Abstract
The first catalytic asymmetric addition of ynamides to aliphatic and aromatic aldehydes is described. This reaction provides unprecedented access to a diverse family of N-substituted propargylic alcohols that are obtained in high yield and ee in the presence of 10 mol% of zinc triflate and N-methylephedrine The use of apolar solvent mixtures is essential to avoid product racemization and to optimize ee’s without compromising conversion.
The unique synthetic versatility of ynamines and ynamides has attracted increasing attention in recent years. In particular, substituted ynamides have been applied in a wide array of carbon-carbon bond forming reactions and several total syntheses of natural products using these multifaceted building blocks as key intermediates have been reported.1 At first glance, ynamines and their analogues appear to be a hybrid structure of alkynes and enamines. The properties and reactivity of ynamines and ynamides, however, is quite unique and by no means an average of these parent functionalities. The presence of the adjacent electron-donating nitrogen atom generates a strongly polarized triple bond. This polarization drastically affects the reactivity and utility of ynamines and it bears huge synthetic potential. Because ynamines are very sensitive and difficult to handle, the incorporation of an electron-withdrawing group that reduces the electron-donating capacity of the amino moiety is necessary to afford isolable ynamine derivatives and as a means to maintain reaction control during catalytic transformations, Figure 1.
Figure 1.
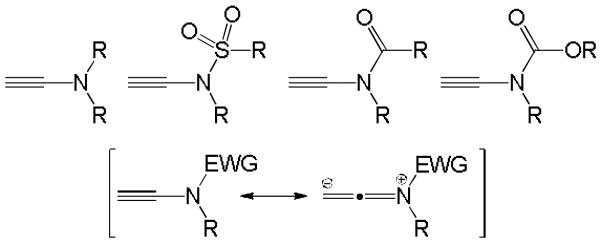
Representative ynamine and ynamide structures.
Witulski,2 Bruckner,3 Saa4 and others introduced various procedures for the synthesis of terminal ynamides, ynesulfonamides and ynecarbamates.1 An impressive variety of reactions with these practical ynamine analogues, including cycloadditions,5 cycloisomerizations,6 homo- and cross-couplings,7 ring-closing metathesis,8 radical additions,9 and titanium-mediated C-C bond formation,10 are known.11 Hsung reported an elegant boron trifluoride promoted two-carbon homologation that yields acrylic amides from aldehydes or ketones upon reaction with terminal ynamides.12 Few examples of nucleophilic additions of lithium or sodium ynamides to aldehydes, imines and ketones toward racemic N-substituted propargylic alcohols have been reported.13 A broadly applicable, mild variant that avoids the use of butyllithium, sodium amide or another strong base has not been developed to date. Moreover, a catalytic enantioselective nucleophilic addition to carbonyl electrophiles is unprecedented. We now wish to introduce a mild catalytic asymmetric ynamide addition to aliphatic and aromatic aldehydes that produces N-substituted propargylic alcohols in high yields and ee’s, Scheme 1
Scheme 1.
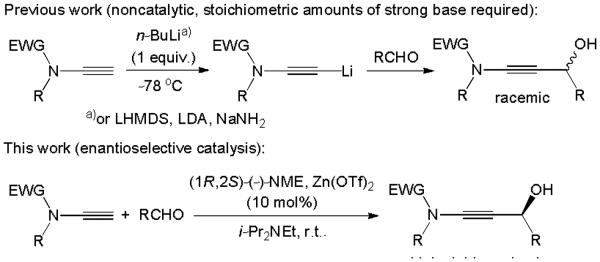
Nucleophilic addition of ynamides to aldehydes.
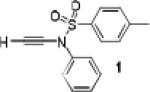
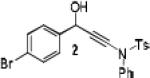
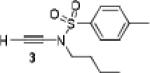
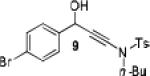
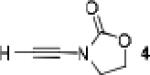
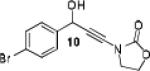
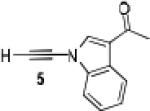
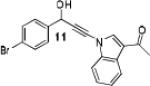
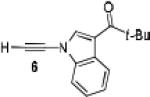
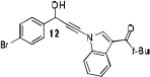
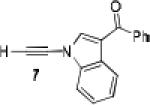
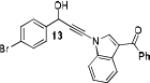
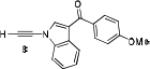
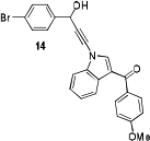
Because little information on the reactivity, in particular with regard to acidity, propensity toward formation of transition metal alkynyl σ-complexes and nucleophilicity, is available for terminal ynamides, we decided to start investigating the possibility of a catalytic enantioselective nucleophilic addition to aldehydes using readily available N-phenyl-N-tosyl ynamide 1 and alkyne addition protocols introduced by Carreira, Trost, Shibasaki and others.14 We were able to prepare ynesulfonamide 1 by three high-yielding steps on the gram scale from commercially available N-tosyl aniline following a literature procedure.3 With this prototype ynamide in hand, we began our search for a catalytic reaction with 4-bromobenzaldehyde by screening a variety of metal salts and chiral ligands, including bisoxazolines, bisoxazolidines, cinchona alkaloids, amino alcohols and diamines, in several solvents.14 We were very pleased to find that the nucleophilic addition occurs in the presence of catalytic amounts of zinc triflate and N-methylephedrine (NME) in toluene at room temperature, providing the N-substituted propargylic alcohol 2 in high yield and 60% ee, see entry 1 in Table 1. Encouraged by this finding, we prepared other ynamides including novel 3-acylindole-stabilized vinylogous ynamides via TBAF-promoted desilylation of TIPS-protected precursors that were obtained as previously described by Stahl.15 As expected, incorporation of the ynamide nitrogen atom into a slightly less electron-withdrawing moiety increases the reactivity while the enantioselectivity of this reaction is substantially reduced. We obtained excellent yields with ynamides 3 and 4 but the ee’s dropped below 40%, entries 2 and 3 in Table 1. The introduction of the indole-derived terminal ynamides 5-8 gave superior results and the ee’s generally improved to 80%, entries 4-7. The reaction with the 3-benzoylindolyl derived ynamide 7 gave the corresponding propargylic alcohol 13 in 87% yield and 77% ee, which seemed particularly promising, entry 6.
Table 1.
Results of the screening of various ynamide nucleophiles.a
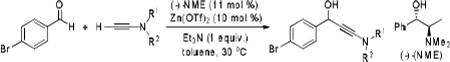
|
| Entry | Ynamide | Product | Time (h) |
Yielda
(%) |
Ee (%) |
|---|---|---|---|---|---|
| 1 |
|
|
2.5 | 87 | 60 |
| 2 |
|
|
3 | 91 | 38 |
| 3 |
|
|
5 | 100 | 27 |
| 4 |
|
|
18 | 68 | 80 |
| 5 |
|
|
18 | 58 | 78 |
| 6 |
|
|
24 | 87 | 77 |
| 7 |
|
|
27 | 60 | 81 |
Based on NMR analysis
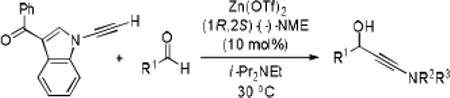
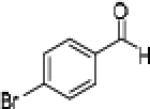
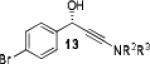
Further optimization of the catalytic asymmetric addition of 7 to bromobenzaldehyde revealed an unexpected feature that to the best of our knowledge has not been observed for additions of ynamines, ynamides or even simple alkynes to aldehydes. During extensive screening of the effects of catalyst loading, solvents and base on the yield and enantioselectivity, we observed that the ee of 13 decreases over time when the reactions were allowed to proceed to full completion. We rationalized that this could be a result of product racemization which one would expect to have an increasing impact at high conversion and at extended reaction times. Indeed, stirring of enantioenriched 13 under typical reaction conditions proved that the C-C bond formation is reversible.16 Comparison of bases showed that the reaction is sluggish when DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) and DMAP (4-dimethylaminopyridine) are used while the best results were obtained with sterically hindered amines and 2,6-lutidine. The presence of the latter, however, was found to strongly promote the racemization reaction which was most evident in THF and diethyl ether. The ee of 13 dropped from 93% to only 23% when the reaction was performed in the presence of one equivalent of 2,6-lutidine in THF for 20 hours. We then discovered that the undesirable effect of the concomitant racemization on the ee of the ynamide addition products can be substantially reduced or eliminated when the reaction is carried out in apolar solvents. Because 13 precipitates in toluene and most of its derivatives are barely soluble in hexane/toluene mixtures, racemization can easily be avoided with these solvent choices even when relatively long reaction times are required to achieve high yields. Under optimized conditions, we were able to produce 13 in 97% yield and 93% ee and we decided to not further optimize the reaction with the other ynamides, entry 1 in Table 2.
Table 2.
Scope of the zinc catalyzed enantioselective ynamide addition.a
|
| Entry | Aldehyde | Product | Time (h) |
Yielda
(%) |
Ee (%) |
|---|---|---|---|---|---|
| 1b |
|
|
18 | 97 | 93 |
| 2 |
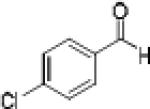
|
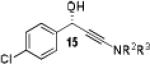
|
16 | 92 | 95 |
| 3 |
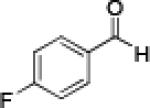
|
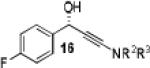
|
18 | 85 | 93 |
| 4 |
|
|
18.5 | 87 | 88 |
| 5 |
|
|
13 | 92 | 71 |
| 6b |
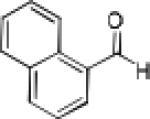
|
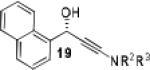
|
18 | 92 | 96 |
| 7 |
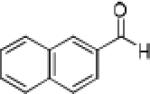
|
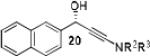
|
17 | 92 | 90 |
| 8 |
|
|
18 | 87 | 84 |
| 9 |
|
|
15 | 93 | 95 |
| 10 |
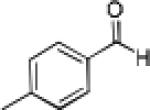
|
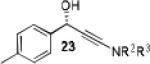
|
20 | 80 | 88 |
| 11 |
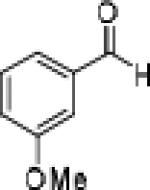
|
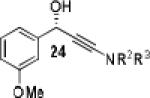
|
26 | 92 | 87 |
| 12 |
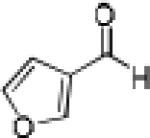
|
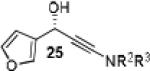
|
21 | 81 | 87 |
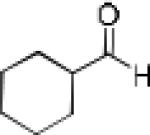
| 13b |
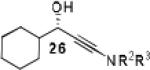
|
|
15 | 89 | 90 |
| 14 |
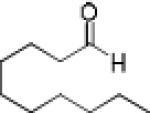
|
|
13 | 87 | 95 |
Standard conditions: ynamide (0.20 mmol), aldehyde (0.30 mmol), Zn(OTf)2 (10 mol%), (−)-NME (11 mol%) in 0.5 mL of toluene/hexane (1:1).
Isolated yields.
Toluene. The absolute configuration of 13 was determined by crystallographic analysis, see ESI. All other assignments are by analogy.
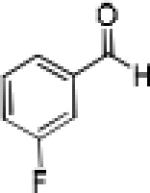
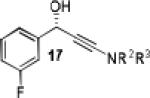
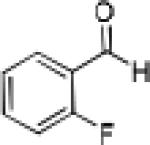
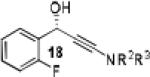
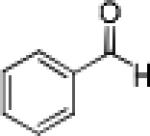
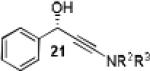
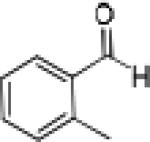
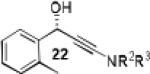
Having optimized the catalytic reaction between bromobenzaldehyde and 7 toward 3-(4-bromophenyl)-3-hydroxy-1-(3-benzoylindolyl)propyne, 13, we finally employed a variety of aldehydes in our method to determine the substrate scope. The reaction with aromatic substrates generally proceeded with high yields and ee’s, entries 1-12 in Table 2. Screening of several other halogenated benzaldehyde derivatives showed that the corresponding products 15-18 can be obtained with excellent results with the exception of 2-fluorobenzaldehyde which was obtained in high yield but somewhat lower ee, entries 2-5. This cannot be attributed to steric hindrance because the ynamide addition to 2-tolyl aldehyde produced 22 in 93% yield and 95% ee, entry 9. We were able to extend this method to aliphatic aldehydes. The reaction with cyclohexanecarboxaldehyde and decanal afforded 26 and 27 in 87-89% yield and 90-95% ee, respectively, entries 13 and 14. Finally, we were successful with growing single crystals of 13 and 20 prepared as described in Table 2 using (1R,2S)-(−)-N-methylephedrine as chiral ligand by slow evaporation of a chloroform solution. Crystallographic analysis of 13 revealed S-configuration, Figure 2 and ESI.
Figure 2.
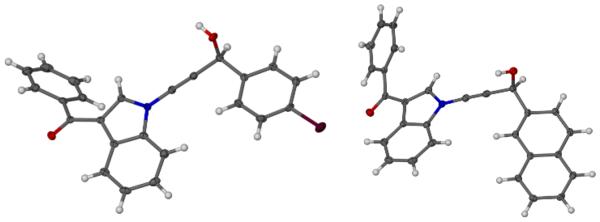
Crystal structures of 13 (left) and 20 (right). Selected crystallographic separations [Å] for 13: C(sp)-C(sp): 1.191, N-C(sp) 1.358. 20: C(sp)-C(sp): 1.191, N-C(sp) 1.359.
Standard conditions: ynamide (0.20 mmol), aldehyde (0.30 mmol), Zn(OTf)2 (10 mol%), (−)-NME (11 mol%) in 0.5 mL of toluene/hexane (1:1). [a] Isolated yields. [b] Toluene. The absolute configuration of 13 was determined by crystallographic analysis, see ESI. All other assignments are by analogy.
We then investigated if the presence of a chiral center in the substrate is tolerated. In fact, the Zn(II)-(−)-NME catalyzed reaction between (R)-citronellal and ynamide 7, gave 28 in 88% yield and 98% de within 3 hours, Scheme 2. The same aldehyde reacted more slowly and with lower diastereoselectivtiy when (+)-NME was used as chiral ligand under otherwise identical conditions. As a result, we isolated 29 in only 56% yield and 73% ee. The reduced yield is mostly a result of slow conversion and 25% of the unreacted ynamide were recovered after 3 hours. These results show that the sense of asymmetric induction is overwhelmingly controlled by the chiral catalyst while the chirality in the substrate may still have a distinctive effect on the reaction outcome. The Zn(II)-(−)-NME catalyzed reaction with the (R)-enantiomer of citronellal represents a matched pair whereas the reduced reaction rate and the lower asymmetric induction observed with Zn(II)-(+)-NME is in accordance with a mismatched pair.17
Scheme 2.
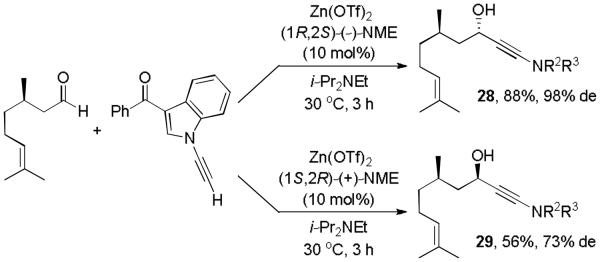
Diastereoselective ynamide addition to citronellal.
In summary, we have developed the first catalytic asymmetric addition of ynamides to aldehydes. The zinc catalyzed method is operationally simple, applicable to aliphatic and aromatic aldehydes, proceeds under mild conditions at room temperature, and provides practical access to a variety of N-substituted propargylic alcohols that are obtained in high yields and ee’s. The use of apolar solvent mixtures proved essential to avoid product racemization and to achieve high ee’s without compromising conversion. The unique reactivity and diversity of the polar ynamide functionality bear remarkable potential for asymmetric synthesis. The introduction of terminal ynamides to catalytic enantioselective additions described herein is expected to provide unprecedented entries to complex chiral building blocks.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from the National Institutes of Health (GM106260).
Footnotes
Electronic Supplementary Information (ESI) available: Synthetic procedures, and spectroscopic and cystallographic details. See http://dx.doi.org/10.1039/b000000x/
Notes and references
- 1.(a) Zificsak CA, Mulder JA, Hsung RP, Rameshkumar C, Wei L-L. Tetrahedron. 2001;57:7575. For excellent reviews, see: [Google Scholar]; (b) Evano G, Coste A, Jouvin K. Angew. Chem. Int. Ed. 2010;49:2840. doi: 10.1002/anie.200905817. [DOI] [PubMed] [Google Scholar]; (c) DeKorver KA, Li H, Lohse AG, Hayashi R, Lu Z, Zhang Y, Hsung RP. Chem. Rev. 2010;110:5064. doi: 10.1021/cr100003s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang X-N, Yeom H-S, Fang L-C, He S, Ma Z-X, Kedrowski BL, Hsung RP. Acc. Chem. Res. 2013 doi: 10.1021/ar400193g. DOI: 10.1021/ar400193g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witulski B, Goessmann M. Chem. Commun. 1999:1879. [Google Scholar]
- 3.Bruckner D. Tetrahedron. 2006;62:3809. [Google Scholar]
- 4.Rodriguez D, Castedo L, Saa C. Synlett. 2007:1963. [Google Scholar]
- 5.(a) IJsselstijn M, Cintrat J-C. Tetrahedron. 2006;62:3837. [3+2] Cycloadditions: [Google Scholar]; (b) Li H, You L, Zhang X, Johnson WL, Figueroa R, Hsung RP. Heterocycles. 2007;74:553. [Google Scholar]; (c) Li H, Hsung RP. Org. Lett. 2009;11:4462. doi: 10.1021/ol901860b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhang X, Li H, You L, Tang Y, Hsung RP. Adv. Synth. Catal. 2006;348:2437. [Google Scholar]; (e) Zhang X, Hsung RP, Li H. Chem. Commun. 2007:2420. doi: 10.1039/b701040k. [DOI] [PubMed] [Google Scholar]; (f) Kim JY, Kim SH, Chang S. Tetrahedron Lett. 2008;49:1745. [Google Scholar]; (g) Zhang X, Hsung RP, Li H, Zhang Y, Johnson WL, Figueroa R. Org. Lett. 2008;10:3477. doi: 10.1021/ol801257j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Martinez-Esperon MF, Rodriguez D, Castedo L, Saa C. Org. Lett. 2005;7:2213. doi: 10.1021/ol050609a. [4+2] Cycloadditions: [DOI] [PubMed] [Google Scholar]; (i) Witulski B, Goessmann M. Synlett. 2000:1793. [2+2+1] Cycloadditions: [Google Scholar]; (j) Witulski B, Stengel T. Angew. Chem. Int. Ed. 1999;38:2426. [2+2+2] Cycloadditions: [PubMed] [Google Scholar]; (k) Witulski B, Stengel T, Fernandez-Hernandez J. Chem. Commun. 2000:1965. [Google Scholar]; (l) Witulski B, Alayrac C. Angew. Chem. Int. Ed. 2002;41:3281. doi: 10.1002/1521-3773(20020902)41:17<3281::AID-ANIE3281>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]; (m) Dateer RB, Shaibu BS, Liu R-S. Angew. Chem. Int. Ed. 2012;51:113. doi: 10.1002/anie.201105921. [DOI] [PubMed] [Google Scholar]
- 6.(a) Marion F, Coulomb J, Courillon C, Fensterbank L, Malacria M. Org. Lett. 2004;6:1509. doi: 10.1021/ol049530g. [DOI] [PubMed] [Google Scholar]; (b) Marion F, Coulomb J, Servais A, Courillon C, Fensterbank L, Malacria M. Tetrahedron. 2006;62:3856. [Google Scholar]; (c) Hashmi AS, Rudolph M, Bats J, Frey W, Rominger F, Oeser T. Chem. Eur. J. 2008;14:6672. doi: 10.1002/chem.200800210. [DOI] [PubMed] [Google Scholar]; (d) Couty S, Meyer C, Cossy J. Tetrahedron. 2009;65:1809. [Google Scholar]
- 7.(a) Rodriguez D, Castedo L, Saa C. Synlett. 2004:377. Homocoupling reactions: [Google Scholar]; (b) Rodriguez D, Castedo L, Saa C. Synlett. 2004:783. Negishi cross-couplings: [Google Scholar]; (c) Martinez-Esperon MF, Rodriguez D, Castedo L, Saa C. Tetrahedron. 2006;62:3843. [Google Scholar]; (d) Tracey MR, Zhang Y, Frederick MO, Mulder JA, Hsung RP. Org. Lett. 2004;6:2209. doi: 10.1021/ol0493251. Sonogashira couplings: [DOI] [PubMed] [Google Scholar]; (e) Dooleweerdt K, Ruhland T, Skrydstrup T. Org. Lett. 2009;11:221. doi: 10.1021/ol802477d. [DOI] [PubMed] [Google Scholar]; (f) Couty S, Liegault B, Meyer C, Cossy J. Org. Lett. 2004;6:2511. doi: 10.1021/ol049302m. Heck reactions: [DOI] [PubMed] [Google Scholar]; (g) Couty S, Liegault B, Meyer C, Cossy J. Tetrahedron. 2006;62:3882. [Google Scholar]
- 8.Saito N, Sato Y, Mori M. Org. Lett. 2002;4:803. doi: 10.1021/ol017298y. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee B, Litvinov DN, Kang J, Bettale JD, Castle SL. Org. Lett. 2010;12:2650. doi: 10.1021/ol1008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Tanaka R, Hirano S, Urabe H, Sato F. Org. Lett. 2003;5:67. doi: 10.1021/ol027209x. [DOI] [PubMed] [Google Scholar]; (b) Tanaka R, Yuza A, Watai Y, Suzuki D, Takayama Y, Sato F, Urabe M. J. Am. Chem. Soc. 2005;127:7774. doi: 10.1021/ja050261e. [DOI] [PubMed] [Google Scholar]; (c) Tanaka D, Sato Y, Mori M. J. Am. Chem. Soc. 2007;129:7730. doi: 10.1021/ja071954t. [DOI] [PubMed] [Google Scholar]
- 11.(a) Witulski B, Buschmann N, Bergstraesser U. Tetrahedron. 2000;56:8473. Other examples of important reactions of terminal ynamides: Hydroboration: [Google Scholar]; (b) Bhunia S, Chang C-J, Liu R-S. Org. Lett. 2012;14:5522. doi: 10.1021/ol302621z. Oxoarylations and cyclizations: [DOI] [PubMed] [Google Scholar]; (c) Yang L-Q, Wang K-B, Li C-Y. Eur. J. Org. Chem. 2013:2775. [Google Scholar]; (d) Couty S, Meyer C, Cossy J. Synlett. 2007:2819. [Google Scholar]; (e) AlRashid ZF, Hsung RP. Org. Lett. 2008;10:661. doi: 10.1021/ol703083k. [DOI] [PubMed] [Google Scholar]; (f) Frederick MO, Mulder JA, Tracey MR, Hsung RP, Huang J, Kurtz KCM, Shen L, Douglas CJ. J. Am. Chem. Soc. 2003;125:2368. doi: 10.1021/ja021304j. Nucleophilic alkylations with lithiated ynamides: [DOI] [PubMed] [Google Scholar]
- 12.You L, Al-Rashid ZF, Figueroa R, Ghosh SK, Ti G, Lu T, Hsung RP. Synlett. 2007:1656. [Google Scholar]
- 13.(a) Joshi RV, Xu Z-Q, Ksebati MB, Kessel D, Corbett TH, Drach JC, Zemlicka J. J. Chem. Soc. Perkin Trans. 1. 1994;10:1089. [Google Scholar]; (b) Rodriguez D, Castedo L, Saa C. Synlett. 2007:1963. [Google Scholar]; (c) Egi M, Yamaguchi Y, Fujiwara N, Akai S. Org. Lett. 2008;10:1867. doi: 10.1021/ol800596c. [DOI] [PubMed] [Google Scholar]; (d) Wang X-N, Winston-McPherson GN, Walton MC, Zhang Y, Hsung RP, DeKorver KA. J. Org. Chem. 2013;78:6233. doi: 10.1021/jo400960e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wang X-N, Hsung RP, Rui Q, Fox SK, Lv M-C. Org. Lett. 2013;15:2514. doi: 10.1021/ol400989x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Ryosuke M, Kobayashi S. Acc. Chem. Res. 2008;41:292. doi: 10.1021/ar700098d. We generally prefer using 4-bromobenzaldehyde over benzaldehyde during initial screening due to its superior shelf life. The reaction did not occur in the absence of a transition metal or under conditions typically used for nucleophilic additions with enamides or enecarbamates. For stereoselective C-C bond formations with enamides and enecarbamates see. [DOI] [PubMed] [Google Scholar]; (b) Anand NK, Carreira EM. J. Am. Chem. Soc. 2001;123:9687. doi: 10.1021/ja016378u. For examples of catalytic asymmetric addition reactions with terminal alkynes: [DOI] [PubMed] [Google Scholar]; (c) Li X, Lu G, Kwok WH, Chan ASC. J. Am. Chem. Soc. 2002;124:12636. doi: 10.1021/ja025541y. [DOI] [PubMed] [Google Scholar]; (d) Takita R, Yakura K, Ohshima T, Shibasaki M. J. Am. Chem. Soc. 2005;127:13760. doi: 10.1021/ja053946n. [DOI] [PubMed] [Google Scholar]; (e) Trost BM, Weiss AH, von Wangelin AJ. J. Am. Chem. Soc. 2006;128:8. doi: 10.1021/ja054871q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Gao G, Wang Q, Yu X-Q, Xie R-G, Pu L. Angew. Chem. Int. Ed. 2006;45:122. doi: 10.1002/anie.200500469. [DOI] [PubMed] [Google Scholar]; (g) Wolf C, Liu S. J. Am. Chem. Soc. 2006;128:10996. doi: 10.1021/ja062711o. [DOI] [PubMed] [Google Scholar]
- 15.Hamada T, Ye X, Stahl SS. J. Am. Chem. Soc. 2008;130:833. doi: 10.1021/ja077406x. [DOI] [PubMed] [Google Scholar]
- 16. The decrease of the ee of 13 was found to coincide with the formation of the starting materials.
- 17.Wolf C, editor. Dynamic Stereochemistry of Chiral Compounds. RSC Publishing; Cambridge, UK: 2008. p. 194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


