Abstract
Within human pulmonary artery, neurotrophin growth factors [NTs; e.g. brain-derived neurotrophic factor (BDNF)] and their high-affinity receptors (tropomyosin-related kinase; Trk) and low-affinity receptors p75 neurotrophin receptor (p75NTR) have been reported, but their functional role is incompletely understood. We tested the hypothesis that BDNF is produced by human pulmonary artery endothelial cells (PAECs). In the context of hypoxia as a risk factor for pulmonary hypertension, we examined the effect of hypoxia on BDNF secretion and consequent autocrine effects on pulmonary endothelium. Initial ELISA analysis of circulating BDNF in 30 healthy human volunteers showed that 72h exposure to high altitude (~11,000 ft, alveolar PO2=100mmHg) results in higher BDNF compared to samples taken at sea level. Separately, in human PAECs exposed for 24h to normoxia vs. hypoxia (1–3% O2), ELISA of extracellular media showed increased BDNF levels. Furthermore, quantitative PCR of PAECs showed 3-fold enhancement of BDNF gene transcription with hypoxia. In PAECs, BDNF induced NO production (measured using an NO-sensitive fluorescent dye DAF2-DA) that was significantly higher under hypoxic conditions, an effect also noted with the TrkB agonist 7,8-DHF. Importantly, hypoxia-induced NO was blunted by neutralization of secreted BDNF using the chimeric TrkB-Fc. Both hypoxia and BDNF increased iNOS (but not eNOS) mRNA expression. In accordance, BDNF enhancement of NO in hypoxia was not blunted by 50 nM L-NAME (eNOS inhibition) but substantially lower with 100μM L-NAME (eNOS and iNOS inhibition). Hypoxia and BDNF also induced expression of hypoxia inducible factor 1 alpha (HIF-1α), a subunit of the transcription factor HIF-1, and pharmacological inhibition of HIF-1 diminished hypoxia effects on BDNF expression and secretion, and NO production. These results indicate that human PAECs express and secrete BDNF in response to hypoxia via a HIF-1- regulated pathway.
Keywords: Neurotrophin, Tropomyosin related kinase, Nitric oxide, Hypoxia Inducible Factor 1, iNOS, eNOS
1. Introduction
Pulmonary vascular tone represents a balance between vasoconstriction involving arterial smooth muscle and vasodilation mediated largely by endothelial factors such as nitric oxide (NO). Accordingly, enhanced vascular tone as occurs with diseases such as pulmonary hypertension represents, at least in part, an imbalance between vasoconstriction vs. vasodilation [1–4]. In this regard, factors such as hypoxia that are known to induce and exacerbate pulmonary hypertension [5–7] can target pulmonary artery endothelial cells (PAECs) and therefore exacerbate their dysfunction. However, the mechanisms by which hypoxia affects PAECs are still under investigation. In this regard, some studies have shown that hypoxia reduces endothelial NO production and decreases expression of the constitutive eNOS enzyme [8, 9] while others demonstrate increases in the oxygen-sensitive, inducible, iNOS enzyme [10, 11]. Whether PAEC-derived NO per se is increased or decreased with hypoxia, and through what mechanisms appears to vary between studies [12, 13]. Furthermore, it is important to consider whether hypoxia itself can alter circulating or locally produced factors that in turn affect PAEC function, e.g. production of NO.
In recent years, there has been increasing interest in the family of growth factors called neurotrophins beyond their well-recognized effects in the nervous system where they are involved in regulation of neurogenesis, neuronal growth, differentiation and survival. [14–16] There is now increasing evidence that neurotrophins are expressed in peripheral tissues including the systemic and pulmonary vasculature [17–21]. In particular, the neurotrophin brain-derived neurotrophic factor (BDNF) has been localized to the adventitia and intima of human pulmonary arteries [22], suggesting a potential local role for endothelium-derived BDNF. We recently showed that exogenous BDNF can induce NO generation in human PAECs and modulate contraction of pulmonary artery tissue rings [18]. Here, BDNF is thought to function predominantly via its high-affinity tropomyosin-related kinase (TrkB) receptor, and not the low-affinity p75NTR receptor [23]. Whether BDNF is actually produced by PAECs and has local effects in the pulmonary vasculature, and how disease states influence it, are not known. Limited studies in cerebral arteries suggest an endothelial source for BDNF [24] with potential upregulation in hypoxia [25]. However, the downstream consequences of BDNF and/or NO are yet to be determined, especially in the setting of hypoxia induced changes in endothelial biology of the pulmonary vasculature which may further differ from the cerebral circuit.
Mechanisms via which BDNF expression is modulated in non-neuronal tissues are still under investigation, and are likely to be cell- and context-specific. In the brain, hypoxia increases BDNF expression [25, 26], while BDNF has been shown to play a role in cardiac myocyte survival following ischemia/infarction [27]. In neuroblastoma cells [28] and in rat airway cell lines [29], TrkB expression is mediated partly by exposure to low oxygen tensions. In terms of hypoxia, an obvious consideration is the transcription factor hypoxia-inducible factor (HIF-1), involved in regulation multiple proteins and cellular functions [30, 31]. The TrkB gene is known to have HIF-1 binding elements [32], and HIF-1 may therefore be important in at least modulating the effects of hypoxia on BDNF/TrkB signaling. Whether HIF-1 modulates BDNF as well is not known.
In the present study, we hypothesized that BDNF is produced by PAECs and that hypoxia acting via HIF1 enhances BDNF production and signaling. In this regard, we also examined what effect BDNF has on hypoxia pathways, towards understanding what role neurotrophins play in hypoxia effects on the pulmonary endothelium in the context of diseases such as pulmonary hypertension.
2. Materials and Methods
2.1. Hypoxia Exposure in Humans
Under a Mayo Clinic Institutional Review Board-approved study related to altitude sickness, blood samples from 30 healthy volunteers were collected as part of a protocol assessing physiological activity and sleep under conditions of normoxia (21% O2; McMurdo Station, Antarctica; elevation 112 ft) vs. hypoxia (~15% O2; morning after 2nd night at altitude at the South Pole Station; elevation ~11,000 ft, alveolar PO2=100mmHg; average O2 saturation 88%). Studies conformed to the rules and regulations of the Declaration of Helsinki. Blood samples were centrifuged, supernatant (blood serum) transferred to cryovials and stored at −80°C. Serum samples were analyzed for BDNF levels using a human BDNF Quantikine ELISA (R&D Systems, Minneapolis).
2.2. Isolation and culture of human PAECs
Human PAECs were isolated as previously described [18]. Pulmonary artery tissue samples from patients undergoing lung surgery (typically lobectomies, pneumectomies) at Mayo Clinic Rochester for non-infectious focal pathologies were utilized. Patient histories were used to identify those without pre-existing pulmonary hypertension from any cause. Normal lung areas distal from the pathologic area were identified with the help of the surgical pathologist and branch pulmonary arteries (<5 mm) were dissected. These procedures were approved by the Mayo Clinic Institutional Review Board, and since no patient identifiers were retained, the studies were considered minimal risk.
Dissected pulmonary artery tissue was cleaned of blood clots and adventitia. The endothelial surface was exposed and cells were scraped onto a 60mm petri dish containing EGM-2 medium and filtered through 100μm filter. Cell suspensions were incubated with Dynabeads Protein G (Invitrogen, Grand Island, NY) tagged with rat anti-CD31 antibody (BD Pharmingen, Franklin Lakes, NJ) for 1h in 4°C and then processed through a magnetic particle separator Dynal MPC-L (Invitrogen) [33]. Cells were then transferred into 6-well plates and grown in 95% air/ 5% CO2 in 37°C in EBM-2 medium (Lonza, Allendale, NJ) enriched with EGM-2 BulletKit (Lonza). Cells of up to 3 passages of subculture were used for experiments, performed under serum-free conditions.
2.3. Hypoxia exposure
PAECs cultured in EGM-2 media and serum deprived prior to experimentation were exposed to hypoxia (1–3% O2) or normoxia (21% O2), under 5% CO2 and 37°C for 24h. Although the human studies as above were conducted at 15% O2 for 72h (a level allowable by our Institutional Review Board), the extent of hypoxemia was substantial. While it would have been ideal to perform the in vitro work at 15% O2, this level of hypoxia is insufficient to promote HIF1α expression and subsequent downstream events such as HIF1 transcriptional activity. Therefore, instead of trying to match O2 levels under in vivo vs. in vitro conditions (or vice versa), we selected an in vitro level that would provide meaningful results in testing our hypotheses.
For secreted BDNF measurements, PAECs were plated in 60mm plates with the same volume (3 ml) of EGM-2 medium with or without the HIF-1 inhibitor 400083 (EMD Millipore, Billerica, MA), and exposed for 24h to normoxia or hypoxia. The inhibitor is an amidophenolic compound that blocks the transcriptional activity of HIF-1 by selectively blocking the accumulation of HIF-1α protein, with no effect on HIF-1α mRNA (or on HIF-1β protein). Immediately thereafter, the culture media were collected and the cells separately harvested for other experiments. Concentration of BDNF in the medium was then determined using ELISA.
2.4. BDNF ELISA
Circulating plasma BDNF in human volunteers as well as BDNF secreted by humans PAECs were measured using the Quantikine Human BDNF ELISA assay kit, according to the manufacturer's instructions, and using a FlexStation3 microplate reader (Molecular Devices, Sunnyvale, CA) set to 450 nm (wavelength correction set to 540 nm). Absorbance readings from test samples were compared to the manufacturer-provided standard calibration curve.
2.5. Western Blot Analysis
PAECs were rinsed with PBS and mechanically harvested into cell lysis buffer (Cell Signaling Technologies, Beverly, MA) and subjected for sonication and centrifugation. Supernatants were collected and protein concentration measured using DC protein Assay kit (BioRad, Hercules, CA). Standard SDS-PAGE was performed with approximately 30μg of samples loaded into Criterion Precast TGX gels (Bio-Rad, Hercules, CA). Proteins were transferred onto PVDF membrane, which was then blocked for at least 1h in Li-Cor buffer (Li-Cor Biosciences, Lincoln, NE). Antibodies used for immunoblotting were: anti-iNOS (Cell Signaling,), anti-eNOS and anti-eNOS pS1177 (BD Biosciences, San Jose, CA), anti-arginase 1 and -arginase 2 (Santa Cruz Biotechnologies, Santa Cruz, CA), anti-HIF-1α and -HIF-2 (Abcam, Cambridge, MA). Protein detection and densitometry were done on Odyssey infrared imaging system, using the Odyssey Application software v3.0 (Li-Cor Biosciences).
2.6. RNA isolation and Q-PCR
PAECs were treated, under either normoxia or hypoxia, with 1 nM BDNF (R&D Systems), 1μg/ml TrkB-Fc chimera (R&D Systems), 10μM HIF-1α inhibitor, or 1μM 7,8-Dihydroxyflavone (7,8-DHF; Tocris, Minneapolis, MN), a BDNF agonist. Total RNA was isolated from these cells, using the RNeasy micro kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was synthesized using Transcriptor reverse transcription kit (Roche, Indianapolis, IN), and was amplified using an LC480 LightCycler (ABI; Carlsbad, CA), and primers listed in Table 1. Real-Time PCR was performed in duplicates per cDNA template, and data for all cDNAs in a category (normoxia, or hypoxia; untreated controls or agonist/inhibitor-treated) were pooled for statistical analysis. All PCR reactions went through 60 amplification cycles. The ratio of fold change in expression of the mRNA of interest for each sample was calculated by normalization of cycle threshold [C(t)] values of the target gene (e.g. BDNF, iNOS etc) to the reference gene (S16) using the comparative C(t) (ΔΔC(t)) method. Data are reported as the ΔC(t) and the average ratio of fold change in mRNA of interest corrected for reference gene. Normoxic, untreated controls were used as calibrator for quantification.
Table 1.
Primers used in PCR analysis of Normoxic and Hypoxic PAECs
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| eNOS | 5'CATCTCTACCGCGACG3' | 5'CGTATGCGGCTTGTCA3' |
| HIF1α | 5'TGAGCTTGCTCATCAGTTGCCACTTC3' | 5'CATGTCACCATCATCTGTGAGAACC3' |
| iNOS | 5'ATGTACCCTCGGTTCTG3' | 5'CGGACTTTGTAGATTCTGC3' |
| P75NTR | 5'CACATAGACTCCTTTACCCA3' | 5'GCATCGGTTGTCGGAA3' |
| S16 | 5'ATCAAGGTGAACGGGC3' | 5'ACGATGGGCTTATCGG3' |
| TrkB-FL | 5'ACTACTACAGGGTCGG3' | 5'CCCTAGCCTAGAATGTCC3' |
| TrkB-T1 | 5'CCACTGGATGGGTAGC3' | 5'CCTGAGAGTTACCTCTGC3' |
2.7. NO imaging
The techniques for real-time measurement of intracellular NO in PAECs have been previously described [18]. Briefly, PAECs were incubated with the cell-permeant fluorescent dye 4,5-diaminofluorescein diacetate DAF-2DA (Calbiochem, San Diego, CA) at room temperature for 60 min, and imaged using a real-time fluorescence imaging system MetaFluor (Molecular Devices) and standard fluorescein filters. Baseline DAF-2 fluorescence levels were used to assess the longer-term effect of factors such as hypoxia or inhibitors. Cells were then perfused with 1nM BDNF (which served as an “agonist” for NO production) and real-time fluorescence changes measured for 10 min using intracellular regions of interest.
2.8. Statistical analysis
PAECs from at least 7 patients were used, although all protocols were not performed in all samples. Each protocol was repeated at least 4 times with at least 3 different patient samples. Two-way ANOVA with Bonferroni correction was used as a statistical test and significance was established at p<0.05. All values are expressed as means ± SE.
3. Results
3.1. Hypoxia enhances BDNF
In normal, healthy volunteers, exposure to 15% hypoxia for 72h significantly increased serum BDNF levels, compared to normoxia in the same individuals (Figure 1A). While there was some variability in the extent of change in circulating BDNF, there was approximately a 3-fold increase with hypoxia.
Figure 1.
Effect of hypoxia on circulating and pulmonary artery endothelial cell (PAEC)-derived brain derived neurotrophic factor (BDNF) in humans. (A) ELISA for BDNF in peripheral blood serum samples from healthy individuals was performed before (control) and following exposure to 15% hypoxia for 72 h (see methods for details). In most individuals, hypoxia significantly increased serum BDNF. (B) A similar in vitro ELISA of supernatants from human PAECs exposed to <5% hypoxia for 24h showed increased secretion of BDNF following hypoxia, even when corrected for protein content due to any potential increase in cell number due to hypoxia. Values are means ± SE (n=30 in (A) and 5 in (B)). *Significant difference from normoxia (p<0.05).
Consistent with the increase in circulating BDNF in vivo, we found that BDNF secretion by human PAECs also significantly increased in vitro with exposure to hypoxia for 24h (Figure 1B). PAECs secreted a detectable quantity of BDNF even under normoxic conditions, however hypoxia enhanced BDNF secretion by ~2-fold, even when corrected for the total protein content given that cell numbers may have changed with hypoxia.
Since enhanced BDNF secretion could involve altered BDNF expression (in addition to release of pre-formed intracellular BDNF), we examined changes in BDNF mRNA in PAECs under normoxia vs. hypoxia using Q-PCR, and found that hypoxia augments mRNA level of BDNF by ~3-fold (Figure 2A). Consistent with the modulation in the mRNA levels, BDNF protein expression is also elevated by hypoxia (Figure 2B). Furthermore, if BDNF is of physiological relevance in PAECs, its signaling would involve TrkB and/or p75NTR. We have previously shown that both the full length and truncated isoforms of TrkB are expressed in human PAECs [18]. Therefore we quantified hypoxia-induced changes in the ratio of TrkB-FL vs. TrkB-T1 and found that with hypoxia, TrkB-FL/TrkB-T1 ratio increases substantially (Figure 2C). However, p75NTR mRNA levels did not increase following hypoxia (Figure 2D).
Figure 2.
Hypoxia-BDNF interactions in PAEC expression of BDNF and its receptors. Measurement of mRNA levels for BDNF, its high affinity receptor tropomyosin related kinase (TrkB, truncated T1 and full length FL forms) and low affinity receptor p75NTR in PAECs under normoxia vs. 24h hypoxia showed that hypoxia augments both BDNF (A) and TrkB-FL (C; in relation to the T1 isoform). In contrast, p75NTR expression was unchanged (D). Hypoxia also increased BDNF protein expression in PAEC lysates (B). Interestingly, acting via TrkB, BDNF caused its own upregulation (A and B) as well as that of TrkB (C) as evidenced the effect of exogenous human recombinant BDNF or activation of TrkB only using the flavanoid agonist 7,8-DHF. These effects of the BDNF/TrkB system were substantially enhanced in the presence of hypoxia. Neutralization of extracellular (presumably PAEC-secreted) BDNF using the chimeric TrkB-Fc protein blunted PAEC expression of TrkB under both hypoxic conditions. Values are means ± SE (n=7 patient samples). *Significant difference from normoxia, # significant effect of BDNF, 7,8-DHF or TrkB-Fc (p<0.05).
3.2. Hypoxia enhances BDNF signaling
In normoxic PAECs exposed to 1nM human recombinant BDNF, there was a significant increase in TrkB-FL/TrkB-T1 ratio, indicating that BDNF enhances expression of its own receptor. This concentration was based on previous reports of circulating serum BDNF levels of ~20 ng/ml which translates to ~1 nM [34–36]. Under hypoxic conditions, there was further increase in this ratio (Figure 2C). Here, a role for extracellular BDNF derived from the PAECs was tested by exposing cells to TrkB-Fc, a soluble BDNF neutralizer (thus blocking TrkB activation), prior to hypoxia treatment. As shown in Figure 2C, increased TrkB-FL/TrkB-T1 ratio caused by hypoxia was abrogated by TrkB-Fc. Conversely, treating cells with the TrkB agonist 7,8-DHF upregulated TrkB-FL/TrkB-T1 ratios to levels comparable to that caused by BDNF, especially under hypoxia (Figure 2C). Consistent with our previous observations [37], control experiments for TrkB-Fc using IgG-Fc did not suppress BDNF effects (data not shown). Furthermore, exposures to BDNF, TrkB-Fc or 7,8-DHF did not alter the expression of p75NTR (Figure 2D), strongly suggesting a specific role for TrkB in BDNF effects. Additionally, we found that both extracellular BDNF as well as 7,8-DHF treatment increased intracellular BDNF mRNA levels, while neutralization of TrkB-Fc decreased it (Figure 2A), suggesting an positive feedback regulation of BDNF of its own expression via TrkB signaling. This was evidenced at both mRNA (Figure 2A) and protein level (Figure 2B).
3.3. HIF-1 is important in hypoxia effects on BDNF/TrkB
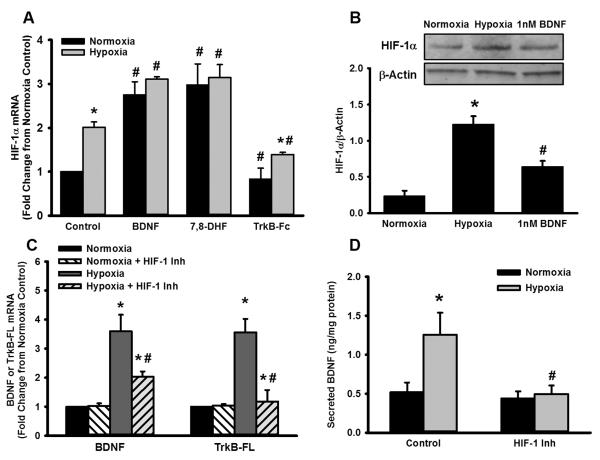
Activation and increased expression of HIF-1α with hypoxia are well-documented [31]. Consistently, PAECs exposed to hypoxia express significantly higher amount of HIF-1α compared to those exposed to 21% O2 (Figure 3A). Interestingly, BDNF upregulates the expression of HIF-1α in both normoxia- and hypoxia-exposed PAECs, although the effect is somewhat greater in the latter (Figure 3A). A similar effect is observed with 7,8-DHF (TrkB agonist), while in contrast, neutralization of extracellular BDNF with TrkB-Fc suppresses hypoxia-induced HIF-1α levels (Figure 3A). Consistently, both hypoxia and BDNF also enhance expression of HIF-1α at the protein level (Figure 3B). This effect is specific for HIF-1α, as treating the cells with HIF-1 inhibitor EMD 400083 blocks hypoxia-induced increase in HIF-1α protein levels. In this regard, previous studies have identified a role for HIF-2 isoform in mediating or modulating the effects of hypoxia [31]. However, we did not observe a detectable modulation in HIF-2 expression in PAECs following hypoxia or BDNF treatment (data not shown). Accordingly, we continued to focus on HIF-1 in subsequent protocols.
Figure 3.
Role of HIF-1α in hypoxia-induced BDNF expression and activity. mRNA analysis (A) and Western blots (B) showed that 24h hypoxia increased PAEC expression of HIF-1α. Interestingly, in both normoxia and hypoxia-exposed PAECs, BDNF also upregulated expression of HIF-1α, with a relatively greater effect in hypoxia (A). A similar effect was observed with the TrkB agonist, while in contrast, neutralization of extracellular BDNF suppressed hypoxia-induced HIF-1α levels. In terms of the role of HIF-1α in hypoxia effects on BDNF and TrkB per se, pharmacological inhibition of HIF-1 (using EMD 400083, see methods) substantially blunted hypoxia enhancement of both BDNF and TrkB mRNA (C), and in accordance, blunted hypoxia-induced enhancement of BDNF secretion (D). Values are means ± SE (n=5 patient samples). *Significant difference from normoxia, # significant effect of BDNF, 7,8-DHF, TrkB-Fc or HIF-1 inhibitor (p<0.05).
Overall, the above data suggest a functional interaction between HIF-1α and BDNF/TrkB signaling. Therefore, we next tested the role of HIF-1α in mediating hypoxia effects of BDNF and TrkB. Pharmacological inhibition with EMD 400083 substantially blunted hypoxia enhancement of both BDNF and TrkB mRNA (Figure 3C), BDNF protein expression (Figure 2B), and BDNF secretion (Figure 3D). It is to be noted that HIF-1 inhibition did not have a substantial influence on baseline BDNF secretion, under normoxic conditions. It is thus likely that during hypoxia, HIF-1 regulates BDNF expression, secretion and signaling.
3.4. HIF-1α-BDNF/TrkB interactions and NO regulation
A primary role for PAECs is production of NO. HIF-1 is known to regulate expression of nitric oxide synthase genes (iNOS, eNOS and nNOS), which are involved in this process [38, 39]. We previously showed that short-term exposure to BDNF can enhance NO production in PAECs [18], but genomic effects of BDNF on NO signaling in the pulmonary artery are not known, particularly in the context of hypoxia. Therefore, we next examined whether BDNF plays a role in eNOS/iNOS and NO signaling in the context of hypoxia. We found that exposure to either hypoxia or BDNF positively regulate NO production in PAECs (Figure 4A). In addition to increasing NO generation under normoxic conditions, BDNF also potentiates the enhancing effect of hypoxia on NO levels. Pre-treatment of PAECs with TrkB-Fc diminished BDNF-induced NO production (Figure 4B). Furthermore, inhibiting HIF-1 with EMD 400083 also causes a decrease in NO, strongly suggesting a role for HIF-1 in hypoxia-mediated NO signaling (Figure 4C). To determine if iNOS or eNOS is the major contributor of NO, we measured NO production in the presence of the inhibitor L-NAME at lower concentration (50 nM; for eNOS) and higher concentration (100 μM; for iNOS). Inhibition of eNOS only slightly blunted the enhanced NO production by hypoxia while blocking iNOS substantially blunted the effects of hypoxia on NO (Figure 4D), indicating that iNOS, rather than eNOS, may be responsible for hypoxia and BDNF-induced NO generation.
Figure 4.
Hypoxia-BDNF interactions in nitric oxide (NO) production by PAECs. NO production in PAECs was measured using diaminofluorescein imaging (see methods). Baseline measurements represented effects of 24h exposures such as hypoxia or inhibitors, while fluorescence responses to BDNF were measured in real-time (A). In PAECs exposed to normoxia vs. hypoxia, exogenous BDNF increased NO generation in normoxia, and potentiated the enhancing effects of hypoxia on NO levels (A) especially at 1 nM that lies in the physiological range. Pre-treatment of PAECs with TrkB-Fc blunted the increase in baseline fluorescence that occurs with hypoxia, while BDNF-induced NO production was also reduced (C). Pharmacologically inhibiting HIF-1 causes a decrease in NO, and blunts the effects of BDNF, strongly suggesting a role for this transcription factor in hypoxia-mediated BDNF signaling (D). To distinguish between the potential roles of iNOS vs. eNOS, the inhibitor L-NAME at lower concentration (50 nM; for eNOS; E) and higher concentration (100 μM; for iNOS; F) was used. Inhibition of eNOS only slightly blunted the enhanced NO production by hypoxia while blocking iNOS substantially blunted the effects of hypoxia on NO. Values in bar graphs are means ± SE (n=5 patient samples). *Significant difference from normoxia, # significant effect of BDNF, TrkBFc, HIF-1 inhibitor, or L-NAME (p<0.05).
Given our findings of altered gene expression by BDNF and hypoxia, we also examined expression of iNOS and eNOS in normoxia vs. hypoxia. While eNOS expression is somewhat reduced by hypoxia (Figure 5A), there is a drastic increase in iNOS mRNA expression under hypoxia (compared to normoxia; Figure 5B). Exposure to BDNF also increases iNOS transcription by several folds, and this is further enhanced in hypoxia. Neutralization of BDNF with TrkB-Fc, or inhibition of HIF-1 eliminates hypoxia-induced increases in iNOS expression, suggesting a link between BDNF and HIF-1 in iNOS regulation. In contrast, eNOS expression was substantially less affected by disrupting BDNF or HIF-1 signaling.
Figure 5.
Role of BDNF in hypoxia effects on eNOS and iNOS in PAECs. With 24h hypoxia, eNOS expression was not significantly affected (A), while iNOS expression substantially increased (B). Exposure to BDNF substantially increased iNOS expression, which was further enhanced in hypoxia (B). Neutralization of BDNF with TrkB-Fc as well as the HIF-1 inhibitor both eliminated increases in iNOS expression, suggesting a link between BDNF and HIF-1 in iNOS regulation. In contrast, eNOS expression was substantially less affected by disrupting BDNF or HIF1 signaling (A). Values are means ± SE (n=4 patient samples). *Significant difference from normoxia, # significant effect of BDNF, 7,8-DHF, TrkB-Fc or HIF-1 inhibitor (p<0.05).
Consistent with the mRNA changes in eNOS and iNOS, we found that eNOS protein levels remained relatively unchanged both under normoxia and hypoxia (data not shown). However, expression level of iNOS protein is distinctly higher with hypoxia than with normoxia (Figure 6A–B). BDNF upregulates iNOS expression both under normoxic and hypoxic conditions, and inhibiting HIF-1 or TrkB signaling attenuates BDNF effects on iNOS expression during hypoxia (Figure 6B).
Figure 6.
Putative mechanisms for hypoxia- and BDNF-induced effects in PAECs. Expression levels of iNOS, and the arginases 1 and 2 were increased by 24h of hypoxia or by BDNF (A). Perturbation of BDNF signaling via TrkB-Fc or of HIF-1 activation via inhibitor blunts increases iNOS (A, B) and arginases 1 (A, C) and 2 (A, D). Values are means ± SE (n=4 patient samples). *Significant difference from normoxia, # significant effect of BDNF, TrkB-Fc or HIF-1 inhibitor (p<0.05).
Our observations of augmented NO accumulation within PAECs following hypoxia or BDNF treatment was somewhat unexpected since both stimuli are thought to be vasoconstrictive in the context of disease. To determine whether other mechanisms could counteract any NO effects in the hypoxic PAEC, we examined the arginase pathway. Arginases 1 and 2 (Arg1 and Arg2) are enzymes that use the same substrate (L-arginine) as NOS enzymes. We found that expression of both Arg1 and 2 are increased by hypoxia, and by BDNF treatment (Figures 6A, C and D). Furthermore, this upregulation of Arg1 and Arg2 is abrogated when HIF-1 or TrkB pathway is blocked.
4. Discussion
PAECs play an important role in regulation of both structure and function of the pulmonary artery via releases of vasoactive molecules such as NO, and factors such as endothelin that are important in vascular contraction and remodeling. In this regard, hypoxia, a major contributing factor in pathogenesis of diseases such as pulmonary hypertension, can act on PAECs, especially given the direct exposure of hypoxic blood to the pulmonary endothelium. Accordingly, understanding the mechanisms by which hypoxia influences PAECs becomes important. The present study demonstrates a novel mechanism, the release of BDNF by PAECs and its potential modulation by hypoxia. Furthermore, an important finding is that BDNF has local effects on PAECs. Here, it appears that BDNF itself modulates the hypoxia-sensitive transcription factor HIF-1 thus priming PAECs in their response to hypoxia, and making BDNF a central player in pulmonary vascular function. Within this context, the interactions between BDNF and hypoxia (via HIF-1α and thus HIF-1) appear to involve the oxygen-sensitive iNOS. However, what was somewhat surprising was our finding that BDNF enhances PAEC NO production, especially in the setting of hypoxia. Whether such effects lead to greater vasodilation in the chronically hypoxic pulmonary artery remains to be determined, where the overall effect on arterial tone may be further modulated by hypoxia and/or BDNF effects on counteracting mechanisms or on vascular smooth muscle. In this regard, our novel but limited findings of increased arginases I and II in the hypoxic or BDNF-exposed PAECs may be particularly relevant in that, depending on substrate availability, the relative effects of arginases on polycations and extracellular matrix proteins in the pulmonary artery may take precedence. Furthermore, parallel hypoxia or BDNF effects on arginases in smooth muscle may be important. Nonetheless, our data underline a locally-produced growth factor with local effects as being a mediator and modulator of hypoxic effects (Figure 7), and thus relevant to the pathogenesis of vascular diseases involving hypoxia, such as pulmonary hypertension.
Figure 7.
Schematic of BDNF-hypoxia interactions in PAECs. Hypoxia can increase circulating BDNF, where PAECs may be a potential source. Hypoxia, acting via HIF-1 can enhance BDNF secretion as well as TrkB expression, which in turn, can increase HIF1α even under normoxia, thus priming PAECs to respond to hypoxia. Both BDNF/TrkB and hypoxia can increase NO production via iNOS.
Although long recognized in the nervous system for their role in neuronal growth and survival [14, 15], there is increasing evidence that neurotrophins, particularly BDNF, as well as Trk and p75NTR receptors are widely distributed in non-neuronal, peripheral tissues including the lung There is now substantial data, including our own, showing the presence of neurotrophins in the lung (see [23] for recent review). In this regard, immunocytochemical and other evidence suggest that BDNF and TrkB receptors are expressed by the pulmonary vasculature [22, 23, 40]. Here, both ligand and receptor have been reported to be present particularly in the intima and adventitia [22]. However, the role of BDNF in the pulmonary artery is still under investigation. For example, as confirmed in this study, we previously showed that both BDNF and TrkB are expressed by human PAECs and BDNF signaling via this high-affinity receptor system is functional in producing NO [18]. However, alterations in BDNF expression and signaling in the context of hypoxia or disease status have not been examined. Here, it is important to note that while upregulation of BDNF by hypoxia has been observed in the cerebral vasculature [25], the downstream effects may be cell- and context-specific, especially, given the myriad of pathways that BDNF can activate. The present study shows that both BDNF and TrkB (but not p75NTR) expression are increased by hypoxia, suggesting a potential functional role for this neurotrophin in hypoxia effects on the pulmonary artery. Here, the data from serum samples of healthy volunteers exposed to hypoxia is relevant in demonstrating that circulating BDNF can also be important in this context. Indeed, the finding that PAECs generate BDNF levels (in ng/mg protein) that are equivalent to circulating levels of ~1 nM raises the intriguing possibility that endothelially-derived BDNF has a large contribution to circulating BDNF. Here, other studies have reported that acute hypoxia can enhance serum concentrations of BDNF. Such increases in BDNF have been associated with beneficial effects such as enhanced cellular survival following stroke or cardiac infarction [27, 41–43]. Whether a similar role for BDNF is relevant to PAECs is not known and remains to be examined, especially if BDNF has differential effects on endothelium vs. smooth muscle. For example, in human airway smooth muscle, exogenous BDNF increases airway contractility and promotes cellular proliferation [44].
The present study shows that PAECs secrete BDNF at baseline and that hypoxia increases BDNF secretion that has autocrine effects on PAECs as demonstrated by the neutralizing effects of the chimeric TrkB-Fc. Furthermore, hypoxia increases both BDNF and TrkB expression, thus facilitating autocrine BDNF effects on PAECs, particularly in the setting of enhanced BDNF release. The mechanisms by which hypoxia enhances BDNF or TrkB expression are not known. Given its importance in hypoxia effects, HIF-1 is a reasonable candidate. However, to the best of our knowledge there are no HIF-1 responsive elements on the BDNF gene. The TrkB gene (NTRK2), on the other hand has twelve putative HREs within a 2kb region upstream of its start site, three of which have been shown to interact with HIF-1α [32] and may therefore be modulated by this mechanism, as suggested by the HIF-1 inhibitor data in our study. Accordingly, we theorize that the enhanced BDNF we observed with hypoxia involves other, indirect mechanisms including NFκB or MAPK that can also be activated in hypoxia. Here, it is also possible that BDNF itself, acting via TrkB, upregulates its own expression, as suggested by the inhibiting effect of TrkB-Fc on BDNF expression even under normoxic conditions. Furthermore, enhanced BDNF expression following TrkB agonism via 7,8-DHF is suggestive of such self-enhancement. Again, mechanisms such as NFκB or MAPK may be relevant, given their role in BDNF/TrkB signaling in other cell types [45]. An alternative pathway may be HIF-2 that can also be activated by hypoxia [31], although there is currently no information on BDNF regulation by this mechanism.
An interesting finding in the current study was the increase in HIF-1α expression by BDNF even under normoxic conditions, with greater expression in hypoxia. At least in the framework of this study, such an effect of BDNF appears to involve enhancement of BDNF and TrkB expression itself, as well as effects on NO production. The mechanisms by which BDNF enhances HIF-1α are not known, but given that the HIF-1α gene is modulated by NFκB, this may be one aspect of BDNF signaling, particularly in the presence of inflammation that may co-exist in pulmonary vascular disease. Although the present study did not specifically examine whether activation of HIF-1α per se by hypoxia is modulated by BDNF, increased HIF-1α expression with chronic BDNF exposure may “prime” PAECs for an exaggerated HIF-1α activation following hypoxia and increased transcriptional activity of HIF-1: a topic that remains to be examined. Furthermore, it will be important to distinguish between BDNF effects on HIF-1 vs. HIF-2, although in pilot studies, we did not find any alteration in HIF-2 levels with BDNF.
We have previously demonstrated that BDNF increases NO production in PAECs via TrkB receptor activation [18]. Interestingly, in the current study we found that hypoxia alone increased NO production in PAECs, which appears to be at odds with the idea of “endothelial dysfunction” and exaggerated vasoconstriction. While it is difficult to definitely support any particular side of a debate on whether hypoxia increases or decreases NO (and by what mechanism) using just our in vitro studies, the important finding here was that BDNF substantially potentiated the effect of hypoxia in terms of NO production, and that too acting via the oxygen/hypoxia-sensitive iNOS. The significance of these findings underscores the possibility that in the setting of hypoxia, enhanced BDNF has autocrine modulating effects of potentiating iNOS-mediated endothelial NO production. Indeed, the finding that BDNF itself increases HIF-1α may be relevant in its priming of hypoxia effects on iNOS. Here, BDNF may act as a co-stimulator for increasing iNOS, akin to cytokines in an inflammatory milieu. More studies are required to determine the mechanisms of such interactions, and whether they lead to enhanced vasodilation. Here, it is possible that other factors induced by hypoxia or BDNF may color the overall effect of NO per se. A particularly relevant aspect is generation of superoxide during hypoxia, which may combine with NO to form peroxynitrite and thus result in vasoconstriction rather than vasodilation. In addition to peroxynitrite, overall NO effects may be limited by enhanced arginase production due to hypoxia [46] reducing substrate availability, thus negating the effects of increased iNOS. This scenario is supported by our novel but limited findings of increased arginases by hypoxia and/or BDNF. These alternative scenarios remain to be further tested.
In addition to effects on tone, PAEC-derived BDNF may have other roles in the response of pulmonary arteries to hypoxia. In airway smooth muscle, BDNF has been shown to enhance [Ca2+]i and contractility, and promote cell proliferation. If such effects also occur in pulmonary vascular smooth muscle, this could regulate vascular remodeling and potentiate the effects of hypoxia on this process. Here, it is important to note a single previous finding that BDNF enhances migration of non-pulmonary endothelial cells [47]. Again, these alternate scenarios remain to be examined.
Highlights
Hypoxia is shown to increase circulating brain-derived neurotrophic factor
Pulmonary endothelium is shown to be a source of BDNF
BDNF can increase endothelial nitric oxide, and enhance hypoxia effects via HIF-1
Hypoxia-BDNF interactions may be relevant to diseases like pulmonary hypertension
Acknowledgements
Martin Helan, M.D. was supported by European Regional Development Fund - Project FNUSA-ICRC (No.CZ.1.05/1.1.00/02.0123) and ICRC Human Bridge - Support of Study Stays of Czech Researchers Abroad: Young Talent Incubator“ (reg. n. CZ.1.07/2.3.00/20.0022), which is financed by the European Social Fund and the state budget of the Czech Republic through The Education for Competitiveness Operational Programme. Additional support by the Foundation for Anesthesia Education and Research (FAER; Hartman), the American Heart Association (Hartman) and the Loretta and Roger Nelson Career Development Award (Hartman), the Flight Attendants Medical Research Institute (FAMRI; Aravamudan) the Mayo Clinic CTSA (NCRR 1 UL1 RR024150), National Science Foundation (BDJ, B-179-M) and HL088029 & HL056470 (YSP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None of the authors have any relevant disclosures.
References
- [1].Frumkin LR. The pharmacological treatment of pulmonary arterial hypertension. Pharmacol Rev. 2012;64:583–620. doi: 10.1124/pr.111.005587. [DOI] [PubMed] [Google Scholar]
- [2].Tajsic T, Morrell NW. Smooth muscle cell hypertrophy, proliferation, migration and apoptosis in pulmonary hypertension. Compr Physiol. 2011;1:295–317. doi: 10.1002/cphy.c100026. [DOI] [PubMed] [Google Scholar]
- [3].Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2010;43:629–34. doi: 10.1165/rcmb.2009-0389TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Humbert M, Gerry Coghlan J, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev. 2012;21:306–12. doi: 10.1183/09059180.00005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McGlothlin D. Classification of pulmonary hypertension. Heart Fail Clin. 2012;8:301–17. doi: 10.1016/j.hfc.2012.04.013. [DOI] [PubMed] [Google Scholar]
- [7].McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21:8–18. doi: 10.1183/09059180.00008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fish JE, Matouk CC, Yeboah E, Bevan SC, Khan M, Patil K, et al. Hypoxia-inducible expression of a natural cis-antisense transcript inhibits endothelial nitric-oxide synthase. J Biol Chem. 2007;282:15652–66. doi: 10.1074/jbc.M608318200. [DOI] [PubMed] [Google Scholar]
- [9].Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem. 2002;277:44085–92. doi: 10.1074/jbc.M205934200. [DOI] [PubMed] [Google Scholar]
- [10].Fagan KA, Morrissey B, Fouty BW, Sato K, Harral JW, Morris KG, Jr., et al. Upregulation of nitric oxide synthase in mice with severe hypoxia-induced pulmonary hypertension. Respir Res. 2001;2:306–13. doi: 10.1186/rr74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fagan KA, Tyler RC, Sato K, Fouty BW, Morris KG, Jr., Huang PL, et al. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol. 1999;277:L472–8. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- [12].Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000;80:1337–72. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- [13].Beleslin-Cokic BB, Cokic VP, Wang L, Piknova B, Teng R, Schechter AN, et al. Erythropoietin and hypoxia increase erythropoietin receptor and nitric oxide levels in lung microvascular endothelial cells. Cytokine. 2011;54:129–35. doi: 10.1016/j.cyto.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barbacid M. Neurotrophic factors and their receptors. Curr Opinion Cell Biol. 1995;7:148–55. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- [15].Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- [16].Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–73. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- [17].Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med. 2012;16:812–23. doi: 10.1111/j.1582-4934.2011.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meuchel LW, Thompson MA, Cassivi SD, Pabelick CM, Prakash YS. Neurotrophins induce nitric oxide generation in human pulmonary artery endothelial cells. Cardiovasc Res. 2011;91:668–76. doi: 10.1093/cvr/cvr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L447–56. doi: 10.1152/ajplung.00501.2005. [DOI] [PubMed] [Google Scholar]
- [20].Hoyle GW. Neurotrophins and lung disease. Cytokine Growth Factor Rev. 2003;14:551–8. doi: 10.1016/s1359-6101(03)00061-3. [DOI] [PubMed] [Google Scholar]
- [21].Nassenstein C, Kerzel S, Braun A. Neurotrophins and neurotrophin receptors in allergic asthma. Prog Brain Res. 2004;146:347–67. doi: 10.1016/S0079-6123(03)46022-6. [DOI] [PubMed] [Google Scholar]
- [22].Ricci A, Greco S, Amenta F, Bronzetti E, Felici L, Rossodivita I, et al. Neurotrophins and neurotrophin receptors in human pulmonary arteries. J Vasc Res. 2000;37:355–63. doi: 10.1159/000025751. [DOI] [PubMed] [Google Scholar]
- [23].Prakash YS, Thompson MA, Meuchel L, Pabelick CM, Mantilla CB, Zaidi S, et al. Neurotrophins in lung health and disease. Expert Rev Respir Med. 2010;4:395–411. doi: 10.1586/ers.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim H, Li Q, Hempstead BL, Madri JA. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J Biol Chem. 2004;279:33538–46. doi: 10.1074/jbc.M404115200. [DOI] [PubMed] [Google Scholar]
- [25].Wang H, Yuan G, Prabhakar NR, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from PC12 cells in response to oxidative stress requires autocrine dopamine signaling. J Neurochem. 2006;96:694–705. doi: 10.1111/j.1471-4159.2005.03572.x. [DOI] [PubMed] [Google Scholar]
- [26].Wilkerson JER, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009;217:116–23. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Okada S, Yokoyama M, Toko H, Tateno K, Moriya J, Shimizu I, et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arterioscler Thrombosis Vasc Biol. 2012;32:1902–9. doi: 10.1161/ATVBAHA.112.248930. [DOI] [PubMed] [Google Scholar]
- [28].Nakamura K, Martin KC, Jackson JK, Beppu K, Woo C-W, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1α in neuroblastoma cells. Cancer Res. 2006;66:4249–55. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- [29].Sciesielski LK, Paliege A, Martinka P, Scholz H. Enhanced pulmonary expression of the TrkB neurotrophin receptor in hypoxic rats is associated with increased acetylcholine-induced airway contractility. Acta Physiologica. 2009;197:253–64. doi: 10.1111/j.1748-1716.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- [30].Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–42. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Martens LK, Kirschner KM, Warnecke C, Scholz H. Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator of the TrkB neurotrophin receptor gene. J Biol Chem. 2007;282:14379–88. doi: 10.1074/jbc.M609857200. [DOI] [PubMed] [Google Scholar]
- [33].Yu J, Taylor L, Wilson J, Comhair S, Erzurum S, Polgar P. Altered expression and signal transduction of endothelin-1 receptors in heritable and idiopathic pulmonary arterial hypertension. J Cell Physiol. 2012 doi: 10.1002/jcp.24132. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–23. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- [35].Jevtovic S, Karlovic D, Mihaljevic-Peles A, Seric V, Vrkic N, Jaksic N. Serum Brain-derived neurotrophic factor (BDNF): the severity and symptomatic dimensions of depression. Psychiatria Danubina. 2011;23:363–9. [PubMed] [Google Scholar]
- [36].Karczewska-Kupczewska M, Kowalska I, Nikolajuk A, Adamska A, Zielinska M, Kaminska N, et al. Circulating brain-derived neurotrophic factor concentration is downregulated by intralipid/heparin infusion or high-fat meal in young healthy male subjects. Diabetes Care. 2012;35:358–62. doi: 10.2337/dc11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sathish V, Vanoosten SK, Miller BS, Aravamudan B, Thompson MA, Pabelick CM, et al. Brain-derived neurotrophic factor in cigarette smoke-induced airway hyperreactivity. Am J Respir Cell Mol Biol. 2013;48:431–8. doi: 10.1165/rcmb.2012-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hu R, Dai A, Tan S. Hypoxia-inducible factor 1 alpha upregulates the expression of inducible nitric oxide synthase gene in pulmonary arteries of hyposic rat. Chin Med J (Engl) 2002;115:1833–7. [PubMed] [Google Scholar]
- [39].Matrone C, Pignataro G, Molinaro P, Irace C, Scorziello A, Di Renzo GF, et al. HIF-1alpha reveals a binding activity to the promoter of iNOS gene after permanent middle cerebral artery occlusion. J Neurochem. 2004;90:368–78. doi: 10.1111/j.1471-4159.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- [40].Ricci A, Felici L, Mariotta S, Mannino F, Schmid G, Terzano C, et al. Neurotrophin and neurotrophin receptor protein expression in the human lung. Am J Respir Cell Mol Biol. 2004;30:12–9. doi: 10.1165/rcmb.2002-0110OC. [DOI] [PubMed] [Google Scholar]
- [41].Westbroek EM, Pawlikowska L, Lawton MT, McCulloch CE, Young WL, Kim H. Brain-derived neurotrophic factor Val66Met polymorphism predicts worse functional outcome after surgery in patients with unruptured brain arteriovenous malformation. Stroke. 2012;43:2255–7. doi: 10.1161/STROKEAHA.112.663096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lazarovici P, Cohen G, Arien-Zakay H, Chen J, Zhang C, Chopp M, et al. Multimodal neuroprotection induced by PACAP38 in oxygen-glucose deprivation and middle cerebral artery occlusion stroke models. J Mol Neurosci. 2012;48:526–540. doi: 10.1007/s12031-012-9818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mackin P, Gallagher P. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Intl J Cardiol. 2005;105:352. doi: 10.1016/j.ijcard.2005.06.063. [DOI] [PubMed] [Google Scholar]
- [44].Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L447–L56. doi: 10.1152/ajplung.00501.2005. [DOI] [PubMed] [Google Scholar]
- [45].Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med. 2012;16:812–23. doi: 10.1111/j.1582-4934.2011.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Krotova K, Patel JM, Block ER, Zharikov S. Hypoxic upregulation of arginase II in human lung endothelial cells. Am J Physiol Cell Physiol. 2010;299:C1541–8. doi: 10.1152/ajpcell.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Matsuda S, Fujita T, Kajiya M, Takeda K, Shiba H, Kawaguchi H, et al. Brain-derived neurotrophic factor induces migration of endothelial cells through a TrkB-ERK-integrin alphaVbeta3-FAK cascade. J Cell Physiol. 2012;227:2123–9. doi: 10.1002/jcp.22942. [DOI] [PubMed] [Google Scholar]