Abstract
Mu opioid receptors (MOP-r) play an important role in the rewarding and locomotor stimulatory effects of heroin. The aim of the current study was to determine whether infusion of small interfering RNAs (siRNA) targeting MOP-r into the midbrain could knock down MOP-r mRNA and affect heroin-induced locomotor activity or heroin-induced conditioned place preference. Ten week old male C57BL/6J mice were surgically implanted bilaterally with guide cannulae directed between the substantia nigra and ventral tegmental area. After 4 days recovery, mice were infused bilaterally with siRNAs that target the MOP-r (2mM × 0.75 μl/side/day for 3 days) or control siRNA. Seven days after the last infusion, a procedure for conditioned place preference was begun with four heroin (3mg/kg i.p.) administration sessions alternating with four saline sessions. While heroin induced an increase in locomotor activity in all groups, siRNAs targeting specific regions of MOP-r significantly attenuated this effect. Of particular interest, mice infused with specific siRNAs targeting the MOP-r failed to develop and express conditioned place preference to heroin, or showed a significantly attenuated preference. These alterations in reward related behaviors are likely due to the reduction in MOP-r mRNA and protein, shown in separate studies by in situ hybridization and autoradiography using the same MOP-r- siRNA infusions. Taken together, these studies demonstrate the utility of siRNA in the neurobiological study of specific components of the reward system and should contribute to the study of other complex behaviors.
Keywords: siRNAs, locomotor activity, conditioned place preference, heroin, mouse, MOP-r
Introduction
Mu opioid receptors (MOP-r) are widely distributed throughout the central nervous system (Thompson et al., 1993; Minami et al., 1994) and play an important role in the reinforcing effects of drugs of abuse. This is supported by studies in mice with MOP-r deletion or MOP-r deficiency. For example, mice lacking the MOP-r gene showed loss of morphine-induced analgesia, reward effect and withdrawal symptoms (Matthes et al., 1996). Intracerebroventricular morphine self-administration and morphine-induced locomotor activity were significantly decreased in the mice with lower MOP-r numbers compared to normal mice (Becker et al., 2000). As expected, heroin-induced conditioned place preference and locomotor activity were absent in MOP-r knock out mice (Contarino et al., 2002). Furthermore, ethanol intake was significantly lower in MOP-r deficient mice, in either oral or intravenous ethanol self-administration studies (Becker et al., 2002; Roberts et al., 2000). Also, a low dose of cocaine failed to induce conditioned place preference in the MOP-r knockout mice, but did in wild type mice (Becker et al., 2002). Taken together, these findings suggest that MOP-r in the central nervous system is necessary in mediating the rewarding properties of many drugs of abuse.
The mesolimbic and nigrostriatal dopaminergic systems are hypothesized to mediate the rewarding effect of drugs of abuse (Di Chiara and Imperato, 1988; Di Chiara and North, 1992; Nestler 1992). MOP-r in the substantia nigra (SN) and ventral tegmental area (VTA) are mostly expressed in the GABAergic interneurons (Di Chiara and North, 1992; Johnson and North, 1992). Activation of MOP-r hyperpolarizes and inhibits GABAergic neurons. This would disinhibit SN and VTA dopamine neurons, increase dopamine neuronal firing and induce dopamine release (Matthews and German, 1984; Di Chiara and North, 1992; Johnson and North, 1992; Margolis et al., 2003). Behavioral studies show that intra-SN or VTA administration of opiates produces conditioned place preference and sustains self-administration behaviors (Bals-Kubik et al., 1993; Baumeister et al., 1993; Devine and Wise, 1994; David et al., 2002), suggesting that SN and VTA dopamine neurons are involved in opiate reward (Pierce and Kumaresan, 2006). Thus, knockdown of MOP-r with siRNAs in these brain areas may provide mechanistic information with site specificity on the neurobiological substrates of these effects.
Small interfering RNA (siRNA) causes sequence-specific gene silencing and elicits down-regulation of gene expression in mammalian cells. In recent years, siRNA knockdown has become a standard method to study functions of proteins of interest. SiRNAs have also been used in the development of treatments of a variety of diseases including animal models of neurological diseases (for review, see (Farah, 2007)). Since the application of RNA interference technology in basic scientific research, or for potential therapeutic administration in the central nervous system, depends largely on the method of delivery, we were interested in determining whether infusion of siRNAs targeting MOP-r can lead to a depletion MOP-r protein in the midbrain, where the dopaminergic neurons are located, and block an increase in locomotor activity and the development of a conditioned place preference resulting from MOP-r activation.
We first infused synthetic unmodified siRNAs, targeting specific sequences for mouse MOP-r, directly into the SN and VTA of the adult C57BL/6J mouse, and then examined how this affected the locomotor response to heroin and the development of heroin-induced conditioned place preference. Then, in different groups of mice, we measured changes in MOP-r mRNA levels and receptor density in the brain of C57BL/6J mice following infusions of one of the siRNAs that had produced behavioral effects. Finally, we examined the effect of another behaviorally active siRNA directly infused into the striatum, a brain region expressing dense MOP-r (Thompson et al., 1993; Minami et al., 1994). We found that infusion of siRNA targeting MOP-r in the midbrain blocked or attenuated heroin induced conditioned place preference and locomotor activity. Infusion of each of the behaviorally active siRNAs was shown to decrease MOP-r mRNA levels and receptor density in the infused brain regions.
These studies demonstrate the utility of siRNA in the neurobiological study of specific components of the reward system and should contribute to the study of other complex behaviors. Further, they should demonstrate the usefulness of unmodified siRNA as a research tool in future studies. Studies such as these could shed light on the potential of siRNAs as therapeutic agents.
Materials and Methods
Subjects
Male C57BL/6J mice (ten week, Jackson Laboratory, Bar Harbor, ME) weighing 22–25 g were individually housed with free access to food and water in a light-(12:12 hour light/dark cycle, lights on at 7:00 am) and temperature-(25 °C) controlled room. The experimental protocols used were approved by the Institutional Animal Care and Use Committee of The Rockefeller University.
Plasmid
Oprm1 psiCHECK plasmid was created by LR clonase recombination between psiCHECK DEST LR and pENTR4 Oprm1. pENTR4 Oprm1 was generated by PCR amplification of rat brain cDNA with oligonucleotides 32.33 and 43.8 (see below) and ligation of digested PCR product into SalI and NotI sites of pENTR4.
RNA oligonucleotides
All siRNA duplexes consisted of two complementary 21-nucleotide RNA strands with 3′ dTdT overhangs designed by our laboratories and chemically synthesized (Dharmacon, Lafayette, CO). SiRNA strands were annealed in artificial cerebral spinal fluid (CMA, North Chelmsford, MA) and injected at a concentration of 2 mM. siRNA sequences were as follows:
“MUMR1”: (sense) 5′-GGCUCAACUUGUCCCACGUdTdT, (anti-sense) 5′-ACGUGGGACAAGUUGAGCCdTdT;
“MUMR2”: (sense) 5′-CCACAUGGUACUGGGAGAAdTdT, (anti-sense) 5′-UUCUCCCAGUACCAUGUGGdTdT;
“MORD2”: (sense) 5′-CAAGAUCGUGAUCUCAAUAdTdT-3′, (anti-sense) 5′-UAUUGAGAUCACGAUCUUGdTdT;
“MORD4”: (sense) 5′-CAAGAUCGUGAUCUCAAUAdTdT-3′, (anti-sense) 5′-UAUUGAGAUCACGAUCUUGdTdT;
GFP4: (sense) 5′-GCAUCAAGGUGAACUUCAAdTdT-3′, (anti-sense) 5′-UUGAAGUUCACCUUGAUGCdTdT;
GL2: (sense) 5′-CGUACGCGGAAUACUUCGAdTdT-3′, (anti-sense) 5′-UCGAAGUAUUCCGCGUACGdTdT.
DNA oligonucleotides:
32.33: 5′-ACGCGTCGACATGGACAGCAGCACCGGCCCAG
43.8: 5′-ATAAGAATGCGGCCGCTTAGGGCAATGGAGCAGTTTCTGCCTC
Restriction sites are underlined.
Selection and prevalidation of siRNAs
SiRNAs were designed to target regions of the sequence of the mRNA of MOP-r which are identical in the mouse and rat (Oprm1: accession number: NM_011013 and NM_013071, respectively) using standard criteria (Reynolds et al., 2004). The siRNAs were prevalidated using an in vitro dual-luciferase assay to assess the degree of knockdown of the MOP-r mRNA. Briefly, a cDNA was cloned into psiCHECK-2 and resulting plasmids were cotransfected with specific and control (GFP4) siRNAs into HEK293 cells according to manufacturer’s protocol. Three siRNA duplexes, “MUMR1”, “MORD2” and “MORD4”, resulted in an in vitro knockdown of more than 85% (Figure 1) and were chosen for in vivo infusion experiments. As control siRNA duplexes, GFP4, green fluorescent protein (control 1), and GL2, firefly luciferase (control 2), were used.
Figure 1.
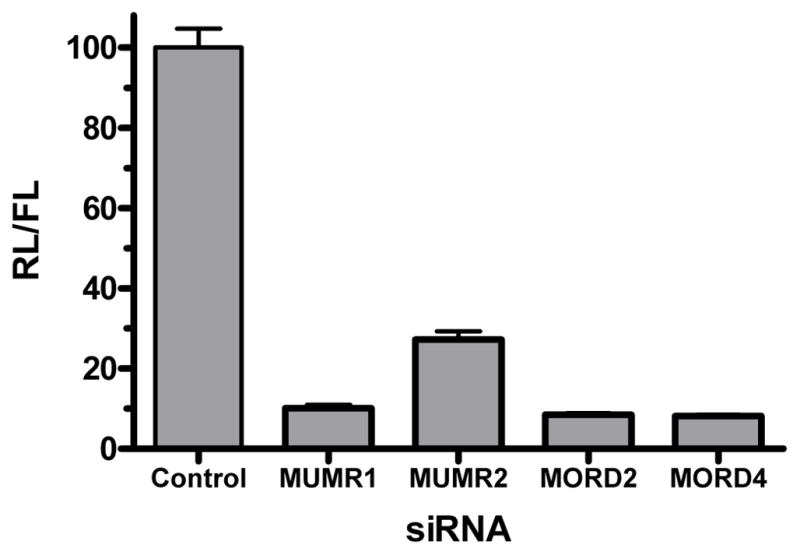
In vitro validation of knockdown by siRNAs targeting specific regions of the mRNA for MOP-r. Control and MOP-r-specific siRNAs were co-transfected with psiCHECK Oprm1 plasmid into HEK293 cells. Luciferase activities were measured and results are shown as ratios of renilla/firefly luciferase (RL/FL) activity normalized to control siRNA transfection (in %), indicating effect of knockdown by specific siRNAs.
Surgical procedures
After 1 week of acclimation, mice were anaesthetized with a combination of xylazine (8.0 mg/kg i.p.) and ketamine (80 mg/kg i.p.) and placed in a stereotaxic frame (David Kopf, Topanga, CA). Guide cannulae (CMA/7, North Chelmsford, MA) were implanted bilaterally between the substantia nigra and ventral tegmental area (coordinates from Bregma: A = −308 mm, L = ± 2mm and V= 4mm at 15 degree (Franklin and Paxinos, 1997). In some groups, guide cannulae (CMA/7, North Chelmsford, MA) were implanted into the striatum (coordinates from Bregma: A = 0.65 mm, L = ± 2.00 mm and V= 3.00 mm (Franklin and Paxinos, 1997). The guide cannulae were fixed to the skull by dental acrylic. Mice were allowed 4–5 days to recover after surgery.
siRNA infusion procedures
Intracranial infusions of siRNA were performed in mice recovered from surgery. In the first series of behavioral studies, one of three different siRNA duplexes (“MUMR1”, “MORD2” or “MORD4”) targeting specific sequences in the mouse gene for MOP-r were infused into each side of the brain between the substantia nigra and ventral tegmental area (SN/VTA) 1.5 μl/day (0.75 μl/side), for 3 consecutive days, in separate groups of mice. As control siRNA duplexes, GFP4 or GL2 targeting GFP and firefly luciferase, respectively, were infused into the same regions of control mice. The infusion pump delivered the siRNA (2mM) at 0.2μl/min for 7.5 min during each infusion. Mice were anaesthetized with a combination of xylazine (8.0 mg/kg i.p.) and ketamine (80 mg/kg i.p.) to prevent movement during infusion.
In the second set of studies, a behaviorally active siRNA duplex (“MUMR1”) was infused into the SN/VTA of mice (or GL2 siRNA duplex in control mice) using the procedure described above. Mice were sacrificed 1 or 7 days after the last infusion of siRNA. Their brains were frozen at −80°C and sectioned to determine, by in situ hybridization and receptor binding autoradiography, whether there were changes in the mRNA and protein levels of the targeted molecule in the infused brain regions.
In the third set of studies, the other behaviorally active siRNA (“MORD2”) was infused into the striatum of experimental mice (or GL2 siRNA in control mice). Changes in the MOP-r mRNA levels and receptor density were determined in mice sacrificed 1 or 7 days after the last injection.
Behavioral testing
The experiments were performed in dimly lit, sound-attenuated mouse place preference chambers (model ENV-3013, MED Associates, St. Ablans, VT) with three distinct compartments that could be separated by removable doors. Locomotor activity was monitored by infrared photobeams in each compartment (Zhang et al., 2004). The locomotor response and the development of conditioned place preference to heroin of mice infused bilaterally with siRNA were studied beginning 7 days after the last infusion since earlier studies found that behavioral change occurred approximately 7–9 days after siRNA infusion (Thakker et al., 2004; Thakker et al., 2005). The study used an unbiased, counterbalanced design in which mice were randomly assigned to either the heroin or saline compartment on the first day. Half the animals had white and half had black chambers as the heroin-paired side. During the pre-conditioning session, each mouse was placed in the center gray compartment with free access to the black and white compartments. The time spent and the locomotor activity within each compartment were recorded for 30 min. Locomotor activity was assessed as the number of “crossovers” defined as breaking the beams at either end of the conditioning compartment. During conditioning sessions, mice were confined to one compartment for 30 min after heroin (3 mg/kg, i.p.) or into the other compartment after saline injection. The mice were injected with heroin and saline on alternate sessions, for a total of eight conditioning sessions with four heroin and four saline trials for each animal. The post-conditioning test session was performed on the day after the last conditioning session, and was identical to the preconditioning session. The difference in time spent in the heroin-paired compartment between the pre- and post-conditioning sessions was used to determine the development of conditioned place preference to heroin. Behavioral experiments were conducted during the first half of the light phase of the diurnal cycle (all experiments were performed between 9:00 am and 12:00 pm).
In situ hybridization
Hybridization probes
Brain sections were subjected to an in situ hybridization protocol as described earlier (Mathieu-Kia and Besson, 1998). 33P-labeled antisense riboprobe was used to detect MOP-r. The DNA template for the probe was generated from cDNA fragments of MOP-r (Genbank accession number AB047546, from 789 to 1612 nucleotide).
Localization of MOP-r mRNA by in situ hybridization histochemistry
Mice were decapitated 1 or 7 days after the last infusion and their brains were frozen at −80° C. Coronal sections (15 μm) were cut at −16 °C and mounted onto Superfrost/Plus glass slides (Fisher Scientific, Pittsburgh, PA). The brain sections were fixed for 10 min at 4 ° C in 4% paraformaldedyde/0.1 M sodium phosphate buffer (pH 7.2) and washed for 10 min in 0.1 M sodium phosphate buffer. Sections were then acetylated at room temperature for 10 min with 0.25% acetic anhydrite dissolved in acetylation buffer. Slides were washed twice in dH2O for 1 min and air-dried. Sections were hybridized overnight at 65 ° C in a humidified environment with a solution consisting of Denhart’s solution, 50% formamide, 10% dextran sulfate, 10mM dTT, 100 μg/ml of sheared and denatured salmon sperm DNA, 400 μg/100ul of tRNA, 10% SDS and 1mM EDTA and 6 × 106 cpm/ml oligonuceotide probe. The hybridization solution was applied in a volume of 100 μl and the slide was coverslipped. The next day, sections were soaked in 4 X SSC/1mM DTT solution to remove the cover slips. Slides were subsequently washed in 4 X SSC/1mM DTT solution at RT for 60 min, 50% formamide/2x SSC, pH 7 at 65 °C for 30 min followed by RNase digestion (20 μg/ml) at room temperature for 60 min. A final 15 min wash in 1 x SSC at 60 °C was carried out followed by a 15 min wash in 0.1 x SSC at 60 °C. The slides were quickly dehydrated in ascending ethanol concentrations and air dried at room temperature. The slides were then opposed to X-ray films (Bmax, Kodak, Rochester, NY) for 3–4 weeks.
Quantification of MOP-r mRNA levels
Quantitative analyses of messenger RNA were performed on autoradiograph films by quantifying grey levels on the exposed film in regions corresponding to the substantia nigra and ventral tegmental area, or striatum (see Fig. 2). The hybridization signals were quantified on both hemispheres by optical density measurement with a computerized image processing system (MCID, Imaging Research, St. Catharines, Canada). Four to seven sections per animal were quantified and the values were averaged to generate an optical density value that corresponds to mRNA abundance per mouse. Then a mean optical density was calculated for each group of mice. Each treatment group consisted of four to eight mice.
Figure 2.
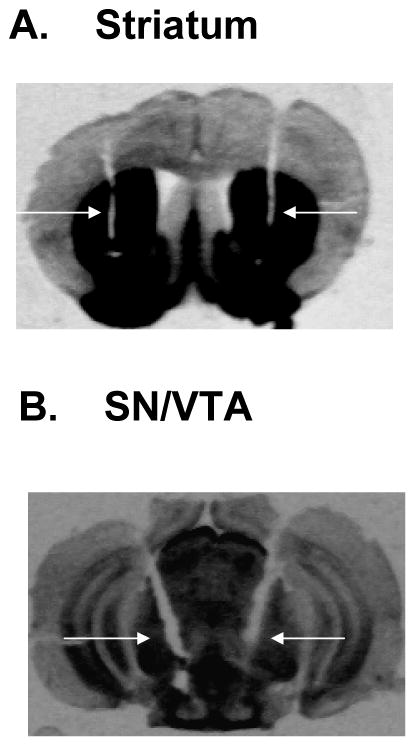
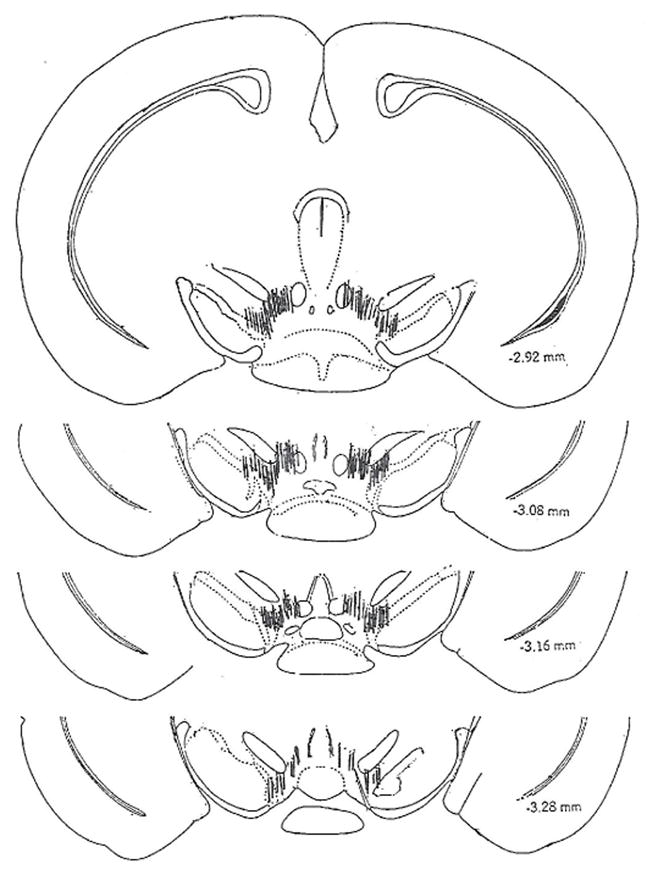
Placement of the guide cannulae for siRNA infusion are shown in Fig 2. The micrograph of a 15 micron section shows the guide cannulae in the striatum (A), or substantia nigra/ventral tegmental area (B) of representative mice used in these studies. Arrows indicate the tracks of the guide cannulae. C. Schematic illustration of infusion cannulae tips within the SN/VTA. The coronal sections depicted are adapted from the atlas by Franklin and Paxinos (1997).
Autoradiography
MOP-r densities in sections were measured by autoradiography as described earlier (Unterwald et al., 1989). Briefly, brain sections were pre-incubated in 50 mM Tris-HCl (pH 7.4) at room temperature for 30 min and then in 50 mM Tris-HCl (pH 7.4) containing tritiated opioid ligands for 60 min at 4°C. MOP-r were labeled specifically with 5 nM of [3H][D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin (DAMGO, New England Nuclear, 60 Ci/mmol). Nonspecific binding was determined on adjacent sections incubated with the tritiated ligand solution as described above but in the presence of 10 μM naloxone. Brain sections were then washed six times at 4°C in 50 mM Tris-HCl (pH 7.4) and then air dried. The labeled sections along with tritium standards (Amersham Life Sciences) were opposed to Bmax film (Kodak, Rochester, NY) for 8 weeks. Exposed autoradiographic films were analyzed as described above. Exposed films were analyzed on the computerized image processing system mentioned above (MCID; Imaging Research). Mean optical density of each brain region was obtained from four to seven sections per subject. Optical densities produced on film by the tritium microscale standards allow us to convert optical density into fmol of radioligand bound per mg of wet-mass tissue equivalent (fmol/mg).
Statistical Analysis
The effect of each siRNA on locomotor activity in each chamber during the four heroin conditioning sessions was evaluated by two-way analysis of variance (Infusion X Trial) with repeated measures on the second factor. The effect of each siRNA that targets MOP-r on the formation of heroin-induced conditioned place preference, and on mRNA and protein levels in each brain region were evaluated by unpaired t-tests.
Results
Cannulae placement
Figure 2A is a photomicrograph of a tissue section from the brain of a representative mouse used in the study, showing cannulae tracks left in the striatum.
Figure 2B is a photomicrograph of a tissue section from the brain of a representative mouse used in the study, showing cannulae tracks left in the SN/VTA. Of a total of 78 mice started in this study, 68 were used in the final analyses; 10 were not included due to misplacement of cannulae.
The ventral points of the cannulae showing the infusion site in SN/VTA are presented in Figure 2C. The infusion cannulae tips were plotted on stereotaxic maps of the SN/VTA −2.92, −3.08, −3.16, 3.28 mm posterior to the bregma, according to the atlas of Franklin and Paxinos (Franklin and Paxinos, 1997).
Effects of siRNAs targeting MOP-r in the SN/VTA on heroin induced locomotor activity
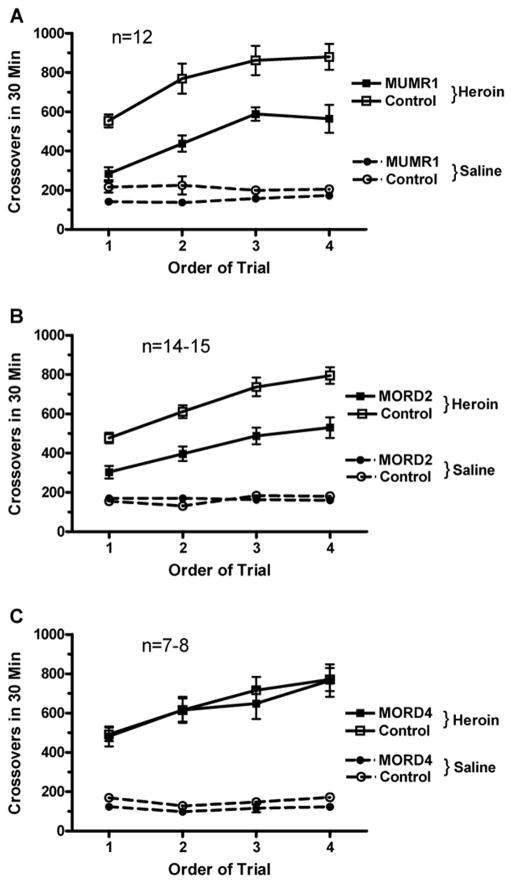
The effect of siRNAs targeting mouse MOP-r in the SN/VTA on heroin induced locomotor activity in the conditioning chamber, measured as number of crossovers from one end of the compartment to the other, is shown in Figure 3. Two-way ANOVA (Infusion X Trial) showed a significant main effect of siRNA “MUMR1” infusion, F (1, 22) = 19.67, p < 0.005 (Panel A). Infusion of siRNA (“MUMR1”) attenuated the heroin induced increases in locomotor activity. There was also a significant increase in locomotor activity over the four conditioning sessions, F (3, 66) = 31.08, p < 0.000001. Mice in both groups showed development of locomotor sensitization in response to repeated heroin injections.
Figure 3.
The effect of siRNAs targeting mouse MOP-r on heroin-induced locomotor activity in the conditioning chamber, measured as number of crossovers from one end of the compartment to the other, is shown in Fig 3. A. There was a significant increase in locomotor activity over the four conditioning sessions. Two-way ANOVA showed a significant main effect of siRNA. Infusion of siRNA (“MUMR1”) attenuated the heroin induced increases in locomotor activity. Mice in both groups showed development of locomotor sensitization in response to repeated heroin injections.
B. Two-way ANOVA showed that there was also a significant effect of the siRNA (“MORD2”) infusion. There was also a significant main effect in heroin conditioning trial in locomotor activities, showing locomotor sensitization induced by heroin in both groups of mice. C, two-way ANOVA showed that there was no significant effect of the “MORD4” siRNA infusion. However, there was a significant main effect in heroin induced locomotor activities in heroin conditioning trials.
Two-way ANOVA (Infusion x Trial) showed that there was also a significant effect of an infusion of another siRNA (“MORD2”), F (1, 27) = 23.23, p < 0.0005 (Panel B). Thus, in mice pretreated with a different siRNA targeting MOP-r, heroin induced a lower locomotor activity than mice pretreated with control siRNA. There was also a significant main effect of Conditioning Trial, F (3, 81) = 46.45, p < 0.000001, showing locomotor sensitization induced by heroin in both groups of mice.
In contrast, pretreatment with the third prevalidated siRNA targeting MOP-r (“MORD4”) had no effect on heroin-induced locomotor activity; only a significant effect of sensitization across the trials was found (Panel C).
Effect of MOP-r siRNAs on formation of heroin induced conditioned place preference
The development of conditioned place preference to heroin in mice infused with siRNAs targeting mouse MOP-r is shown in Figure 4. Mice pre-infused with “MUMR1” showed blockade of increases in time spent on the heroin-paired side, compared with controls mice infused with control siRNA, p < 0.005 (panel A). Similarly, mice pre-infused with “MORD2” showed a significantly smaller increase in time spent in the heroin-paired chamber compared to controls, p < 0.05, shown in Panel B. In contrast, mice infused with “MORD4” showed no effect of this siRNA on the formation of conditioned place preference (Panel C).
Figure 4.
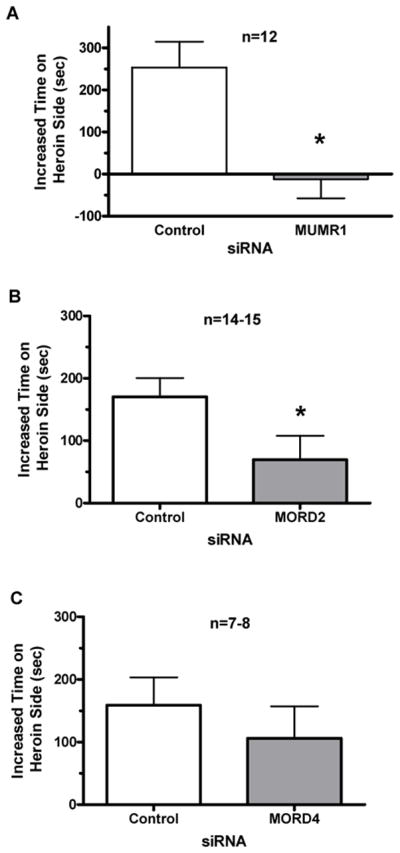
Effect of siRNA targeting mouse MOP-r on heroin-induced conditioned place preference expressed as mean (+SEM) increased time spent in the conditioning chamber are shown in Fig 4. A. A two-tailed t-test showed that pretreatment with siRNA targeting mouse MOP-r (“MUMR1”) prior to heroin blocked the formation of a conditioned place preference. B. Pretreatment with siRNA targeting mouse MOP-r (“MORD2”) attenuated the formation of conditioned place preference induced by heroin. C. Infusion of siRNA (“MORD4”) targeting mouse MOP-r did not affect the formation of conditioned place preference induced by heroin.
The behavioral effect of “MUMR1” and “MORD 2” were examined in two separate studies. The behavioral response did not differ across these cohorts and these were combined for statistical analysis. Two of the three siRNAs, targeting MOP-r show significant attenuation of heroin-induced locomotor activity and blockade or attenuation of heroin-induced conditioned place preference, while the third showed no effect. Thus, infusion of different siRNAs, targeting different sequences of the mouse MOP-r mRNA, that had been effective in the in vitro studies, showed different behavioral effects.
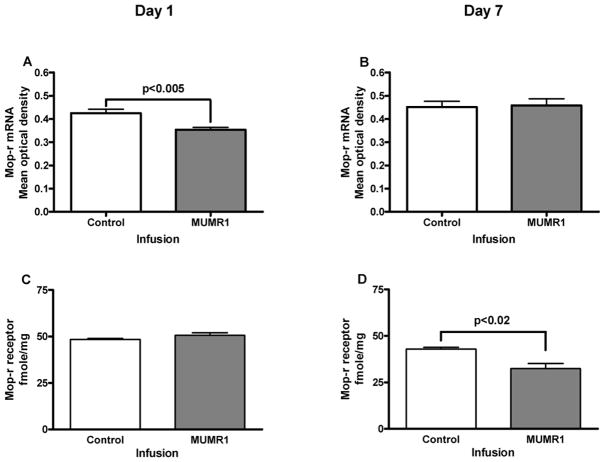
Effect of siRNA (“MUMR1”) targeting MOP-r on MOP-r mRNA levels and receptor density in the mouse midbrain
The effect of “MUMR1” siRNA on MOP-r mRNA levels is shown in Figure 5. A t-test revealed that levels of MOP-r mRNA of mice infused with “MUMR1” siRNA in the SN/VTA were significantly lower than those of the mice injected with the control siRNA, t (8) = 3.51, p < 0.005 (Panel A) on the first day after siRNA infusions. This effect was not found 7 days after the infusions (Panel B).
Figure 5.
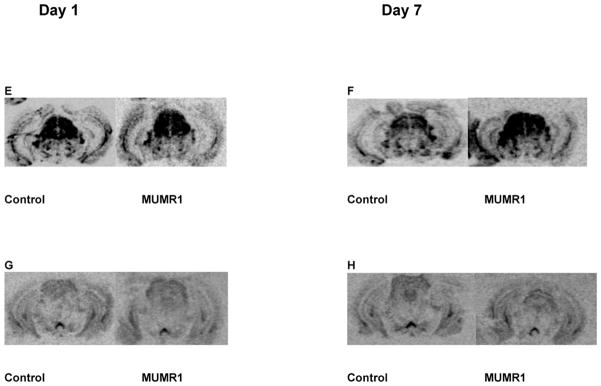
The effect of siRNA (“MUMR1”) on MOP-r mRNA levels and receptor density is shown in Fig 5. A. Levels of MOP-r mRNA of mice infused with “MUMR1” siRNA in the SN/VTA were significantly lower than those of the mice injected with the control siRNA, on the first day after infusions (see also a photomicrograph from a representative mouse in 5E). B. There was no difference in levels of MOP-r mRNA between the two groups 7 days after infusions (see also a photomicrograph from a representative mouse in 5F). C. There was no statistical difference in MOP-r density of the two groups on the first day after infusions (see also a photomicrograph from a representative mouse in 5G). D. There was a significant difference in MOP-r density between the siRNA (“MUMR1”) injected group and the control group, 7 days after infusions (see also a photomicrograph from a representative mouse in 5H).
The quantitative distributions of MOP-r labeled with [3H] DAMGO (5 nM) in coronal sections of SN/VTA from mice infused with siRNA (“MUMR1”) targeting MOP-r or non-specific siRNA are also shown in Fig 5. A t-test showed that there was no significant difference between the groups on the first day after infusion (Panel C). However, there was a significant difference between the siRNA (“MUMR1”) infused group and the control group, t (14) = 2.47, p < 0.02 (Panel D) on the seventh day after infusions. Thus, infusions of this MOP-r targeted siRNA significantly lowered the MOP-r density in the SN/VTA 7 days later.
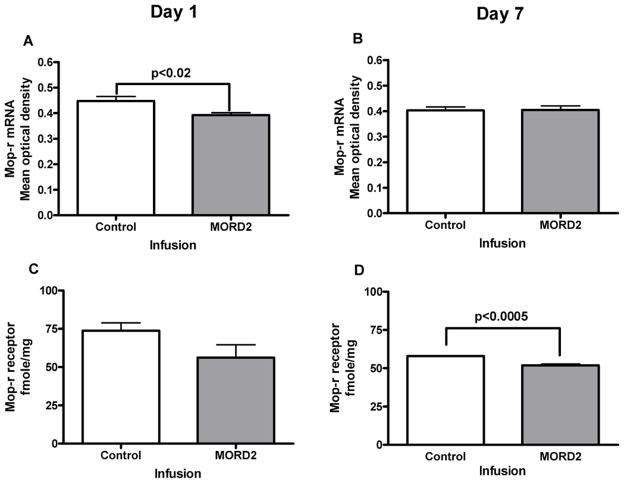
Effects of siRNA (“MORD2”) targeting mouse MOP-r on MOP-r mRNA and receptor density in the mouse striatum
The effect of “MORD2” siRNA on striatal MOP-r mRNA levels is shown in Fig 6. In mice infused with siRNA, striatal MOP-r mRNA levels were significantly lower than those of the controls, t (10) = 2.69, p < 0.02 on the first day after infusions (Panel A). Thus, siRNA (“MORD2”) targeting MOP-r led to decreased levels of MOP-r mRNA across the striatum. The same effect was not found 7 days after infusions (Panel B).
Figure 6.
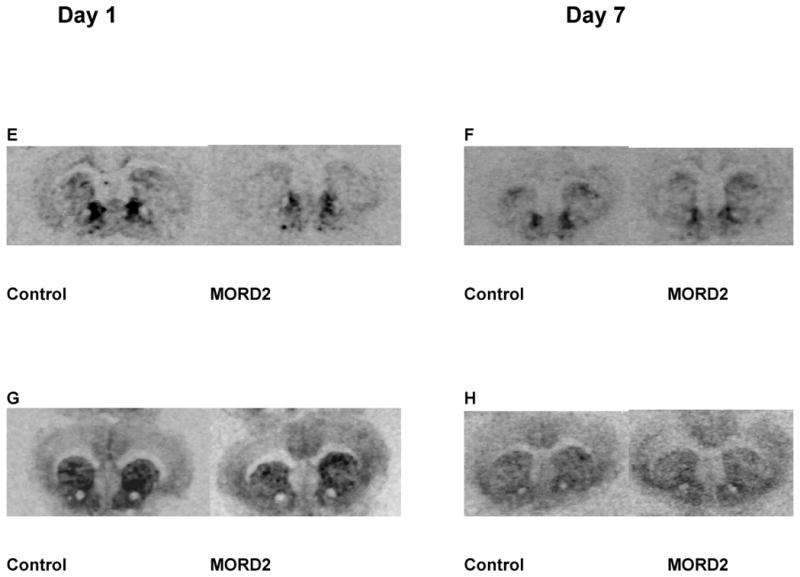
The effect of siRNA (“MORD2”) on striatal MOP-r mRNA levels and density is shown in Fig 6. A. Striatal MOP-r mRNA levels in mice infused with siRNA were significantly lower than those of the controls 1 day after infusions (see also a photomicrograph from a representative mouse in 6E). B. Infusions of siRNA into the mouse striatum did not change the MOP-r mRNA levels 7 day after infusions (see also a photomicrograph from a representative mouse in 6F). C. There was not significant difference between the two groups in striatal MOP-r density at day 1 after infusions (see also a photomicrograph from a representative mouse in 6G). D. Striatal MOP-r receptor density levels were significantly lowered in siRNA (“MORD2”) groups than that of the controls (see also a photomicrograph from a representative mouse in 6H).
The effect of siRNA (“MORD2”) on the striatal MOP-r density is also shown in Fig 6. The striatal MOP-r density in mice infused with siRNA appeared to be lower than that of the control mice on the first day after infusions, however, there was no statistically significant difference between the two groups. Seven days after the infusions, striatal MOP-r density from mice infused with siRNA (“MORD2”) was significantly lower compared to those of the control groups, t (6) = 6.45, p< 0.0005 (Panel D).
Discussion
In the current study, we found that infusion of siRNAs targeting mouse MOP-r in the midbrain, where mesolimbic and nigrostriatal dopaminergic neurons are located, attenuated heroin-induced increases in locomotor activity and blocked or attenuated conditioned place preference in adult C57BL/6J mice. In separate mice, delivery of the same siRNAs into the same brain region resulted in a decrease in MOP-r mRNA levels and receptor density in the area surrounding the site of infusion. Furthermore, infusion of another behaviorally effective siRNA into the mouse striatum, where mesolimbic and nigrostriatal dopaminergic terminals are innervated, also led to a decrease in MOP-r mRNA levels and receptor density.
Opioids such as heroin produce their pharmacological effects primarily through the MOP-r (Kieffer, 1999; Kieffer and Gaveriaux-Ruff, 2002; Surratt et al., 1994). Pharmacological studies have shown that most opioids, such as heroin, have highest affinity for MOP-r, (e.g. Pasternak, 1993) rather than delta and kappa opioid receptors. The MOP-r are critical sites for opioid actions of reward (Wise, 1996; Matthes et al., 1996; Sora et al., 2001) as well as analgesia (Matthes et al., 1996; Sora et al., 1997) tolerance to antinociception (Aghajanian, 1978) and physical dependence (Johnson and McCagh, 2000; Matthes et al., 1996). MOP-r are widely distributed in the CNS, including the mesolimbic and nigrostriatal dopaminergic systems. The current study involved delivery of siRNA directly into the midbrain (SN/VTA) in the behavioral studies because it is well known that opiates activate MOP-r and inhibit GABAergic interneurons in this brain region, which disinhibits dopaminergic neurons (Johnson and North, 1992b). This may underlie the behavioral changes following opioid administration.
In the behavioral studies, infusion of siRNAs targeting MOP-r to the midbrain led to attenuation of the locomotor activity and blocked or attenuated the development of conditioned place preference induced by heroin. The results are consistent with behavioral changes found in global MOP-r knock out mice (Becker et al., 2000). It is intriguing that infusion of siRNA into the SN/VTA, where dopamine cell bodies are located, produces a behavioral consequence similar to that of the global MOP-r knockout mice, suggesting that MOP-r in the midbrain play a key role in heroin induced rewarding and locomotor stimulating effects (see David et al., 2007).
To determine whether such changes in behavior are related to alteration in the target molecule, siRNAs that showed behavioral effects were delivered into the midbrain to examine whether they resulted in reduced levels of MOP-r mRNA or receptor density. We found that repeated infusion of a siRNA (“MUMR1”) targeting MOP-r resulted in a decrease in mouse MOP-r mRNA levels (Fig. 5) on the first day after the siRNA infusion and a decrease in MOP-r receptor density on the seventh days after the infusion. To further explore this effect, a second siRNA (“MORD2”) that also produced attenuation of heroin’s behavioral effects was infused into the striatum, a brain region with high levels of MOP-r mRNA in rodents (Thompson et al., 1993; Minami et al., 1994). Again, decreases in MOP-r mRNA levels were found in the region infused proceeding observed reductions in receptor density (Fig. 6). This is consistent with the results from earlier studies showing that infusion of non-viral siRNAs targeting different genes led to a decrease of the target gene mRNA levels (Thakker et al., 2004, 2005, 2006).
There are differences in siRNA delivery methods between the current studies and the earlier studies. First, in our studies, siRNAs were infused into a specific brain region known to have abundant MOP-r whereas in earlier studies, siRNAs targeting different genes were infused either into the intrathecal space or the dorsal third ventricle (Dorn et al., 2004; Thakker et al., 2004, 2005, 2006). Second, in the current studies, siRNAs targeting MOP-r were infused into a brain region of interest repeatedly over a short period of time whereas in earlier studies siRNAs were constantly infused into intrathecal space or ventricles by osmotic minipumps. Third, the current studies used artificial cerebral spinal fluid as a vehicle, because cerebral spinal fluid is present naturally in the brain, whereas the earlier studies used phosphate-buffered saline for delivery. Since siRNA was specifically infused into the midbrain (an area between the substantia nigra and the ventral tegmental area) or into the striatum in these studies, the targeted gene knockdown effect was localized to the targeted brain regions (Fig. 5 & 6). In contrast, when siRNAs were pumped into either the intrathecal space or ventricle in the earlier studies, the genes knock down effects were widespread (Thakker et al., 2004; Thakker et al., 2005; Hoyer et al., 2006).
Our delivery of siRNA directly into a specific brain region allows the use of the lowest possible concentrations of siRNA. High concentrations of siRNA have been known to cause immune reactions, off target effects and saturation of the microRNA pathway (Snove et al., 2004; Grimm and Kay, 2006).
The infusion target was selectively chosen to include both the SN and VTA since MOP-r are present in the GABAergic neurons controlling dopamine neurons located in both SN and VTA. We purposely delivered a large amount of siRNA (0.75 ul/side/day) to achieve the maximum knockdown effect. The molecular weight of the infused siRNA is quite large (approximately 7035 kd). Previous studies showed that when smaller molecules such as lidocaine or tetrodotoxin (TTX) (molecular weights of 234.3 or 319 kd, respectively) were delivered into the brain (e.g. Boehnke and Rasmusson, 2001) the effective spread for lidocaine or TTX were approximately 1 and 2 mm, respectively. Based solely on the size of our siRNA constructs, we estimate that the infused siRNA might affect neurons within approximately 0.5 mm of the infusion site. Also, some of the infused siRNA may have been degraded by RNAase, further minimizing any diffusion effects.
One important observation in our studies is that the behavioral consequences induced by infusion of siRNAs targeting different sequences of the MOP-r gene are different, even though in vitro tests had demonstrated that all three siRNAs efficiently cleaved the MOP-r mRNA in HEK293 cells. One possibility is that certain siRNAs targeting a specific sequence of a gene requires a higher concentration to achieve down regulation. Constant application of siRNA with osmotic minipumps may circumvent this. However, our findings clearly show that results derived from in vitro studies may not predict the outcome for an in vivo behavioral study.
In conclusion, infusion of siRNAs targeting mouse MOP-r directly into the midbrain attenuated heroin-induced increases in locomotor activity and blocked or attenuated the formation of conditioned place preference in adult C57BL/6J mice. These behavioral changes are likely to be due to knockdown of MOP-r mRNA resulting in decreased receptor density at the infusion site, shown in our studies, demonstrating the utility of siRNA in the neurobiological study of specific components of the reward system.
Acknowledgments
This work was supported by NIH-NIDA P60 DA05130 to Dr. Mary Jeanne Kreek.
Footnotes
Disclosure/Conflict of Interest
The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Baumeister AA, Hurry M, Curtis W, Chaney TM, Wolf E, Leoni RR. The antinociceptive and motivational effects of intranigral injection of opioid agonists. Neuropharmacology. 1993;32:1299–1303. doi: 10.1016/0028-3908(93)90024-w. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Brodemann R, Kraus J, Peters B, Schroeder H, Thiemann W, Loh HH, Hollt V. Morphine self-administration in mu-opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:584–589. doi: 10.1007/s002100000244. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Hollt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J Neurosci Method. 2001;105:133–141. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- Contarino A, Picetti R, Matthes HW, Koob GF, Kieffer BL, Gold LH. Lack of reward and locomotor stimulation induced by heroin in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2002;446:103–109. doi: 10.1016/s0014-2999(02)01812-5. [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology. 2002;160:307–317. doi: 10.1007/s00213-001-0981-2. [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci. 1994;14:1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, North RA. Neurobiology of opiate abuse. Trends Pharmacol Sci. 1992;13:185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- Farah MH. RNAi silencing in mouse models of neurodegenerative diseases. Curr Drug Deliv. 2007;4:161–167. doi: 10.2174/156720107780362276. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. New York: Academic Press, Inc; 1997. [Google Scholar]
- Grimm D, Kay MA. Therapeutic short hairpin RNA expression in the liver: viral targets and vectors. Gene Ther. 2006;13:563–575. doi: 10.1038/sj.gt.3302727. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Thakker DR, Natt F, Maier R, Huesken D, Muller M, Flor P, HVDP, Schmutz M, Bilbe G, Cryan JF. Global down-regulation of gene expression in the brain using RNA interference, with emphasis on monoamine transporters and GPCRs: implications for target characterization in psychiatric and neurological disorders. J Recept Signal Transduct Res. 2006;26:527–547. doi: 10.1080/10799890600929663. [DOI] [PubMed] [Google Scholar]
- Johnson RE, McCagh JC. Buprenorphine and naloxone for heroin dependence. Curr Psychiatry Rep. 2000;2:519–526. doi: 10.1007/s11920-000-0012-8. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Kia AM, Besson MJ. Repeated administration of cocaine, nicotine and ethanol: effects on preprodynorphin, preprotachykinin A and preproenkephalin mRNA expression in the dorsal and the ventral striatum of the rat. Brain Res Mol Brain Res. 1998;54:141–151. doi: 10.1016/s0169-328x(97)00338-0. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- Minami M, Onogi T, Toya T, Katao Y, Hosoi Y, Maekawa K, Katsumata S, Yabuuchi K, Satoh M. Molecular cloning and in situ hybridization histochemistry for rat mu-opioid receptor. Neurosci Res. 1994;18:315–322. doi: 10.1016/0168-0102(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW. Pharmacological mechanisms of opioid analgesics. Clin Neuropharmacol. 1993;16:1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nature Biotechnology. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. Mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Snove O, Jr, Nedland M, Fjeldstad SH, Humberset H, Birkeland OR, Grunfeld T, Saetrom P. Designing effective siRNAs with off-target control. Biochem Biophys Res Commun. 2004;325:769–773. doi: 10.1016/j.bbrc.2004.10.097. [DOI] [PubMed] [Google Scholar]
- Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR. Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology. 2001;25:41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt CK, Johnson PS, Moriwaki A, Seidleck BK, Blaschak CJ, Wang JB, Uhl GR. -mu opiate receptor. Charged transmembrane domain amino acids are critical for agonist recognition and intrinsic activity. J Biol Chem. 1994;269:20548–20553. [PubMed] [Google Scholar]
- Thakker DR, Natt F, Husken D, Maier R, Muller M, van der Putten H, Hoyer D, Cryan JF. Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc Natl Acad Sci U S A. 2004;101:17270–17275. doi: 10.1073/pnas.0406214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker DR, Natt F, Husken D, van der Putten H, Maier R, Hoyer D, Cryan JF. siRNA-mediated knockdown of the serotonin transporter in the adult mouse brain. Mol Psychiatry. 2005;10:782–789. 714. doi: 10.1038/sj.mp.4001687. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat mu opioid receptor. Neuron. 1993;11:903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Tempel A, Koob GF, Zukin RS. Characterization of opioid receptors in rat nucleus accumbens following mesolimbic dopaminergic lesions. Brain Res. 1989;505:111–118. doi: 10.1016/0006-8993(89)90120-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]