Abstract
Rationale: Most virus-induced attacks of asthma are caused by rhinoviruses (RVs).
Objectives: To determine whether people with asthma are susceptible to an increased viral load during RV infection.
Methods: Seventy-four children (4–18 yr old) were enrolled; 28 with wheezing, 32 with acute rhinitis, and 14 without respiratory tract symptoms. Nasal washes were evaluated using quantitative polymerase chain reaction for RV to judge viral load along with gene sequencing to identify strains of RV. Soluble intercellular adhesion molecule-1, IFN-λ1, and eosinophil cationic protein in nasal washes, along with blood eosinophil counts and total and allergen-specific IgE in sera, were also evaluated. Similar assessments were done in 24 young adults (16 with asthma, 8 without) who participated in an experimental challenge with RV (serotype 16).
Measurements and Main Results: Fifty-seven percent of wheezing children and 56% with acute rhinitis had nasal washes testing positive for RV. The geometric mean of viral loads by quantitative polymerase chain reaction in washes from wheezing children was 2.8-fold lower, but did not differ significantly from children with rhinitis (7,718 and 21,612 copies of viral RNA per microliter nasal wash, respectively; P = 0.48). The odds for wheezing were increased if children who tested positive for RV were sensitized to one or more allergens (odds ratio, 3.9; P = 0.02). Similarly, neither peak nor cumulative viral loads differed significantly in washes from adults with asthma compared with those without asthma during the experimental RV challenge.
Conclusions: During acute symptoms, children infected with RV enrolled for wheezing or acute rhinitis had similar viral loads in their nasal washes, as did adults with and without asthma infected with RV-16 experimentally.
Keywords: asthma, intercellular adhesion molecule-1, quantitative polymerase chain reaction, rhinitis, rhinovirus
At a Glance Commentary
Scientific Knowledge on the Subject
Previous viral load studies, performed predominantly in vitro, suggest that the asthmatic airway may be more susceptible to rhinovirus infection, more permissive of increased viral replication, and defective in viral clearance.
What This Study Adds to the Field
In this investigation, viral loads evaluated in nasal washes in vivo were not significantly different among children and adults with asthma compared to nonasthmatic individuals with acute rhinitis during infections with rhinovirus.
Rhinoviruses (RVs) account for most virus-induced exacerbations of asthma among children and young adults (1–3). A wide diversity of RV genotypes cause recurrent infections that lead to attacks of wheezing that begin in infancy, and these early childhood episodes of wheeze with RV are associated with an increased risk for developing asthma as children grow older (4–7). After 3 years of age, RVs are associated with up to 70% of asthma exacerbations among children living in both temperate and tropical climates (2, 8, 9). The mechanisms underlying the role of RV in provoking these attacks remain poorly understood; however, it is clear that most children and adults who experience RV-induced exacerbations are atopic and have high titers of serum IgE antibody (Ab) to allergens, such as dust mite, which have been shown to significantly increase the risk of wheezing with RV (9–11).
Using in vitro methods, several studies have demonstrated that the production of innate, antiviral type I and type III IFNs in bronchial epithelial cells from patients with asthma is reduced compared with levels secreted by cells from the lower airway of patients without asthma following infection with RV (12, 13). This impaired antiviral response correlated inversely with increasing quantities of RV-RNA detected by quantitative polymerase chain reaction (qPCR) in culture supernatants (12, 13). In two other studies, the secretion of innate IFNs from plasmacytoid dendritic cells in peripheral blood was significantly decreased in cells from subjects with asthma compared with those without after stimulation with influenza in one study, or RV in the other (14, 15). Additionally, the production of these cytokines correlated inversely with serum IgE levels or FcεRIα expression on plasmacytoid dendritic cells, and IgE cross-linking on plasmacytoid dendritic cells before stimulation with influenza or RV further diminished this innate response. Taken together, these observations suggest that people with asthma may be at risk for higher viral loads and symptoms affecting their respiratory tract during RV infection.
In the present study, we used two different study designs to compare viral loads among individuals with and without asthma during an RV infection. These studies included the evaluation of (1) children treated for exacerbations of wheezing along with children with acute rhinitis; and (2) young adults, with and without asthma, who were inoculated experimentally with RV-16 and then monitored over time through peak symptoms and symptom resolution. In both studies, viral load was assessed by qPCR in nasal washes and the results analyzed in relation to total and allergen-specific serum IgE; blood eosinophil counts; and the levels of IFNλ1, eosinophil cationic protein (ECP), and soluble intercellular adhesion molecule-1 (sICAM-1) in nasal secretions. Some of the results have previously been included in published abstracts (16, 17).
Methods
Pediatric Study
Study participants included 74 children (4–18 yr old) who were evaluated at the University of Virginia Medical Center in a cross-sectional, case-controlled study. The children enrolled included 28 who required treatment with at least a nebulized β2 agonist for wheezing heard by a physician on auscultation; 32 with acute rhinitis (onset of symptoms ≤ 4 d), but with no cough or wheeze; and 14 without respiratory tract signs or symptoms (control subjects). None of the wheezing children had taken systemic steroids before their visit, one was hospitalized for further management, and the remainder were discharged home on 3–5 days of oral steroids. Most (93%) of the wheezing children, 41% of those with acute rhinitis, and 57% of control subjects were enrolled in the emergency room (ER). The others were enrolled in the general pediatric clinics. Most of the control subjects without respiratory symptoms were evaluated for trauma, gastrointestinal complaints, and fever.
Demographic information and patient characteristics were obtained from family members by an administered questionnaire focused on the child’s past medical history, immediate family history for allergic disease (i.e., parents and siblings), and tobacco smoke exposure at home. Children with chronic lung disease, congenital heart disease, immunodeficiency, or oncologic disorders were not enrolled. Informed consent was obtained from parents, and informed assent was obtained from children 7 years of age or older. This study was approved by the institutional review board at the University of Virginia.
Assessments for Total IgE, Allergen-Specific IgE Ab, and Blood Eosinophil Counts
Blood (5–7 ml) was obtained by venipuncture and sera were analyzed for total IgE and IgE Ab to dust mite (Dermatophagoides pteronyssinus and Dermatophagoides farinae), Alternaria, cockroach, ragweed, Timothy grass, Bermuda grass, oak, cat, and dog allergens using the Phadia ImmunoCap assay (Phadia/ThermoFisher, Hercules, CA). Sera with greater than or equal to 0.35 IU/ml of IgE Ab to any of the allergens tested were considered positive for sensitization.
sICAM-1, IFN-λ1, and ECP in Nasal Washes
Concentrations of sICAM-1 were measured in nasal washes by ELISA (R&D Systems, Minneapolis, MN) (detection limit = 0.35 ng/ml) as was IFN-λ1 (IL-29) (Affymetrix/eBioscience, San Diego, CA) (detection limit = 2.0 pg/ml). Nasal wash samples sufficient for measuring IFN-λ1 were not available from subjects enrolled in the RV-16 challenge study. Measurements of ECP in nasal wash supernatants were done using the Phadia ImmunoCap assay (detection limit = 2.0 ng/ml).
Virology
Viral RNA was extracted from nasal washes and reversed-transcribed as previously described (2). The resulting cDNA was used for viral identification and RV load assessments at the University of Wisconsin-Madison. Respiratory viruses were identified with a high throughput multiplex PCR assay (18). PCR and DNA sequencing was used to identify RV strains (19). A pan-RV qPCR assay was used to measure viral load (20). For subjects infected with RV-16, RNA was extracted from serial dilutions of an available pool of RV-39, which, like RV-16, is a RV-A serotype. The extractions were done using Qiamp RNA isolation kits (Qiagen, Crawley, UK) and cDNA was generated as previously described (21). The cDNA was amplified using primers and probes specific for conserved regions of RV and detected by qPCR (RV forward 5′-GGCCCTGAATGTGGCTAA-3′; RV reverse 5′-ATCCCCGCAATTGCTCGTTAC-3′; probe 5′-FAM/ CTTGCAGCCAATGCA-BHQ-3′) (Integrated DNA Technologies, Coralville, IA). The PCR mix consisted of iQ 2X supermix (BioRad, Uppsala, Sweden), cDNA, and 200 μM of each primer. Using 27 nasal wash samples previously genotyped for RV strains (9), these primers and probes detected all RV-A strains (n = 13) including RV-16, but did not detect RV-C strains (n = 14). Nasal wash samples from the experimental RV-16 challenge were evaluated in duplicate and the values compared with the standard curve to determine the copies of viral RNA per microliter of nasal wash supernatant. The neat concentration of the pool used in this analysis contained 5.0 × 107 copies of viral RNA per microliter nasal wash.
Experimental Infections with RV-16
Subjects (18–30 yr old) who participated in the experimental challenges with RV-16 included 16 with mild, physician-diagnosed asthma, all of whom were atopic and had positive methacholine challenges. Eight subjects without asthma, all with negative methacholine and negative prick skin tests, were included as control subjects. Additional subject characteristics, confirmation of institutional review board approval and informed consent, and details of symptom scores reported by the subjects were described previously (22). Each subject was inoculated with 300 TCID50 of RV-16, and nasal washes from each subject were collected before RV inoculation and on Days 1, 2, 3, 4, 7, 10, 14, and 21 during the infection. Information about the production and safety testing for the RV-16 inoculum used in this study is provided in the online supplement.
Statistical Analyses
Categorical data were summarized as counts and percentages, and continuous scaled data were summarized by the mean or the geometric mean of the measurement distribution and a 95% confidence interval.
Pediatric study.
Binary cross-sectional outcome variables were analyzed by exact logistic regression. Confidence interval construction for the odds ratio was based on the exact methods of the LOGISTIC procedure of SAS version 9.2 (SAS Institute Inc., Cary, NC). Continuous scaled cross-sectional outcome variables were analyzed by one-way and two-way analysis of variance. A Bonferroni type I error adjustment was used for multiple test comparisons pertaining to a single outcome variable. The Welch version of the two-sample Student t test was used as the pivotal quantity for between-group hypothesis tests.
Experimental RV challenge study.
Longitudinal continuous scaled outcome variables from the experimental challenge study were summarized by cumulative outcome values. The cumulative outcome values were analyzed by repeated measures analysis of variance. Between-group comparisons of the mean cumulative outcome values for the different stages of RV infection were formulated by linear contrasts of the least squares means.
Correlation analyses.
Relationships between continuous scaled outcome variables were assessed by the nonparametric Spearman rank correlation coefficient. For all analyses, a two-sided P less than or equal to 0.05 decision rule was used as the null hypothesis rejection criterion for all hypothesis-driven tests.
Results
Children Evaluated for Wheezing and Acute Rhinitis (Pediatric Study)
Patient characteristics are summarized in Table 1. More children seen for wheezing had a history of wheezing and other allergy-related symptoms than those with acute rhinitis or children without respiratory symptoms. Most children with wheezing and rhinitis were on Medicaid and had higher rates of exposure to environmental tobacco smoke at home. Those enrolled for wheezing had significantly higher geometric mean levels of total serum IgE and blood eosinophil counts than those with acute rhinitis or control subjects (Table 1). Additionally, 86% of the children with wheezing had serum IgE Ab to one or more of the allergens tested (odds ratio for wheezing compared with nonwheezing children, 8.4; P < 0.001). The atopic status of subjects enrolled is shown in more detail in Table E1 in the online supplement.
Table 1:
Patient Characteristics: Children Evaluated for Wheezing and Acute Rhinitis compared to Control Subjects
| Wheezing (n = 28) | Acute Rhinitis (n = 32) | Control Subjects (n = 14) | |
|---|---|---|---|
| Mean age, yr | 8.9† | 10.5 | 11.8 |
| Age range, yr | 4–18 | 4–18 | 5–18 |
| Male, % | 64* | 34 | 64 |
| Race/ethnicity, % W/AA/H/O | 21/64/4/10† | 41/50/6/3 | 64/36/0/0 |
| Medicaid,1 % | 61 (23) | 85 (26)‡ | 44 (9) |
| Previous history for wheezing, % | 93***,††† | 34‡ | 0 |
| Previous history for allergic symptoms,2 % | 46**,†† | 38‡‡ | 0 |
| Family history for allergy,3 % | 71 | 50 | 64 |
| ETS,4 % | 61 | 59 | 46 |
| ETS exposure from mothers, % | 50***,††† | 31 | 29 |
| Total serum IgE,5 IU/ml | 260 (144–469)***,††† | 57 (33–100) | 38 (14–98) |
| Serum IgE antibody to allergen,6 % positive | 86***,†††,* | 45 | 31 |
| Total eosinophil counts,5 cells/mm3 | 362 (215–608)† | 156 (96–253) | 120 (59–248) |
Definition of abbreviations: AA = African American; ETS = environmental tobacco smoke; H = Hispanic; O = other; W = white.
Symbols marking significant differences between groups are:
Wheezing versus acute rhinitis: *P < 0.05; **P < 0.01; ***P < 0.001.
Wheezing versus children without respiratory symptoms (control subjects): †P < 0.05; ††P < 0.01; †††P < 0.001.
Acute rhinitis versus control subjects: ‡P < 0.05; ‡‡P < 0.01.
Percentage of children whose families were covered by Medicaid. The number of children with data available is shown in parenthesis.
Percentage of children reporting symptoms of atopic dermatitis, or food allergy, conjunctivitis, or persistent or seasonal rhinitis.
Percentage of children from families with at least one parent or sibling with a current or previous history for asthma, seasonal rhinitis or conjunctivitis, eczema, or food allergy.
Percentage of children exposed to tobacco smoke from one or more individuals living at home.
Total IgE levels and blood eosinophil counts are reported as geometric means followed by 95% confident intervals in parentheses.
Percentage of children who had at least one positive test for serum IgE antibody to the allergens tested.
Virus Identification
The percentages of children enrolled with wheezing or acute rhinitis who tested positive for RV were 57.1 and 56.2%, respectively. By comparison, the prevalence of subjects with positive tests to other viruses was low (Table 2). Among those testing positive for RV, the number of days between the onset of cold symptoms and the day of enrollment reported by wheezing children and their parents compared with those with rhinitis was 3.0 and 2.5 days, respectively (P = 0.54). Most wheezing children who tested positive for RV had IgE Ab to one or more allergens (14 of 16 subjects, 87.5%), and the odds ratio for wheezing compared with those with acute rhinitis based on having a positive test for RV together with sensitization (IgE Ab) to one or more allergens was 3.9 (P < 0.02).
Table 2:
Viral Analyses: Children Evaluated for Wheezing and Acute Rhinitis compared to Control Subjects
| Wheezing (n = 28) | Acute Rhinitis (n = 32) | Control Subjects (n = 14) | |
|---|---|---|---|
| RT-PCR for RV, % positive | 57.1 (n = 16)* | 56.2 (n = 18)† | 7.1 |
| RV-A, % positive | 50 | 59 | 0 |
| RV-C, % positive | 50 | 35 | 100 |
| RV-B, % positive‡ | 0 | 6 [2] | |
| Other viruses, % positive§ | 21 [6] | 6 [2] |
Definition of abbreviations: RT-PCR = reverse transcriptase polymerase chain reaction; RV = rhinovirus.
Symbols marking significant differences between groups are:
Wheezing children versus control subjects: *P < 0.01.
Acute rhinitis children versus control subjects: †P < 0.01.
Only two children with acute rhinitis (shown in brackets) tested positive for RV-B strains, one of whom also tested positive for RV-A.
Six wheezing children (shown in brackets) tested positive for viruses other than RV: two for enterovirus (human enterovirus-68); one for adenovirus C; one for adenovirus E; one for adenovirus C and RV (Group A); and one for adenovirus C, metapneumovirus, and parainfluenzavirus 1. Two children with rhinitis (shown in brackets) tested positive for virus other than RV: one for coronavirus and the other for adenovirus B and E.
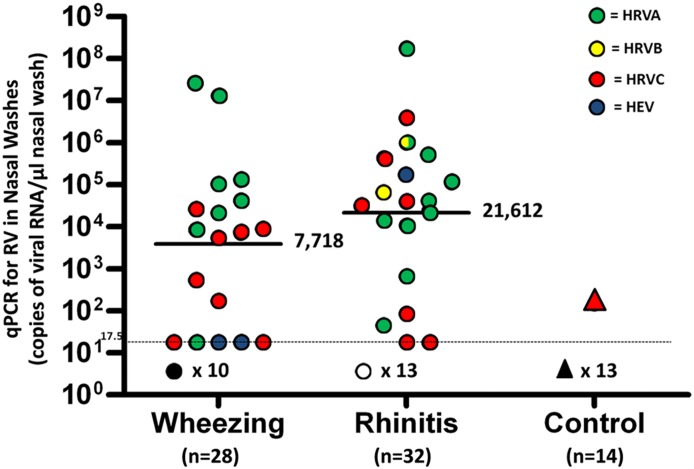
There was no significant difference in viral load between wheezing children and those with acute rhinitis, although the geometric mean value for qPCR was 2.8-fold lower in the washes from wheezing children (Figure 1). RV-A and RV-C strains were the most common types detected. Each species (RV-A and RV-C) accounted for 50% of the positive tests for RV among wheezing children, whereas RV-A strains were more common among the children with rhinitis, although this difference was not statistically significant (Table 2).
Figure 1.
Quantitative polymerase chain reaction (qPCR) for rhinovirus (RV) in nasal washes from children with wheezing and acute rhinitis. Geometric mean values and 95% confidence intervals for viral load expressed as copies of viral RNA per microliter of nasal wash were 7,718 (95% confidence interval, 870–68,485) in washes from wheezing children and 21,612 (95% confidence interval, 2,915–160,250) in washes from children with rhinitis. P = 0.48. HEV = human enterovirus; HRVA, HRVB, and HRVC = human rhinovirus types A, B, and C.
RV Analyses in Relation to sICAM-1, ECP, and IFN-λ1 in Nasal Washes
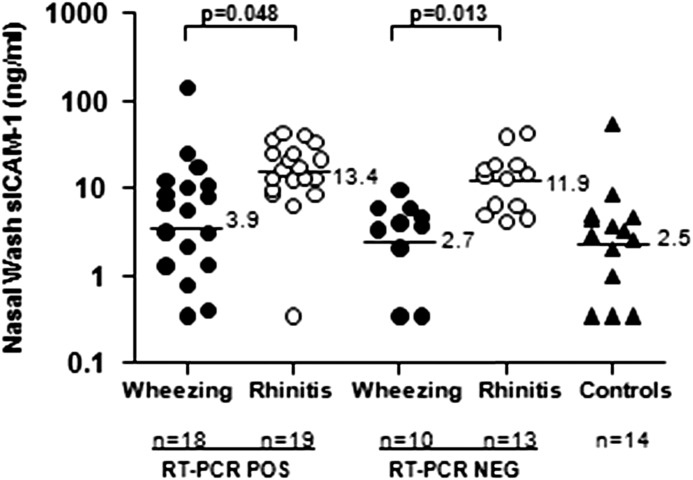
Lower levels of sICAM-1 were detected in washes from children treated for wheezing compared with washes from the children with rhinitis, among those who tested positive or negative for RV (Figure 2). Overall, qPCR values for RV correlated better with levels of sICAM-1 in washes from children with wheezing and rhinitis among those who tested positive for RV-A strains (n = 13; rs = 0.54; P = 0.05) than for those who tested positive for RV-C strains (rs = 0.35; P = 0.22). An inverse correlation was observed between sICAM-1 and total serum IgE among the wheezing children (rs = −0.41; P = 0.03) but not for those with rhinitis (rs = 0.06; P = 0.73).
Figure 2.
Nasal wash levels of soluble intercellular adhesion molecule-1 (sICAM-1) in children with wheezing and acute rhinitis. sICAM-1 values are shown as solid circles for wheezing children, open circles for children with rhinitis, and solid triangles for children without respiratory tract symptoms. Geometric mean values are noted to the right of the horizontal lines for each group. NEG = negative; POS = positive; RT-PCR = reverse transcriptase polymerase chain reaction.
The levels of IFN-λ1 tended to be lower in nasal washes from wheezing children, including those testing positive for RV, than from those with rhinitis, although the differences were not significant (see Table E2). Values for nasal ECP were higher in washes from children with wheezing and with rhinitis among those who tested positive for RV, but did not differ significantly between the wheezing and rhinitis groups (see Table E2). Correlations between qPCR for RV and ECP levels or IFN-λ1 in washes from the children enrolled with wheezing and acute rhinitis were not strong (rs = 0.27, P = 0.02, and rs = 0.32, P = 0.02, respectively).
Experimental RV Challenges
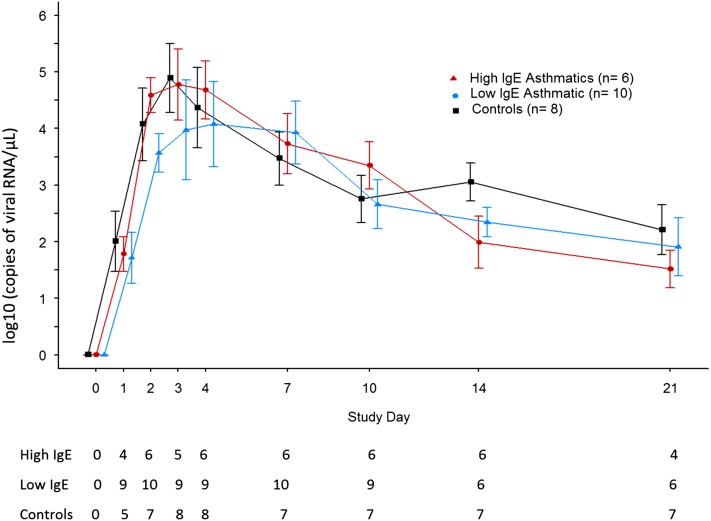
To evaluate how viral loads change over time, qPCR for RV was compared in washes from young adults with asthma (n = 16) and control subjects without asthma (n = 8) who were infected with RV-16 experimentally. The subjects with asthma included six with high levels of serum IgE (range, 371–820 IU/ml) and 10 with lower levels (range, 29–125 IU/ml). No significant differences in viral load were observed during the infection between these groups (Figure 3); however, based on cumulative viral load assessments, there was a trend for lower viral loads among the subjects with asthma who had high levels of total IgE throughout the infection (Table 3). Cumulative viral loads early in the infection (i.e., adding together Days 1 and 2 values after virus inoculation), and during peak cold symptoms (Days 3, 4, and 7 values combined), during symptom resolution (Days 10, 14, and 21 values combined), and cumulative viral loads during the entire 21 days of monitoring after virus inoculation did not differ statistically between groups (Table 3; see Figure E1). Similar to the children with wheezing and acute rhinitis, the levels of sICAM-1 correlated with qPCR values during peak symptoms: rs = 0.39, P = 0.06 on Day 3; rs = 0.48, P < 0.02 on Day 4; and rs = 0.44, P < 0.04 on Day 7.
Figure 3.
Quantitative polymerase chain reaction for rhinovirus in nasal washes from subjects with asthma and control subjects without asthma after inoculation with rhinovirus-16. The geometric mean of quantitative polymerase chain reaction values are indicated by symbols and standard error bars for each day that nasal washes were collected. The number of washes testing positive for virus at each time point for each group is shown beneath the figure.
Table 3:
Cumulative Viral Load Evaluated by qPCR during an Experimental Infection with RV-16*
| Subjects with Asthma/High IgE† (n = 6) | Subjects with Asthma/Low IgE‡ (n = 10) | Subjects without Asthma§ (n = 8) | |
|---|---|---|---|
| Early infection (Days 1 and 2) | 1,523 (602–7,319) | 15,569 (3,865–85,104) | 6,453 (744–55,097) |
| Peak symptoms (Days 3, 4, 7) | 48,582 (24.6–784.2 × 103) | 124,216 (38.3–1,064.1 × 103) | 110,791 (5.5–1,611.8 × 103) |
| Symptom resolution (Days 10, 14, 21) | 710 (355–1,236) | 2,208 (515–7,234) | 1,989 (874–4,652) |
| Total viral load overall | 65,657 (27.4–792.3 × 103) | 306,769 (184.2–1,151.7 × 103) | 164,615 (11.3–1,662.4 × 103) |
Definition of abbreviations: qPCR = quantitative polymerase chain reaction; RV = rhinovirus.
qPCR values in copies of viral RNA/μl are expressed as geometric means, followed by interquartile ranges in parentheses.
Total IgE values for the subjects with asthma with high IgE were 371–820 IU/ml.
Total IgE values for the subjects with asthma with low IgE were 29–125 IU/ml.
Total IgE values for the control subjects without asthma were 6–38 IU/ml.
Discussion
This investigation used two different study designs to compare viral loads in vivo among children and adults infected with RV to determine whether the asthmatic airway is susceptible to increased viral load, or difficulties clearing the infection. Although impairments in innate antiviral immunity together with increased viral replication have previously been observed in ex vivo experiments (12–15), we did not detect significant differences in viral loads in washes from the children with asthma who had naturally acquired infections with RV, or from the adults with asthma challenged experimentally with RV compared with infected individuals without asthma who were enrolled in these studies.
This is the first study to evaluate viral loads among children treated for exacerbations of wheezing compared with those with acute rhinitis. Based on qPCR assessments, the viral load in the upper airway, where RV replicates vigorously, was similar in nasal washes from the wheezing and rhinitis groups, although viral shedding among the wheezing children tended to be lower. The prevalence of positive tests for RV was essentially the same in both groups. Thus, the odds ratio for wheezing based only on a positive test for RV was not significant among these children. However, similar to our previous studies in Charlottesville and Costa Rica, the combination of a positive test for RV and sensitization (IgE Ab) to one or more aeroallergens increased the risk for wheezing significantly (2, 3, 9, 10).
Two previous studies, both done in adults, reported similar viral loads in individuals with and without asthma infected with RV. One study was a prospective evaluation of adults who were evaluated when they experienced acute cold symptoms (20). In that study, urgent care was not needed, so the investigators were able to compare viral loads in both nasal washes and induced sputum samples and no differences in viral loads between the subjects with asthma and subjects without asthma were observed. Similar to the children with natural colds and acute symptoms in our study, it is difficult to be certain when the infections among these individuals started. The other previous study was an experimental RV-16 challenge of adults (23). Like our study, similar viral loads were observed among allergic subjects with asthma and volunteers without asthma during the infection. Another experimental inoculation study also reported no significant asthma-related differences in viral loads evaluated in samples from the upper and lower airway, although the amount of virus detected was numerically greater in those with asthma compared with those without (24).
What differentiates our assessments from previous studies is that we examined viral loads in individuals infected with RV using two different study designs, which led to results that were in agreement. Additionally, two different methods were used to analyze data from the experimental RV challenges including a comparison of viral loads at each time point when nasal washes were collected, and a comparison of cumulative viral loads between the subjects with and without asthma, an approach that provides more power to detect differences between the two groups. The cumulative viral load assessments were evaluated during the early phase of the infection when innate immune responses begin, during peak symptoms, during symptom resolution when acquired antiviral immune responses contribute to viral killing and clearance, and during the entire 21 days after virus inoculation.
Almost all wheezing children in our pediatric study required treatment in the ER, but they did not vary significantly with respect to the severity of their symptoms at the time of enrollment. However, in the RV-16 challenge study, we previously reported that the subjects with asthma and high levels of total IgE had lower respiratory tract symptoms that were significantly greater than symptoms reported by the subjects with asthma and low IgE levels or by the control subjects without asthma, despite having viral loads that were, if anything, lower throughout the infection in the present evaluation. Additionally, the subjects with asthma with high and low IgE levels had respiratory tract symptoms (both upper and lower) that were increased compared with control subjects during the period of symptom resolution, even though the viral loads were not significantly different from the control subjects without asthma. Taken together, the results indicate that viral load is not likely to influence the persistence of symptoms in the subjects with asthma, which supports the hypothesis that the prolongation of symptoms may result from the amplification of allergic inflammation provoked by RV.
In keeping with previous studies, we observed IFN-λ1 levels that were lower, but the differences were not significant, in nasal washes from wheezing children than in washes from the children with acute rhinitis (13, 15, 25). In addition, two recent studies have not been able to confirm that IFN-λ1 expression is lower in epithelial cells from the asthmatic airway (26, 27). We also measured levels of sICAM-1 in washes from the children and adults in our studies because of its role as the cell-surface receptor used to infect airway epithelial cells by most (90%) RV-A and RV-B serotypes. In the pediatric study, significantly lower levels of sICAM-1 were observed in washes from wheezing children compared with washes from those with acute rhinitis, and we previously reported that the same was true for levels of sICAM-1 in washes from the adults with asthma with high IgE levels who were experimentally challenged with RV-16, a RV-A serotype that uses ICAM-1 as its receptor for infection (22). Decreased expression, before and after RV infection, of membrane-bound ICAM-1 on bronchial epithelial cells from subjects with asthma compared with cells from control subjects without asthma was also reported previously (12). In the nasal washes from children infected with RV-A strains known to bind ICAM-1 in the present study, and from adults infected experimentally with R-16 (an RV-A strain) during peak symptoms, viral loads correlated with sICAM-1 levels, whereas viral loads and sICAM-1 in washes from wheezing children infected with RV-C strains did not correlate significantly. Together, the data suggest that the receptor used by RV to infect epithelial cells may influence viral load for the RV-A viruses, but this observation may or may not apply to infections with RVs that do not use ICAM-1 as their receptor (e.g., RV-C strains, or RV-A strains that infect by the low-density lipoprotein receptor).
The capacity of RV infection to stimulate higher ECP levels in nasal washes from children without asthma who had acute rhinitis and positive tests for RV was observed, and was also noted previously in our experimental challenges with RV-16 (22). It is not yet clear whether the production of ECP is unique to infections with RV or whether other viral pathogens can also induce this response. The role of ECP in the pathogenesis of symptoms during an RV infection, and to what extent the production of ECP contributes to the amplification of allergic inflammation in the atopic host deserves further study.
To what extent the ability of RVs to infect the lower airway plays a role in the pathogenesis of wheezing is still unclear. Similar viral loads were observed in induced sputum samples from adults with and without asthma in the studies cited previously (20, 23). Additionally, two bronchoscopic evaluations of adults challenged with RV-16 experimentally were not able to distinguish differences between the extent of RV infection in the lower airway of hosts with and without asthma by immunohistochemistry or in situ hybridization (28, 29). As yet, studies have not addressed whether the extent of RV replication within epithelial cells is more diffuse as compared with the “patchy” distribution of infected cells previously reported in the upper respiratory tract of individuals without asthma and allergy (30). One would suspect, however, that viral loads in nasal washes would correlate with the quantity of intracellular RV replication, unless there is enhanced apoptosis to kill infected cells before virus is released in the asthmatic airway.
Our study of children with RV infections was not powered for more detailed comparisons of biomarkers analyzed in the asthmatic and the rhinitic groups, or for the comparison of viral loads between the subgroups of asthmatics or rhinitics infected with RV-A and RV-C strains (each of which accounted for half of the 16 positive tests for RV detected among wheezing children in the pediatric ER study). The pediatric ER study was also not large enough to adjust for the effects of sex, race, or ethnicity. Additional studies are needed to learn how viral loads associated with RV-A and RV-C strains compare. More research is also needed to evaluate the roles of membrane-bound ICAM-1 and sICAM-1 in regulating RV viral load, because the benefit of sICAM-1 in reducing viral load and cold symptoms during an RV-A (serotype 39) infection was previously demonstrated in subjects who received sICAM-1 intranasally at the time of experimental inoculation (31). Additionally, when the method of infecting cells used by RV-C strains is known, similar studies may provide new and important insights.
In conclusion, the results of this investigation suggest that the regulation of viral load during infections with RV is likely to be complex. In the pediatric study, the risk for wheezing with RV was significantly increased in the atopic host; however, the viral loads among the children with and without asthma were similar and the same was true among the adults who were infected with RV experimentally. Taken together, these studies suggest that the asthmatic response to RV is likely to result from inflammatory pathways that are amplified or independently provoked by RV, rather than from a higher viral load in the asthmatic airway.
Acknowledgments
Acknowledgment
The authors thank Lyn Melton and Hayley James for their help transcribing and preparing this manuscript, and Fue Vang and Kristine Grindle for their technical assistance.
Footnotes
Supported by National Institutes of Health grants U01-AI100799, R01-AI020565, R01-AI057438, and U19-AI070364; the Cove Point Foundation; MedImmune, Inc.; Merck; and the University of Virginia Children’s Hospital.
Drs. Kennedy and Shaker contributed equally to this investigation.
Author Contributions: All authors made substantial contributions to the concept and design of the article and the interpretation of the data. All authors revised the article critically for important intellectual content, and gave their final approval of the version to be published.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201310-1767OC on January 28, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TAE, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care: IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 3.Heymann PW, Carper HT, Murphy DD, Platts-Mills TAE, Patrie J, McLaughlin AP, Erwin EA, Shaker MS, Hellems M, Peerzada J, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwane MK, Prill MM, Lu X, Miller EK, Edwards KM, Hall CB, Griffin MR, Staat MA, Anderson LJ, Williams JV, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 5.Linder JE, Kraft DC, Mohamed Y, Lu Z, Heil L, Tollefson S, Saville BR, Wright PF, Williams JV, Miller EK. Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol. 2013;131:69–77, e1–e6. doi: 10.1016/j.jaci.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlinson WD, Waliuzzaman Z, Carter IW, Belessis YC, Gilbert KM, Morton JR. Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J Infect Dis. 2003;187:1314–1318. doi: 10.1086/368411. [DOI] [PubMed] [Google Scholar]
- 9.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, Murphy DD, Odio S, James HR, Patrie JT, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499–1505, e5. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duff AL, Pomeranz ES, Gelber LE, Price GW, Farris H, Hayden FG, Platts-Mills TA, Heymann PW. Risk factors for acute wheezing in infants and children: viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics. 1993;92:535–540. [PubMed] [Google Scholar]
- 11.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, Calabrese F, Caramori G, Ballarin A, Snijders D, et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–1314. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, Gangnon RE, Gill MA, Gern JE, Lemanske RF, Jr, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMeen V, Shaker M, Gern J, Carper H, Murphy D, Vrtis R, Platts-Mills T, Heymann PW. Viral load assessments of rhinovirus (RV) by quantitative RT-PCR in nasal washes from children treated in the emergency department (ED) for Asthma. J Allergy Clin Immunol. 2008;121:S146. [Google Scholar]
- 17.Kennedy JL, Steinke JW, Murphy D, Carper H, Stallings AP, Platts-Mills TAE, Borish L, Heymann PW. Asthmatics infected with rhinovirus demonstrate up-regulation of IL-15. J Allergy Clin Immunol. 2012;129:S200. [Google Scholar]
- 18.Lee WM, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Shult PA, Prudent JR, Gern JE. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF, Jr, Shult PA, Gern JE. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denlinger LC, Sorkness RL, Lee WM, Evans MD, Wolff MJ, Mathur SK, Crisafi GM, Gaworski KL, Pappas TE, Vrtis RF, et al. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am J Respir Crit Care Med. 2011;184:1007–1014. doi: 10.1164/rccm.201103-0585OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinke JW, Crouse CD, Bradley D, Hise K, Lynch K, Kountakis SE, Borish L. Characterization of interleukin-4-stimulated nasal polyp fibroblasts. Am J Respir Cell Mol Biol. 2004;30:212–219. doi: 10.1165/rcmb.2003-0071OC. [DOI] [PubMed] [Google Scholar]
- 22.Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TAE, Hayden FG, Gwaltney JM, Jr, Hatley TK, Owens AM, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–1016. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 23.DeMore JP, Weisshaar EH, Vrtis RF, Swenson CA, Evans MD, Morin A, Hazel E, Bork JA, Kakumanu S, Sorkness R, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16 J Allergy Clin Immunol 2009124245–252.e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller EK, Hernandez JZ, Wimmenauer V, Shepherd BE, Hijano D, Libster R, Serra ME, Bhat N, Batalle JP, Mohamed Y, et al. A mechanistic role for type III IFN-λ1 in asthma exacerbations mediated by human rhinoviruses. Am J Respir Crit Care Med. 2012;185:508–516. doi: 10.1164/rccm.201108-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, Finkbeiner WE, Dolganov GM, Widdicombe JH, Boushey HA, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390, e2. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 30.Arruda E, Boyle TR, Winther B, Pevear DC, Gwaltney JM, Jr, Hayden FG. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 31.Turner RB, Wecker MT, Pohl G, Witek TJ, McNally E, St George R, Winther B, Hayden FG. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA. 1999;281:1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]