Abstract
OBJECTIVE
Fecal microbiota transplantation (FMT) has been suggested as a new treatment to manage Clostridium difficile infection (CDI). With use of a mathematical model of C. difficile within an intensive care unit (ICU), we examined the potential impact of routine FMT.
DESIGN, SETTING, AND PATIENTS
A mathematical model of C. difficile transmission, supplemented with prospective cohort, surveillance, and billing data from hospitals in the southeastern United States.
METHODS
Cohort, surveillance, and billing data as well as data from the literature were used to construct a compartmental model of CDI within an ICU. Patients were defined as being in 1 of 6 potential health states: uncolonized and at low risk; uncolonized and at high risk; colonized and at low risk; colonized and at high risk; having CDI; or treated with FMT.
RESULTS
The use of FMT to treat patients after CDI was associated with a statistically significant reduction in recurrence but not with a reduction in incident cases. Treatment after administration of high-risk medications, such as antibiotics, did not result in a decrease in recurrence but did result in a statistically significant difference in incident cases across treatment groups, although whether this difference was clinically relevant was questionable.
CONCLUSIONS
Our study is a novel mathematical model that examines the effect of FMT on the prevention of recurrent and incident CDI. The routine use of FMT represents a promising approach to reduce complex recurrent cases, but a reduction in CDI incidence will require the use of other methods to prevent transmission.
Clostridium difficile is a frequent source of healthcare-associated infections (HAIs), especially among patients who receive treatment regimens that involve antibiotics1 or proton pump inhibitors (PPIs)2,3 or who have other conditions that disrupt normal gut microbiota. The rate of C. difficile infection (CDI) in the United States has been increasing since 2000, and CDI caused an estimated 336,565 cases in 2009.4 In some healthcare facilities, CDI has eclipsed methicillin-resistant Staphylococcus aureus as the leading source of HAI.5 Of special concern is the development of recurrent CDI, which may be a complicated, long-term condition typified by repeated bouts of severe diarrhea.
Because altering the indigenous microbiota of the intestinal tract causes CDI, there has been an interest in recolonizing the intestinal tract with introduced donor bacteria obtained from either healthy donor stool6,7 or a synthetically derived pure culture.8 This procedure, referred to as fecal microbiota transplantation (FMT), restores the bacterial ecology that keeps C. difficile in check. Both uncontrolled case reports7,8 and a small clinical trial6 have shown encouraging results; however, FMT is still largely reserved for specialized intervention in difficult or refractory cases. Furthermore, the implications of routine intestinal recolonization as a standard course of treatment for the prevention of recurrent or incident CDI have not been widely explored. The need for an increased understanding of the potential effects and utility of FMT is especially urgent in light of the US Food and Drug Administration’s increased interest in the procedure and their decision that it falls under the agency’s regulatory purview.9
Mathematical models are ideal for studying such hypothetical scenarios. They can provide a repeatable, quantitative environment with which to evaluate evidence, guide policy creation, discover critical thresholds upon which the success of interventions may depend, and suggest new directions for observational studies and clinical trials. These strengths are difficult or impossible to duplicate with empirical research within a hospital. Critically, one patient’s outcome influences another’s exposure, which violates traditional statistical assumptions of independence. Finally, mathematical models are capable of scaling up the independent, individual-level observations that emerge from clinical research to the population level. In this way, we may study how these individuals interact with one another and influence the transmission process without a risk to patient safety. To evaluate the impact of routine intestinal microbiota recolonization in patients with CDI, we developed a mathematical model that describes the transmission of C. difficile within an intensive care unit (ICU) and has the capability to test the impact of FMT on prevention of recurrent or initial C. difficile infection due to in-hospital transmission.
METHODS
Data
Hospital data were obtained from 3 separate sources, each consisting of patient records between July 1, 2009, and December 31, 2010. The first data set was a cohort of 609 adult patients with incident CDI extracted from prospectively collected HAI surveillance data from 28 community hospitals in the Duke Infection Control Outreach Network (DICON).10 This data set included admission, discharge, and diagnosis times; outcomes that included death and discharge; and patient demographic characteristics. The second data set included weekly surveillance time series from 31 DICON-affiliated hospitals within the DICON network, consisting of the overall number of hospital-onset, healthcare facility–associated CDI cases classified by infection preventionists at DICON member hospitals using Centers for Disease Control and Prevention surveillance criteria,11 whole hospital patient-day denominator data, ICU patient-days, and whether the hospital was using a nonmolecular diagnostic test or a diagnostic test based on polymerase chain reaction (PCR). In total, these series consist of 1,805 cases and 344,471 ICU patient-days. Finally, a third data set included hospital billing records for 452 inpatients discharged from the ICU within the University of North Carolina (UNC) Healthcare System, consisting of discharge times; orders for drugs that place patients at risk for CDI, such as PPIs or fluoroquinolones; coded diagnoses present at hospital admission; and demographic characteristics.
Transmission Model
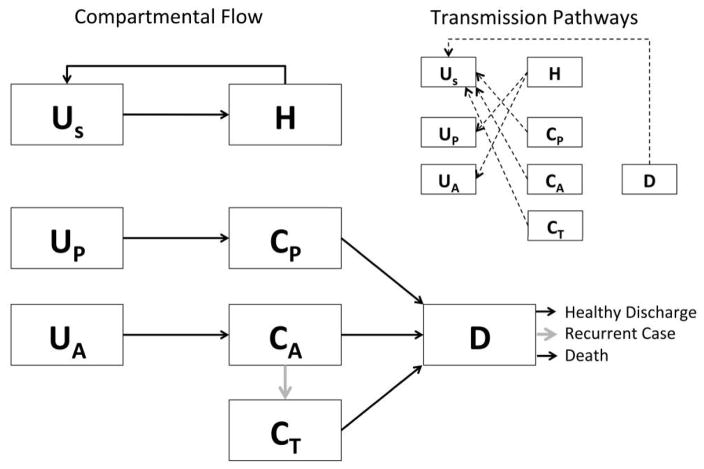
The transmission of C. difficile through an ICU was modeled as a series of compartments representing patient health and treatment states (Figure 1). Healthcare personnel (HCP) were modeled as being either uncontaminated (US) or contaminated (H), representing hands or gloves contaminated by vegetative C. difficile or spores. Patients could be in 1 of 6 compartments. Compartment UP represented uncolonized patients who were not receiving high-risk medication for CDI, and UA represented uncolonized patients who were receiving high-risk medications. Similarly, CP and CA represented low-risk and high-risk patients who had previously been exposed to the organism. Compartment D represented patients who had developed CDI. Finally, some of the scenarios we considered had an additional compartment, CT, which represented patients under prophylactic treatment using FMT administered via colonoscopy to prevent an initial infection.
FIGURE 1.
Schematic representation of the compartmental flow of a mathematical model of the use of fecal microbiota transplantation (FMT) to prevent incident and recurrent Clostridium difficile infection (CDI). Inset indicates the potential routes of bacterial contamination between patients and healthcare workers, whereas gray arrows indicate the movements within the model influenced by the simulated intervention. Healthcare personnel are classified as uncontaminated (US) or contaminated (H), and patients are classified into low risk and uncolonized (Up), low risk and colonized (CP), high risk and uncolonized (UA), high risk and colonized (CA), patients with CDI (D), and patients undergoing FMT (CT).
The interactions and transitions between these compartments were governed by a series of 8 differential equations (detailed in the Appendix). Patients were admitted into UP, UA, CP, CA, or D. Colonized patients (CP and CA) and patients with CDI (D) shed infectious material that may contaminate hands of HCP, and uncontaminated patients (UP and UA) are subsequently colonized when cared for by HCP with contaminated hands. HCP could decontaminate their hands by washing them after contact with either the patient or the environment immediately surrounding them.
Because there is evidence of a surface contamination component to C. difficile transmission,12 contacts between patients and HCP were modeled as direct care tasks, which could involve contact with the patient environment as well as physical interaction between the patient and HCP. Once colonized, patients could progress to CDI (CX to D). All patients were eventually discharged from the hospital. Three possible outcomes were tracked for patients with CDI: death, discharge from the hospital in good health, and discharge from the hospital with subsequent development of recurrent CDI.
The model made several simplifying assumptions. First, all HCP were assumed to interact with all patients within the ICU, and patients were assumed not to interact with each other. It is not known whether disruption of intestinal microbiota places patients at greater risk for developing CDI once colonized or at greater risk for colonization and thus for subsequent infection.13,14 Therefore, colonization once exposed to C. difficile and development of infection after colonization is treated as a single process within the model. Additionally, we assumed that patients who were treated with antibiotics or PPIs were prescribed those medications immediately on arrival into the ICU. Additionally, we assumed that the medication-induced disruption of the normal gut microbiota was immediate and lasted beyond the discontinuation of treatment. This effectively meant that, once a patient was classified as at high risk, they remained so unless an active intervention was made to recolonize their intestinal tract.
Parameterization and Model Calibration
The transmission model was parameterized using a combination of estimates from the literature and the data sets discussed above. Specific values and the sources that they are derived from are described in Table 1. Rates of death and discharge for patients with CDI were estimated from the DICON cohort data, whereas rates of discharge, use of antibiotics and PPIs, and the prevalence of C. difficile infection present at admission were all estimated from the UNC Healthcare in-patient billing data.
TABLE 1.
Parameters for a Mathematical Model of the Use of Fecal Transplantation to Prevent Clostridium difficile Infection (CDI) and Recurrence
| Symbol | Description | Value | Source |
|---|---|---|---|
| ι | Handwashing rate | 9.365 hand washes or glove changes per hour | 20–22 |
| ρ | Contact rate between patients and HCP | 4.244 direct care tasks per patient per hour | 23 |
| σi | Probability that a healthcare provider’s hands are contaminated by contact with a patient of type i | Low risk: 0.35; high risk: 0.35; active infections: 0.50 | 24–27 |
| Ψ | Probability of transmission from contaminated HCP hands to uncontaminated patient’s skin | 0.90a | 24 |
| θi | Discharge rate for an uninfected patient of type i | High risk: 1/12.006 days; low risk: 1/3.318 days | UNC Healthcare billing data |
| ζ | Hourly probability of death for a patient with active CDI | 0.000625 | DICON cohort data |
| γ | Hourly probability of discharge for a patient with active CDI | 0.00188 | DICON cohort data |
| νi | Proportion of admitted patients who are of patient type i | CP: 0.00447; CA: 0.0155; UP: 0.209; UA: 0.727; D: 0.044 | UNC Healthcare billing data1,28 |
| κ | Hazard of developing CDI in low-risk, contaminated patients | 0.000208 | DICON surveillance data |
| τ | Relative risk of developing CDI due to high-risk medication | 3.37 | 2,14,29 |
| Φ | Hourly probability of receiving postmedication FMT to prevent incident infection or recurrence | Antibiotics only: 0.0011; antibiotics and PPIs: 0.00169 | UNC Healthcare billing data30–33 |
| χ | Percentage of eligible patients receiving fecal transplant | 0–100 (varies by scenario) | |
| ω | Probability of a discharged patient developing recurrence | 0.30 | 3 |
| η | Probability of fecal transplant in moving patient to low-risk category | 0.938 | 6 |
NOTE. CA, high-risk and colonized patients; CP, low-risk and colonized patients; D, patients with CDI; DICON, Duke Infection Control Outreach Network; HCP, healthcare personnel; PPI, proton pump inhibitor; UA, high-risk and uncolonized patients; Up, low-risk and uncolonized patients; UNC, University of North Carolina.
Assumed to be highly efficient on the basis of general agreement between skin sampling and hand culture methods, indicating a minimal loss of contamination between touching a patient’s skin and deposition on another surface.
The underlying hazard of developing CDI for low-risk patients was estimated by fitting a deterministic version of the mathematical model above to the DICON surveillance time series. On the basis of previous research indicating a 56% increase in the number of reported cases within those hospitals that switched from nonmolecular to PCR diagnostic tests,15 case numbers were inflated by 1.56 for weeks during which nonmolecular tests were in use. This adjusted time series was then transformed into a weighted average of the cumulative number of CDI cases in the 31 DICON hospitals to estimate a typical level of infection, which the model was fit to using least squares to obtain the hazard of developing CDI in colonized low-risk patients.
Simulations
The mathematical model described above was applied to a single 12-bed ICU consisting of single patient rooms with 4 registered nurses and a single intensivist, based on average size and staffing information and best-practice guidelines for ICUs.16–18 Admissions were fixed to be equal to discharges to maintain a steady patient population. Several different potential treatment regimens were considered (Table 2). First, we created a baseline scenario, modeling no routine use of fecal transplantation. Second, we modeled a series of scenarios depicting the systematic use of FMT after CDI to prevent recurrent cases, treating 20%, 40%, 60%, 80%, and 100% of cases. Third, we modeled a series of scenarios examining the use of FMT prophylactically to prevent incident infections, treating contaminated high-risk (CA) patients immediately after the conclusion of their treatment regimen, moving them to a new, low-risk category (CT). These scenarios considered treating 20%, 40%, 60%, 80%, and 100% of all high-risk patients or just those patients who received fluoroquinolone antibiotics. Finally, a combination strategy was examined, treating patients with FMT both after CDI and after receipt of high-risk medication.
TABLE 2.
Patient Outcomes from a Mathematical Model of the Use of Fecal Transplantation to Prevent Clostridium difficile Infection and Recurrence in a Simulated 12-Bed Intensive Care Unit over a 1-Year Period
| Scenario, treatment level, % | Median recurrence (25th percentile, 75th percentile) | Pa | Median incidence (25th percentile, 75th percentile) | Pa |
|---|---|---|---|---|
| Baseline, 0 | 2 (0, 6) | 0 (0, 1) | ||
| After infection | ||||
| 20 | 1 (0, 4) | <.001 | 0 (0, 1) | .39 |
| 40 | 1 (0, 4) | 0 (0, 1) | ||
| 60 | 1 (0, 3) | 0 (0, 1) | ||
| 80 | 0 (0, 1) | 0 (0, 2) | ||
| 100 | 0 (0, 1) | 0 (0, 1) | ||
| After receipt of high-risk medication (antibiotics) | ||||
| Antibiotics | ||||
| 20 | 1 (0, 5) | .36 | 0 (0, 1) | .003 |
| 40 | 1 (0, 6) | 0 (0, 1) | ||
| 60 | 1 (0, 6) | 0 (0, 1) | ||
| 80 | 2 (0, 6) | 0 (0, 1) | ||
| 100 | 1 (0, 6) | 0 (0, 1) | ||
| Antibiotics and PPIs | ||||
| 20 | 2 (0, 5.25) | .56 | 0 (0, 1) | .001 |
| 40 | 2 (0, 6) | 0 (0, 1) | ||
| 60 | 1 (0, 5) | 0 (0, 1) | ||
| 80 | 2 (0, 5) | 0 (0, 1) | ||
| 100 | 2 (0, 6) | 0 (0, 1) | ||
| Combined | ||||
| 20 | 1 (0, 5) | <.001 | 0 (0, 1) | .02 |
| 40 | 1 (0, 4) | 0 (0, 1) | ||
| 60 | 1 (0, 3) | 0 (0, 1) | ||
| 80 | 0 (0, 2) | 0 (0, 1) | ||
| 100 | 0 (0, 1) | 0 (0, 1) | ||
NOTE. PPI, proton pump inhibitor.
Kruskal-Wallis 1-way analysis of variance test.
Deterministic models do not fully capture the transmission dynamics of small populations, such as the experience of a single, 12-bed ICU. Therefore, each of the treatment scenarios described above were modeled using 1,000 stochastic simulations of the equation system by means of the Gillespie direct method.19 The effect of this is twofold. First, individuals within the models are treated as discrete units (ie, no fractions of patients exist in compartments). Second, because individuals are treated as discrete units and the model becomes probabilistic, variations attributable to random chance may arise. These random fluctuations play an important role in disease dynamics when modeling a small population. The simulations were run over a 1-year time span.
Two primary outcomes were tracked in all scenarios: the number of incident infections, and the number of infections that developed into recurrent cases. Note that, in many simulations, we expected the number of recurrent cases to be higher than the number of incident infections. The model handled both incident infections that arise in the ICU and prevalent infections at admission, both of which could develop into recurrence. The results of stochastic models are frequently non-normally distributed, and differences between treatment groups were analyzed with nonparametric Kruskal-Wallis tests. Simulations were written in Python 2.7, and all statistical analysis was performed in R, version 2.15.
RESULTS
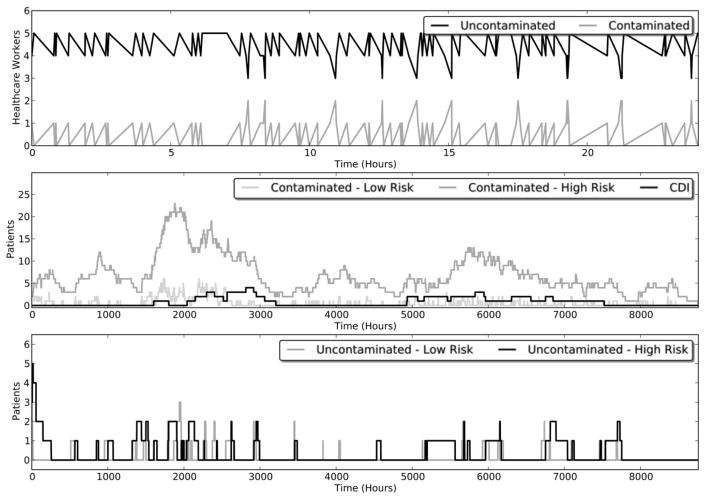
The median and 25th and 75th percentile numbers of recurrent and incident cases for all modeled scenarios are reported in Table 2. The baseline, no-intervention model produced results similar to the known epidemiology of CDI. Infection rates were low, but periodic outbreaks of C. difficile occurred, as did periods of no infection. Despite high levels of patient colonization and sustained transmission of C. difficile within the ICU, hand contamination of HCP was rare and short lived. An example showing the development of a typical simulation over time is shown in Figure 2.
FIGURE 2.
A single stochastic realization of a mathematical model of the use of fecal microbiota transplantation to prevent incident and recurrent Clostridium difficile infection (CDI). The top panel shows the level of hand contamination in healthcare workers over a 24-hour period, whereas the bottom 2 panels depict the number of patients and their current health state over a 1-year period.
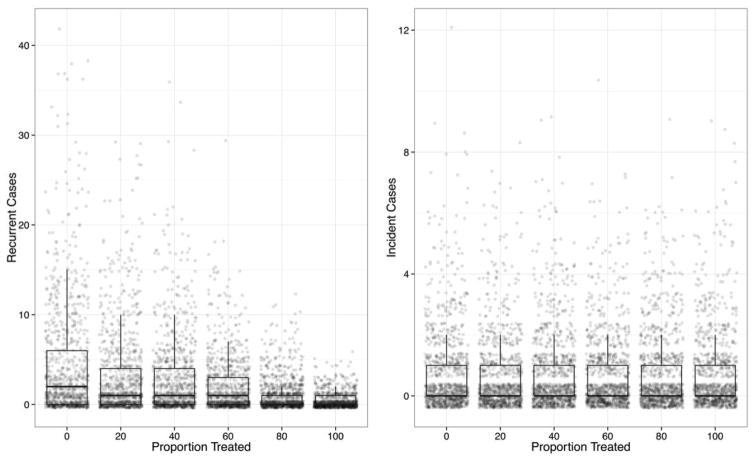
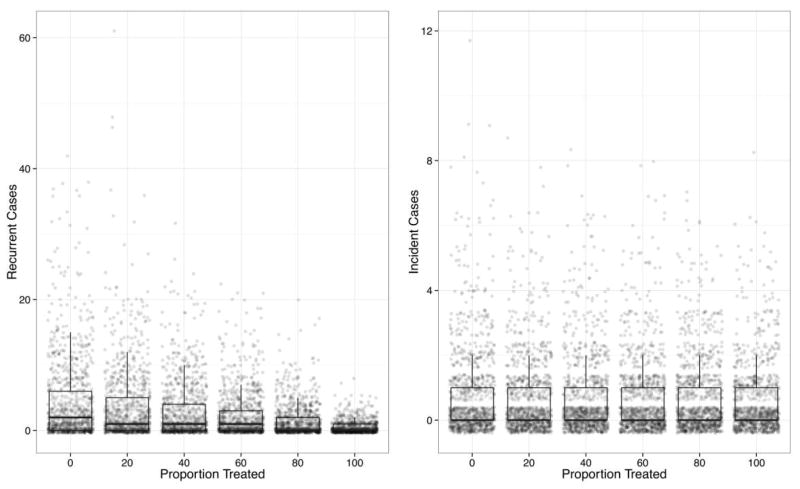
The impact of FMT in preventing recurrence among different proportions of patients after CDI is shown in Figure 3. The treatment resulted in a statistically significant (P < .001) difference in the number of recurrences among the different treatment groups. The median number of recurring cases ranged from 2 (interquartile range [IQR], 0–6) for no treatment to a median of 0 (IQR, 0–1) when 100% of patients were treated. Treatment did not result in a significant difference in the number of incident infections, regardless of what proportion of patients were treated (P = .39). The median number of incident cases was 0 for all scenarios (IQR, 0–1).
FIGURE 3.
Simulated recurrent and incident cases of Clostridium difficile infection (CDI) for 6 levels of fecal microbiota transplantation after CDI to prevent the development of recurrence. All simulation outcomes are shown, with the results summarized with box-and-whisker plots depicting the median, 25th and 75th percentiles, and 1.5 times the interquartile range.
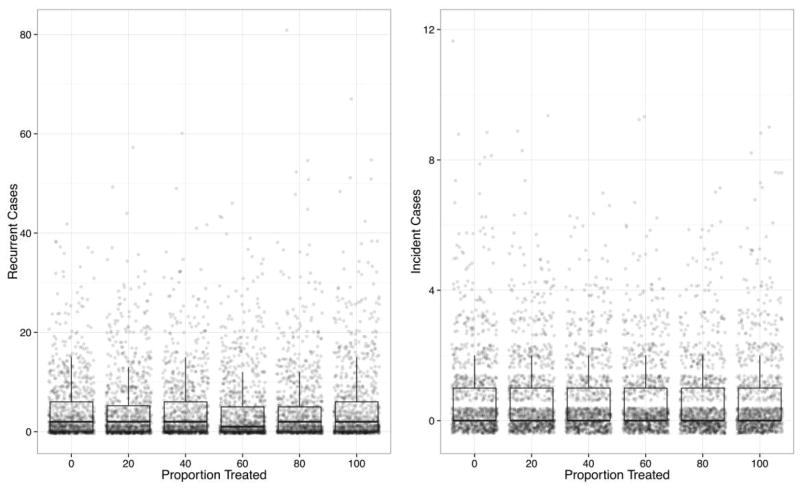
The results of scenarios in which patients were treated prophylactically after discontinuation of antibiotic therapy or PPIs had very similar results to one another. Figure 4 shows the results of the latter scenario for different proportions of treatment. Neither approach resulted in a statistically significant difference in recurrence, regardless of the proportion treated (P = .36 and .56, respectively); all scenarios had a median of 2 recurrent cases except for the scenario with 60% treatment, which had a median of 1 recurrent case.
FIGURE 4.
Simulated recurrent and incident cases of Clostridium difficile infection (CDI) for 6 levels of fecal microbiota transplantation after receipt of high-risk medication to prevent the development of infection and recurrence among patients who received antibiotics or proton pump inhibitors. All simulation outcomes are shown, with the results summarized with box-and-whisker plots depicting the median, 25th and 75th percentiles, and 1.5 times the interquartile range.
The difference in incident infections was statistically significant both when the treatment group was limited to patients who received antibiotics over all levels of treatment (P = .004) and to patients who received antibiotics, PPIs, or both (P = .001). In both treatment scenarios, however, this difference did not result in tangibly different model outcomes from a clinical perspective. In scenarios in which only patients who received antibiotics were treated, all treatment levels had a median of 0 incident cases (IQR, 0–1). Simulations with 0% treatment did have a higher maximum number of incident cases (n = 12) than did models in which 20%–100% of cases were treated (n = 9, 7, 9, 7, and 9, respectively). Similar patterns were seen for simulations in which patients who received both antibiotics and PPIs were treated (data not shown).
Combining both prophylactic treatment and postinfection treatment protocols resulted in a statistically significant difference in recurrent cases over the proportion of patients treated (P < .001). The median number of recurring cases ranged from 2 (IQR, 0–6) for no treatment to a median of 0 (IQR, 0–1) when all patients were treated. This strategy also resulted in a statistically significant difference in incident infections (P = .02), although, as with the purely prophylactic scenarios, this difference did not manifest in a change in median incidence, because all treatment levels had a median number of cases of 0 (IQR, 0–1). However, the no-treatment scenario had a higher maximum number of incident cases (n = 12) compared with treatment levels of 20%–100% (n = 9, 8, 8, 7, and 8, respectively). These results are shown in Figure 5.
FIGURE 5.
Simulated recurrent and incident cases of Clostridium difficile infection (CDI) for 6 levels of combined fecal microbiota transplantation (FMT) after receipt of high-risk medication to prevent the development of infection and recurrence among patients who received antibiotics or proton pump inhibitors and FMT after CDI to prevent the development of recurrence. All simulation outcomes are shown, with the results summarized with box-and-whisker plots depicting the median, 25th and 75th percentiles, and 1.5 times the interquartile range.
DISCUSSION
Our unique study using mathematical modeling found that the widespread use of FMT resulted in a marked reduction in the number of patients discharged from the hospital who would go on to develop recurrent cases of CDI. Importantly, this reduction was seen in all modeled scenarios ranging from relatively low levels of treatment (20% of patients) to very high levels of treatment (100% of patients) with no apparent threshold effect. This widespread evidence of a positive effect suggests that these results should be robust not only to varying levels of treatment but also to lower levels of efficacy, because the two are mathematically equivalent. Future research should evaluate the costs associated with these procedures, to assess whether the decrease in recurrent cases of CDI offsets the economic costs of treatment as well as the risk to the patient. However, given the paucity of empirical studies to establish rates of complication and the lack of an established standard route of FMT administration, no cost-benefit component was included in this preliminary study.
Unsurprisingly, because treatment after CDI to prevent recurrent cases is a process that occurs entirely after infection, the modeled intervention had very little impact on incident hospital-acquired infections. Some secondary effects may be seen if fecal transplantation becomes a regularly used treatment and results in a reduced number of recurrent cases and fewer admissions with prevalent recurring C. difficile infection. Capturing this phenomenon would require modeling not only a single ward but an entire local healthcare system, which is beyond the scope of this study.
The evidence for a positive effect of using FMT after high-risk medications to prevent incident infection is less apparent. Although treating patients who are receiving antibiotics or both antibiotics and PPIs resulted in statistically significant or nearly significant results at an α = 0.05 level, these results appear to have little tangible clinical impact on the number of incident infections and no impact on the occurrence of recurrent infections. There is also very little evidence for a synergistic effect between the 2 treatment strategies. Scenarios that explored the simultaneous use of FMT after CDI and high-risk medication had results that were very similar to those of FMT use after CDI alone. Taken as a whole, these results indicate that routine use of fecal transplantation represents a promising tool to prevent complicated, recurring episodes of CDI, but techniques to recolonize the intestinal tract alone will be insufficient to contain the spread of C. difficile within a hospital ward.
This study is not without limitations. The model is limited to a single ICU in isolation. Although this limited scope allows it to specifically address the complex problems presented in critical care infection control, it is incapable of detecting effects in the hospital as a whole brought about by altering transmission and recurrence within the ICU. Many of the states within the model, such as whether or not a patient has come into contact with C. difficile, are not regularly observed within hospitals, and thus some of the outcomes of the model cannot be directly verified. As with all mathematical models, the results of the study are dependent on the assumptions about the natural history of CDI and the values of the parameters used. The purpose of this study, however, is not to provide precise predictions of future levels of infection but to examine the overall impact of fecal transplantation as a routine treatment option when dealing with C. difficile. Within this scope, the model structure and parameter estimates represent the state-of-the-art knowledge of in-hospital C. difficile transmission. Similarly, the parameter values chosen are not meant to emulate a particular hospital but to provide a reasonable approximation of the experience of an ICU staffed by HCP following good infection control practices.
This study represents, to the best of our knowledge, the first use of a mathematical model to quantify the potential effects of fecal transplantation for the treatment and prevention of CDI. These types of models represent a useful method for evaluating the potential impact of new treatment approaches in areas of limited clinical and empirical evidence. Our results suggest that routine intestinal recolonization is a powerful tool for the prevention of recurrent infection. When combined with other infection control measures, such as improved surface disinfection and antibiotic stewardship, fecal transplantation has great potential to produce a substantial reduction in the burden of C. difficile, especially in reducing highly morbid and difficult-to-manage recurrent infections.
Acknowledgments
We thank J. Smith for her comments and advice.
Financial support. This project was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme. The opinions expressed in this article are those of the authors and do not necessarily represent those of Merck Sharp & Dohme. This project was also supported by a grant from the North Carolina Translational and Clinical Sciences Institute (UL1RR025747).
APPENDIX
The system differential equations that describe the dynamics of Clostridium difficile transmission are detailed below. The definitions and values of the parameters are outlined in Table 1.
Footnotes
Potential conflicts of interest. E.T.L. has received research grant support from Merck. D.J.A. participates on the speaker’s bureau of and has received research grant support from Merck. All other authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
References
- 1.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 2.Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011–1019. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 3.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70(4):298–304. doi: 10.1016/j.jhin.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Agency for Healthcare Research and Quality. HCUPnet; 2006. [Accessed September 17, 2012]. http://hcupnet.ahrq.gov/ [Google Scholar]
- 5.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare–associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 6.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 7.You DM, Franzos MA, Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacterio-therapy. Ann Intern Med. 2008;148(8):632–633. doi: 10.7326/0003-4819-148-8-200804150-00024. [DOI] [PubMed] [Google Scholar]
- 8.Petrof EO, Gloor GB, Vanner SJ, Weese SJ. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: “RePOOPulating” the gut. Microbiome. 2013;1(3) doi: 10.1186/2049-2618-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. US Food and Drug Administration. Federal Register. 2013;78(138):42965–42966. [Google Scholar]
- 10.Anderson DJ, Miller BA, Chen LF, et al. The network approach for prevention of healthcare-associated infections: long-term effect of participation in the Duke Infection Control Outreach Network. Infect Control Hosp Epidemiol. 2011;32(4):315–322. doi: 10.1086/658940. [DOI] [PubMed] [Google Scholar]
- 11.McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile–associated disease. Infect Control Hosp Epidemiol. 2007;28(2):140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 12.Weber D, Rutala W, Miller M, Huslage K. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38(5 suppl 1):S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 13.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7(7):526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 14.Owens RC, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(suppl 1):S19–S31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 15.Moehring RW, Lofgren ET, Anderson DJ. Impact of change to molecular testing for Clostridium difficile infection on healthcare facility–associated incidence rates. Infect Control Hosp Epidemol. 2013;34(10):1055–1061. doi: 10.1086/673144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane RL, Shamliyan TA, Mueller C, Duval S, Wilt TJ. The association of registered nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med Care. 2007;45(12):1195–1204. doi: 10.1097/MLR.0b013e3181468ca3. [DOI] [PubMed] [Google Scholar]
- 17.Ward NS, Read R, Afessa B, Kahn JM. Perceived effects of attending physician workload in academic medical intensive care units: a national survey of training program directors. Crit Care Med. 2012;40(2):400–405. doi: 10.1097/CCM.0b013e318232d997. [DOI] [PubMed] [Google Scholar]
- 18.Thompson DR, Hamilton DK, Cadenhead CD, et al. Guidelines for intensive care unit design. Crit Care Med. 2012;40(5):1586–1600. doi: 10.1097/CCM.0b013e3182413bb2. [DOI] [PubMed] [Google Scholar]
- 19.Gillespie DT. Exact stochastic simulation of coupled chemical reactions. J Phys Chem. 1977;81(25):2340–2361. [Google Scholar]
- 20.Chen LF, Carriker C, Staheli R, et al. Observing and improving hand hygiene compliance: implementation and refinement of an electronic-assisted direct-observer hand hygiene audit program. Infect Control Hosp Epidemiol. 2013;34(2):207–210. doi: 10.1086/669084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oughton MT, Loo VG, Dendukuri N, Fenn S, Libman MD. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect Control Hosp Epidemiol. 2009;30(10):939–944. doi: 10.1086/605322. [DOI] [PubMed] [Google Scholar]
- 22.Jabbar U, Leischner J, Kasper D, et al. Effectiveness of alcohol-based hand rubs for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol. 2010;31(6):565–570. doi: 10.1086/652772. [DOI] [PubMed] [Google Scholar]
- 23.Ballermann MA, Shaw NT, Mayes DC, Gibney RTN, Westbrook JI. Validation of the Work Observation Method By Activity Timing (WOMBAT) method of conducting time-motion observations in critical care settings: an observational study. BMC Med Inform Decis Mak. 2011;11:32. doi: 10.1186/1472-6947-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RLP, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45(8):992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero DM, Nerandzic MM, Jury LA, Jinno S, Chang S, Dons-key CJ. Acquisition of spores on gloved hands after contact with the skin of patients with Clostridium difficile infection and with environmental surfaces in their rooms. Am J Infect Control. 2012;40(6):556–558. doi: 10.1016/j.ajic.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Bobulsky GS, Al-Nassir WN, Riggs MM, Sethi AK, Donskey CJ. Clostridium difficile skin contamination in patients with C. difficile–associated disease. Clin Infect Dis. 2008;46(3):447–450. doi: 10.1086/525267. [DOI] [PubMed] [Google Scholar]
- 27.Sethi AK, Al-Nassir WN, Nerandzic MM, Bobulsky GS, Donskey CJ. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol. 2010;31(1):21–27. doi: 10.1086/649016. [DOI] [PubMed] [Google Scholar]
- 28.Lanzas C, Dubberke ER, Lu Z, Reske KA, Gröhn YT. Epidemiological model for Clostridium difficile transmission in health-care settings. Infect Control Hosp Epidemiol. 2011;32(6):553–561. doi: 10.1086/660013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pépin J, Saheb N, Coulombe MA. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 30.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29(4):745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 32.Giordano P, Weber K, Gesin G, Kubert J. Skin and skin structure infections: treatment with newer generation fluoroquinolones. Ther Clin Risk Manag. 2007;3(2):309–317. doi: 10.2147/tcrm.2007.3.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderhoff BT, Tahboub RM. Proton pump inhibitors: an update. Am Fam Physician. 2002;66(2):273–280. [PubMed] [Google Scholar]