05, **p < .01, ***p < .001.
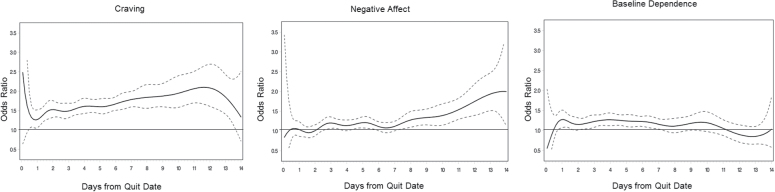
Results from the TVEM analysis addressing the first research question, how craving, negative affect, and baseline dependence are associated with smoking over time, are presented in Figure 1. This figure presents the exponentiated slopes (odds ratios [ORs]) for the associations between these three variables and momentary smoking at points in continuous time during the 2 weeks postquit for the reference group (placebo). As with all ORs, an OR of 1 indicates that odds of the outcome are equal across the presence or absence of a given predictor (i.e., there is no significant association). ORs greater than 1 indicate that the presence or higher quantity of predictor is associated with greater odds of a given outcome, whereas ORs less than 1 indicate lesser odds with higher levels of the predictor. Dotted lines represent 95% CIs that show whether a value is significantly different from 1. These results differ from more traditional analyses in that different ORs are presented at different points in time. We turn first to the trajectory of craving and its associations with smoking. Craving was positively associated with odds of smoking lapse at all points after the first day postquit. As shown by ORs for craving at various points in the plot, the association between craving and smoking increased somewhat over time. For example, a 1 SD increase in craving was associated with 1.7 times greater odds of lapse at the beginning of the second day postquit and about 2 times greater odds in the 12th day postquit. The association between negative affect and smoking became significant around the second day postquit, with greater negative affect associated with greater odds of smoking. This association increased over time. For example, in the first day postquit, there was no significant association between negative affect and smoking; on Day 4, a 1 SD increase in negative affect was associated with about 1.3 times greater odds of smoking, and by Day 13, this increased to about 1.7. The third trajectory, for baseline dependence, was only significant for a short period between Day 3 and Day 7, with the association decreasing after peaking around Day 4. In addition, the time-invariant control variable, gender, was significant, suggesting that women had lesser odds of lapsing during the 2 weeks postquit compared with men (β = −0.13, OR = 0.87, p < .01).
Figure 1.
Time-varying effect model predicting momentary smoking after quit attempt by craving, negative affect, and baseline dependence. Dotted lines indicate 95% confidence intervals. Time is measured and analyzed continuously but is labeled in daily intervals for ease of presentation.
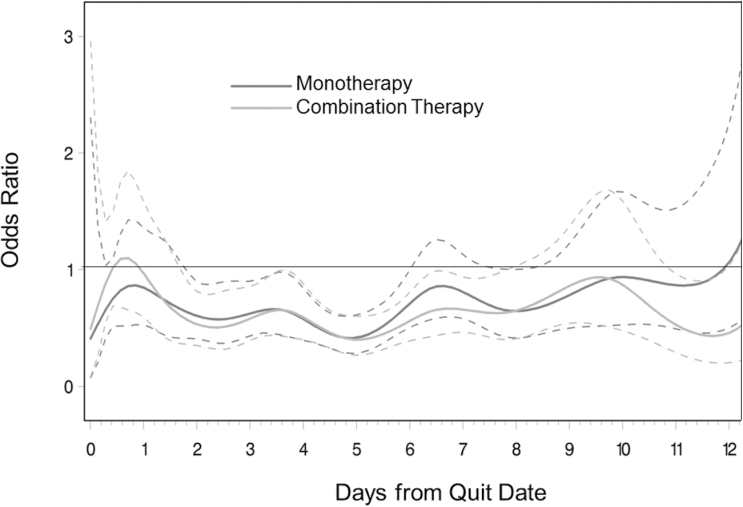
For the second research question, we examined how the impact of treatment on smoking lapse differed over time. These results are presented in two plots (Figures 2 and 3). Figure 2 presents the time-varying association between treatment group (monotherapy or combination therapy) and smoking compared with placebo, which is interpreted in a similar fashion to Figure 1. Points where the upper confidence level is less than 1 indicate periods where individuals in a given treatment group had significantly lower odds of smoking compared with the placebo group. For both treatments, odds of smoking were significantly lower than in the placebo group from Day 2 through Day 6 postquit, and these differences became nonsignificant in the second week postquit. For example, in the second day postquit, individuals in both the monotherapy and combination therapy conditions had about 75% lesser odds of smoking compared with those in the placebo group, whereas during the 10th day postquit, there was virtually no difference in odds of smoking between groups.
Figure 2.
Time-varying effect model predicting momentary smoking after quit attempt by monotherapy and combination therapy compared with placebo. Dotted lines indicate 95% confidence intervals. Time is measured and analyzed continuously but is labeled in daily intervals for ease of presentation.
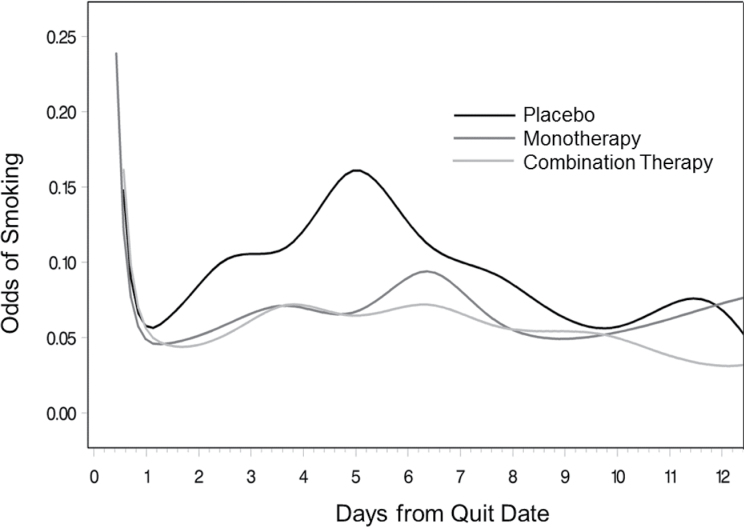
Figure 3.
Odds of smoking in the 2 weeks postquit for monotherapy, combination therapy, and placebo groups. Time is measured and analyzed continuously but is labeled in daily intervals for ease of presentation.
Figure 3 presents an alternate way of presenting these findings: as odds of smoking during the 2 weeks postquit for the three groups. Periods of statistical significance are the same as in Figure 2 and are thus presented without ORs for ease of presentation. This figure demonstrates that odds of smoking were relatively stable across time for individuals in the two treatment groups. However, odds of smoking increased for the placebo group, peaking at Day 5, and then decreased to be roughly equivalent to the treatment groups. At Day 5, when risk of smoking peaked for the placebo group, there were 15% odds of reporting smoking at a given EMA occasion for those in the placebo group, compared with about 6% for those in either treatment group. By Day 10, however, individuals in all three groups who had not currently relapsed had about 5% odds of smoking at a given time.
DISCUSSION
This study examined time-varying predictors of smoking lapses after quit attempt, as well as effects of mono and combination therapies on odds of smoking lapse. We first used a traditional multilevel modeling approach and found that all of our hypothesized predictors were associated with smoking lapse. We then used a TVEM and found that associations changed over time. We found that baseline dependence and momentary craving and negative affect all predicted smoking after quit attempt, but this association differed across the 2 weeks after a quit attempt. Although prior work using more traditional methods (Berkman et al., 2011; Japuntich et al., 2011; Piasecki et al., 2000; Shiffman & Waters, 2004; Zhou et al., 2009) has identified these factors as predictors of lapse, this study showed periods when they were more strongly or weakly associated with smoking. This difference is represented by our series of analyses; for example, in the MLM, a 1 SD increase in baseline dependence was associated with 1.28 greater odds of smoking; in the TVEM, the association varied over time from a high of 1.4 greater odds early in the quit attempt to no effect in the second week. Of the three predictors studied, craving had the strongest influence on smoking, and the association increased over time, peaking around the 12th day after quit attempt. Stronger baseline dependence was associated with greater odds of smoking in the first week postquit, but this association became nonsignificant about 8 days postquit. For negative affect, however, nearly the opposite process was observed; the association between negative affect and smoking steadily increased after about the seventh day postquit. These findings suggest that, among those who can establish initial abstinence, different factors influence smoking at different points in a quit attempt. In the early stages of a quit attempt, nicotine dependence and craving may play stronger roles, but this wears off after about a week or 12 days, respectively. After about a week, the influence of negative affect on smoking lapse increases, suggesting that later lapses may be more strongly related to affective cues. This is consistent with Shiffman’s findings that negative affect predicts smoking at a subsequent time (Shiffman et al., 2007).
We also found that the impact of mono and combination pharmacotherapy differed from placebo across the 2 weeks postquit. Individuals who received either active medication had lesser odds of smoking compared with placebo in the first week postquit, but these differences became nonsignificant by the second week postquit. Coupled with our results on processes involved in quit attempts in the placebo group, this suggests that current smoking cessation medications are most effective early in a quit attempt, when the influence of nicotine dependence and cravings is stronger. These therapies are not as effective in later stages when affect plays a greater role in smoking lapse. Thus, smoking cessation programs may benefit from including or adding additional counseling or support related to ways to improve mood and deal with stressors without smoking during the second week after a quit attempt. Unlike prior studies of longer term relapse (Blondal, Gudmundsson, Olafsdottir, Gustavsson, & Westin, 1999; Bohadana, Nilsson, Rasmussen, & Martinet, 2000; Kornitzer, Boutsen, Dramaix, Thijs, & Gustavsson, 1995; Piper et al., 2009; Smith et al., 2009), the two treatment groups did not differ significantly from each other. Thus, although this study provides some evidence for the impact of treatment over placebo, it does not provide strong evidence for choosing one type of therapy over another. However, because these prior studies, including one using the same sample, examined later relapse and this study focused on smoking shortly after quit attempt, it is possible that these processes may change after the 2 week postquit period, and future research should examine the process in later time periods. Also note that the peak in smoking lapse for the placebo group may seem later than would be expected (i.e., lapse would be expected in the first few days when withdrawal typically peaks), but this sample includes all occurrences of smoking (not just first lapse) and excludes all participants who were unable to establish initial abstinence. There were significantly more placebo condition smokers who were unable to establish initial abstinence and those who were able to do so, despite no active medication, may have been more motivated or less dependent than those who were not able to maintain initial abstinence and therefore, they were able to delay their lapsing beyond the first few days of the quit attempt.
There are several limitations to this study. First, the use of TVEM in this study provides some trade-offs compared with other methods, such as multilevel modeling. Rather than focusing on within-person effects, this method examines associations between variables over time across a large population of individuals. In a sense, it provides a series of snapshots of associations in the population at a given time. Thus, unlike a MLM, it examines the effect of deviations from a population mean, rather than from individuals’ means. However, a future extension is planned, which will be able to model interindividual variability. More substantively, this study only looks at the 2 weeks immediately after a quit attempt, thus providing no information about processes involved in smoking lapse beyond 2 weeks postquit, or how these processes are associated with long-term abstinence. We did not include measurement occasions after an individual had progressed to regular smoking; thus, it is possible that some effects detected were spurious, and future research could aim to better understand differential processes predicting first lapse, later lapses, and full relapse. Although TVEM utilizes all complete data from participants, fewer measurement occasions were completed toward the end of the 2-week period, which could bias the results. In addition, these analyses examined momentary associations between craving, negative affect, baseline dependence, and smoking after quit attempt, and although we interpreted these factors as being predictive of smoking lapse, it is possible that the direction of associations is reversed or bidirectional. For example, negative affect may predict smoking lapse, but people may also feel more negative affect after they have smoked. Future research should attempt to better understand the directionality of this process, although the timing of EMA measurement occasions, due in part to logistical constraints, makes understanding these dynamics problematic.
This study suggests several areas for future research that are beyond the limited scope of this introductory paper. This study focused on any smoking after quit attempt that occurred prior to full relapse. However, it is possible that processes are not the same for all smoking lapses. For example, a first lapse may involve different processes than a later lapse. Future research could utilize methods for jointly examining time-varying and survival processes (Tsiatis & Davidian, 2004) to better understand these different processes. This study focused on momentary occurrences of smoking without differentiating whether the lapses led to relapse or whether the participant reestablished abstinence. Thus, future research should focus on the differential patterns of smoking after initial lapses, including understanding the process of transitioning from lapse to relapse. For simple demonstration purposes, we combined the treatment conditions into monotherapy and combination therapy, yet it is possible that different pharmacotherapies may have different time-varying effects (e.g., bupropion vs. nicotine replacement). Additionally, we did not study varenicline. Future studies could compare these different treatment conditions. Similarly, we did not examine how craving, negative affect, and baseline dependence were differentially associated with smoking for the different treatment groups; future research could examine these predictors by treatment interactions to better understand the mechanisms by which smoking cessation therapies are effective. Finally, we examined a main effect of gender showing that, in the 2 weeks postquit overall, placebo women have lesser odds of smoking. This differs from prior research that shows that women have greater odds of relapse (Perkins, 2001), but this must be interpreted in the context of looking only at women who were able to establish initial abstinence. This may be a result of the shorter time period studied or influenced by removal of occasions after full relapse. Future work should expand on this analysis by examining time-varying effects or interactions by gender to better understand how these processes may differ for men and women.
Despite these limitations, this study shows how examining time-varying effects in EMA data on smoking can provide new insights that are not possible with more traditional methods. Specifically, it shows how different processes are involved in smoking lapse at different periods, as well as how treatment effects differ over time. This provides information that can be used in future smoking cessation programs, such as periods to provide additional psychological support. In addition, this paper provides a first empirical demonstration of logistic TVEM, a technique that can be used to better understand lapse and relapse processes with the increasingly commonly collected EMA data on substance use cessation and recovery programs.
FUNDING
This work was supported by Award Numbers P50-DA010075-16, R21-DA024260, P50-DA0197, and T32-DA017629 from the National Institute on Drug Abuse (NIDA) , P50-CA84724 and R01-CA168676 from the National Cancer Institute (NCI), and M01-RR03186 from The General Clinical Research Centers Program of the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, NCI, the National Institutes of Health, or the National Center for Research Resources.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We thank Tom Piasecki for commenting on an earlier version of this manuscript.
REFERENCES
- Baker T. B., Piper M. E., McCarthy D. E., Bolt D. M., Smith S. S., Kim S.-Y, … Toll B. A. (2007). Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research, 9(Suppl. 4), S555–S570. 10.1080/14622200701673480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E. T., Dickenson J., Falk E. B., Lieberman M. D. (2011). Using SMS text messaging to assess moderators of smoking reduction: Validating a new tool for ecological measurement of health behaviors. Health Psychology, 30, 186–194. 10.1037/a0022201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondal T., Gudmundsson L. J., Olafsdottir I., Gustavsson G., Westin A. (1999). Nicotine nasal spray with nicotine patch for smoking cessation: Randomised trial with six year follow up. British Medical Journal, 318, 285–288. 10.1136/bmj.318.7179.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohadana A., Nilsson F., Rasmussen T., Martinet Y. (2000). Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: A randomized, double-blind, placebo-controlled trial. Archives of Internal Medicine, 160, 3128–3134 [DOI] [PubMed] [Google Scholar]
- Bolt D. M., Piper M. E., Theobald W. E., Baker T. B. (2012). Why two smoking cessation agents work better than one: Role of craving suppression. Journal of Consulting and Clinical Psychology, 80, 54–65. 10.1037/a0026366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2004). Cigarette smoking among adults: United States, 2002. Morbidity and Mortality Weekly Report, 53, 427–431 [PubMed] [Google Scholar]
- Colvin P. J., Mermelstein R. J. (2010). Adolescents’ smoking outcome expectancies and acute emotional responses following smoking. Nicotine & Tobacco Research, 12, 1203–1210. 10.1093/ntr/ntq169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M. C., Bailey W. C., Cohen S. J. (2000). Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service [Google Scholar]
- Fiore M. C., Jaén C. R., Baker T. B., Bailey W. C., Benowitz N. L., Curry S. J, … Wewers M. E. (2008). Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127 [DOI] [PubMed] [Google Scholar]
- Japuntich S. J., Leventhal A. M., Piper M. E., Bolt D. M., Roberts L. J., Fiore M. C., Baker T. B. (2011). Smoking characteristics and smoking-cessation milestones. American Journal of Preventative Medicine, 40, 286–294. 10.1016/j.amepre.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitz H. S., Brigham J., Lessov-Schlaggar C. N., Krasnow R. E., Swan G. E. (2009). Association of tobacco dependence and quit attempt duration with Rasch-modeled withdrawal sensitivity using retrospective measures. Addiction, 104, 1027–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornitzer M., Boutsen M., Dramaix M., Thijs J., Gustavsson G. (1995). Combined use of nicotine patch and gum in smoking cessation: A placebo-controlled clinical trial. Preventive Medicine, 24, 41–47. 10.1006/pmed.1995.1006 [DOI] [PubMed] [Google Scholar]
- Perkins K. A. (2001). Smoking cessation in women. Special considerations. CNS Drugs, 15, 391–411. 1172-7047/01/0005-0391 [DOI] [PubMed] [Google Scholar]
- Peto R., Darby S., Deo H., Silcocks P., Whitley E., Doll R. (2012). Smoking, smoking cessation, and lung cancer in the UK since 1950: Combination of national statistics with two case-control studies. British Medical Journal, 321, 323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki T. M., Fiore M. C., McCarthy D. E., Baker T. B. (2002). Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction, 97, 1093–1108 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Jahng S., Wood P. K., Robertson B. M., Epler A. J., Cronk N. J, … Sher K. J. (2011). The subjective effects of alcohol–tobacco co-use: An ecological momentary assessment investigation. Journal of Abnormal Psychology, 120, 557–571. 10.1037/a0023033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki T. M., Jorenby D. E., Smith S. S., Fiore M. C., Baker T. B. (2003). Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. Journal of Abnormal Psychology, 112, 3–13 [PubMed] [Google Scholar]
- Piasecki T. M., Niaura R., Shadel W. G., Abrams D., Goldstein M., Fiore M. C., Baker T. B. (2000). Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology, 109, 74–86 [DOI] [PubMed] [Google Scholar]
- Piper M. E., Schlam T. R., Cook J. W., Sheffer M. A., Smith S. S., Loh W.-Y, … Baker T. B. (2011). Tobacco withdrawal components and their relations with cessation success. Psychopharmacology, 216, 569–578. 10.1007/s00213-011-2250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. E., Smith S. S., Schlam T. R., Fiore M. C., Jorenby D. E., Fraser D., Baker T. B. (2009). A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry, 66, 1253–1262. 10.1001/archgenpsychiatry.2009.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. (2009). Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment, 21, 486–497. 10.1037/a0017074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Balabanis M. H., Gwaltney C. J., Paty J. A., Gnys M., Kassel J. D, … Paton S. M. (2007). Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence, 91, 159–168. 10.1016/j.drugalcdep.2007.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Waters A. J. (2004). Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology, 72, 192–201. 10.1037/0022-006X.72.2.192 [DOI] [PubMed] [Google Scholar]
- Shiyko M. P., Lanza S. T., Tan X., Li R., Shiffman S. (2012). Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: Differences between successful quitters and relapsers. Prevention Science, 13, 288–299. 10.1007/s11121-011-0264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. S., McCarthy D. E., Japuntich S. J., Christiansen B., Piper M. E., Jorenby D. E, … Jackson T. C. (2009). Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Archives of Internal Medicine, 169, 2148–2155. 10.1001/archinternmed.2009.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C. T., Fant R. V., Fagerström K. O., McGovern J. F., Henningfield J. E. (2001). Combination nicotine replacement therapy for smoking cessation. CNS Drugs, 15, 453–467 [DOI] [PubMed] [Google Scholar]
- Tan X., Shiyko M. P., Li R., Li Y., Dierker L. (2012). A time-varying effect model for intensive longitudinal data. Psychological Methods, 17, 61–77. 10.1037/a0025814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. H., Jr, Hasselblad V., Henley S. J., Thun M. J., Sloan F. A. (2002). Benefits of smoking cessation for longevity. American Journal of Public Health, 92, 990–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun M. J., Heath C. W. (1997). Changes in mortality from smoking in two American Cancer Society prospective studies since 1959. Preventive Medicine, 26, 422–426. 10.1006/pmed.1997.0182 [DOI] [PubMed] [Google Scholar]
- Tsiatis A., Davidian M. (2004). Joint modeling of longitudinal and time-to-event data: An overview. Statistica Sinica, 14, 809–834 [Google Scholar]
- Watson D., Clark L. A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070 [DOI] [PubMed] [Google Scholar]
- Welsch S. K., Smith S. S., Wetter D. W., Jorenby D. E., Fiore M. C., Baker T. B. (1999). Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology, 7, 354–361. 10.1037/1064-1297.7.4.354 [DOI] [PubMed] [Google Scholar]
- Yang J., Tan X., Li R., Wagner A. (2012). TVEM (time-varying effect model) SAS macro suite users’ guide (version 2.0.0) University Park, PA: The Methodology Center, Penn State; Retrieved from http://methodology.psu.edu [Google Scholar]
- Zhou X., Nonnemaker J., Sherrill B., Gilsenan A. W., Coste F., West R. (2009). Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addictive Behaviors, 34, 365–373. 10.1016/j.addbeh.2008.11.013 [DOI] [PubMed] [Google Scholar]