Abstract
Rationale: An increased cancer aggressiveness and mortality have been recently reported among patients with obstructive sleep apnea (OSA). Intermittent hypoxia (IH), a hallmark of OSA, enhances melanoma growth and metastasis in mice.
Objectives: To assess whether OSA-related adverse cancer outcomes occur via IH-induced changes in host immune responses, namely tumor-associated macrophages (TAMs).
Measurements and Main Results: Lung epithelial TC1 cell tumors were 84% greater in mice subjected to IH for 28 days compared with room air (RA). In addition, TAMs in IH-exposed tumors exhibited reductions in M1 polarity with a shift toward M2 protumoral phenotype. Although TAMs from tumors harvested from RA-exposed mice increased TC1 migration and extravasation, TAMs from IH-exposed mice markedly enhanced such effects and also promoted proliferative rates and invasiveness of TC1 cells. Proliferative rates of melanoma (B16F10) and TC1 cells exposed to IH either in single culture or in coculture with macrophages (RAW 264.7) increased only when RAW 264.7 macrophages were concurrently present.
Conclusions: Our findings support the notion that IH-induced alterations in TAMs participate in the adverse cancer outcomes reported in OSA.
Keywords: sleep apnea, intermittent hypoxia, cancer, inflammation, tumor-associated macrophages
At a Glance Commentary
Scientific Knowledge on the Subject
An increased incidence of and mortality from cancer have been recently reported among patients with obstructive sleep apnea (OSA). In a rodent model, intermittent hypoxia can promote melanoma tumor growth and metastasis.
What This Study Adds to the Field
Changes induced by intermittent hypoxia during sleep on host immunity appear to underlie some of the adverse cancer outcomes reported in patients with OSA.
Obstructive sleep apnea (OSA) is a highly prevalent disorder affecting at least 4 to 10% of all adults and is associated with a large array of end-organ morbidities affecting multiple organ systems (1). OSA is characterized by repetitive obstructions of the upper airway during sleep that result in intermittent hypoxia (IH), increased inspiratory efforts, and sleep fragmentation (2), all of which have now been extensively examined in both patients and murine models aiming to identify the mechanisms underlying the extensive cardiovascular, cognitive, and metabolic comorbidities (3). In two recent seminal epidemiological studies, OSA has been associated with enhanced cancer aggressiveness (4) and mortality (5), whereby the cyclical hypoxia that characterizes OSA has been proposed as the major determinant of processes involving tumor invasion and metastasis (6, 7). Initial studies using animal models of OSA suggest that IH during the sleep cycle promotes increased melanoma tumor growth and metastatic potential, thereby lending biological plausibility to the epidemiological studies (8, 9), and further supporting the putative role of hypoxia in tumor biology (10–12). However, the potential mechanisms involved in IH-induced changes in tumor growth have not yet been explored.
It is now well established that the immune system participates in cancer processes, including angiogenesis, invasion, and metastasis (13). Regulatory T cell lymphocytes (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) have been extensively examined and shown to support tumor progression and survival in most cancers (14–16). TAMs are a major component of tumor stroma and can gradually polarize into two functionally distinct phenotypes as dictated by the tumor microenvironment, namely tumor-inhibitory (M1) and tumor-promoting (M2) phenotypes (17). In fact, TAMs can secrete mitogenic factors, proangiogenic cytokines, and immunosuppressive agents in response to hypoxia (16, 18). MDSCs have the ability to suppress T-cell responses and can also differentiate into TAMs within the tumor (19, 20). However, the effects of IH on the host immune system and its possible role in tumor aggressiveness are unknown. Therefore, we hypothesized that the adverse effects of IH on tumor proliferation and invasion would be mediated via alteration in TAM phenotypes rather than via a direct effect on tumor cells.
To assess the effect of IH on the immune system in the context of cancer, we used a lung epithelial tumor model (TC1 cells) and assessed tumor growth under IH or RA conditions, as well as changes in TAMs, MDSCs, and Tregs within the tumor. To determine whether TAMs exhibit altered phenotype and/or function under IH conditions, purified TAMs from tumors were interrogated using quantitative plasma membrane proteomics and M1/M2 cell surface markers with flow cytometry. Isolated TAMs from tumors of mice exposed to RA and IH were further evaluated in the context of tumor proliferation, migration, invasion, and extravasation properties in vitro. Finally, we developed an in vitro model to further investigate the effects of IH on tumor cells using single or coculture approaches with macrophages.
Methods
Animals, In Vivo IH, and Epithelial Lung Tumor Model
Eighty C57BL/6J male mice (7 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME). All experimental procedures were approved by The Institutional Animal Care and Use Committee of the University of Chicago.
Forty mice were placed in commercially designed environmental chambers (21) (see online supplement) and were subjected to IH with alternating cycles of 90 seconds (6% FiO2 followed by 21% FiO2, 20 cycles/h) for 12 h/d. This IH profile is associated with reproducible nadir of oxyhemoglobin saturations in the 65 to 72% range (21). A control group (n = 40) was exposed to continuous circulating room air (RA). Mice were preexposed during 2 weeks to either RA or IH and were then injected with 105 TC1 cells (diluted in 200 μl of phosphate-buffered saline) in the left flank. Every 3 days, tumor volume (V) was estimated by externally measuring its length (L) and width (W) with an electronic caliper (V = W2 × L/2). After 4 weeks from tumor injection, mice were killed and tumors excised and weighed. The presence of invasion toward the skeletal muscle was also assessed. Tumor samples were used for flow cytometry analyses, proteomics, immunohistochemistry (F4/80 staining), and/or for isolation of CD11b+ cells (TAMs) (see online supplement for detailed protocol).
Isolation of Macrophages and Proteomic Analysis
Macrophages were purified using magnetic beads coupled to anti-CD11b antibody (see online supplement).
Plasma membrane proteomics on isolated TAMs (see online supplement) was performed essentially as previously described (22). Proteins were detected by liquid chromatography–electrospray ionization–tandem mass spectrometry and quantified by spectral counting. Differences in relative protein abundance were assessed with t test and G test (22), such that the false discovery rate was less than 0.05. For full details, see online supplement. Purified resting bone marrow–derived macrophages (M0) were classically activated by treatment with LPS (5 ng/ml) and IFN-γ (12 ng/ml) for 24 hours as previously described (22) (for full details, see online supplement).
Quantification of Tregs, MDSCs, and TAMs and Assessment of M1 and M2 Phenotypes by Flow Cytometry
The total number of each cell type and their density (cells/g tumor) was initially assessed from several segments of each tumor using flow cytometry (see online supplement). The median fluorescent intensity (MFI) for CD86 and CD40 were used as M1 markers, whereas CD206 and TFRC served as M2 markers (22, 23).
Effects of TAMs on Proliferation, Migration, Invasion, and Extravasation Properties of TC1 Cells
Proliferation assay.
TC1 cells were cultured either in single culture or coculture with TAMs (ratio 1:4) isolated from tumors of mice exposed to either IH or RA. After 48 hours, both populations were counted by flow cytometry (see online supplement).
Migration tests.
To study the migration properties of TC1 cells and TAMs, two different assays on permeable Transwells were performed: (1) TC1 cells were cultured on top in serum-free medium either in single culture or in coculture with RA- or IH-exposed TAMs. Cells were allowed to migrate to the lower compartment by using 10% fetal bovine serum as chemoattractant for 18 hours. (2) RA- or IH-exposed TAMs were cultured for 24 hours to collect conditioned medium. TC1 cells were placed in the upper chamber, and the conditioned medium was placed in the bottom as chemoattractant. In both migration assays, the cells were harvested after 18 hours from the bottom surface and counted by flow cytometry (see online supplement).
Three-dimensional spheroid invasion test.
To assess the invasiveness of tumor cells in coculture with TAMs, a three-dimensional (3D) spheroid cell invasion assay was used. Green fluorescent protein–TC1 cells were seeded with RA- or IH-exposed TAMs, and the invasion was monitored until Day 6 after adding the invasion matrix (see online supplement). The use of fluorescence microscopy for green fluorescent protein–labeled TC1 cells allowed for discerning tumor cells within the 3D structure. The area of invasion was determined as the area of the spheroid embedded in the invasion matrix minus its corresponding control without invasion matrix.
Transendothelial extravasation assay.
The ability to extravasate the endothelial layer by TAMs and tumor cells either alone or in coculture with TAMs was evaluated using an electrical substrate-impedance sensing system (ECIS; Applied Biophysics, Troy, NY). Mouse brain endothelial bEnd.3 cells were seeded during 24 hours until reaching confluence. Then, TC1 cells and either RA- or IH-exposed TAMs were added alone or together. Resistance values were normalized relative to an undisturbed confluent endothelial monolayer just before to cell additions (see online supplement).
Proliferation of Tumor Cells and Macrophages in Single Culture and Coculture Using an IH In Vitro Model
A total of 5 × 104 macrophages (RAW 264.7) were preexposed to either IH (30 min 5% O2 followed by 30 min 21% O2 balanced in 5% CO2, 1 cycle/h) or RA (21% O2 balanced in 5% CO2) (see online supplement). The IH paradigm used herein is accompanied by progressive reductions or increases in medium FiO2 for 15 minutes followed by stable target FiO2 conditions until the end of the cycle. After 48 hours, RAW 264.7 cells were maintained either in single culture or in coculture with tumor cells (B16F10 or TC1; ratio 1:8) for an additional 48-hour period in the same conditions (IH or RA). In parallel, both tumor cells were also subjected to either IH or RA in isolation. At the end of each experiment, cells were identified, counted, and percentage of RAW 264.7 cells expressing high levels of CD86 and CD206 was assessed by flow cytometry.
Statistical Analysis
Data are presented as mean ± SEM. For in vivo studies, t tests were used to compare between IH and RA groups. Group comparisons for proliferation, migration, invasion, and extravasation assays were conducted using one-way analysis of variance or t tests as appropriate. For in vitro studies, two-way repeated measures analysis of variance was performed where treatment (RA vs. IH) and single versus coculture conditions constituted the primary variables. Student-Newman-Keuls post hoc tests were used for multiple comparisons. A two-tailed P value of < 0.05 was considered to achieve statistical significance.
Results
IH Promotes In Vivo Increased Tumor Growth and Invasion toward Adjacent Tissues
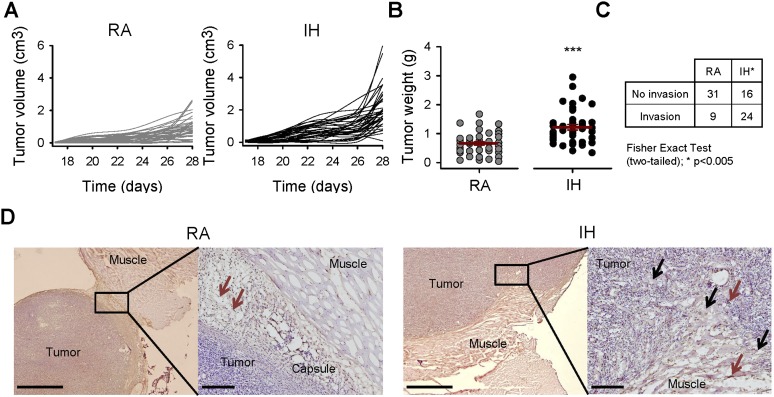
Mice exposed to IH exhibited accelerated tumor growth and weight at Day 28 after tumor injection (0.66 ± 0.06 g in RA vs. 1.22 ± 0.09 g in IH, P < 0.001; Figures 1A and 1B). In addition, the number of animals presenting invasion into the muscle was threefold higher (P = 0.001) in those animals subjected to IH (24 of 40) when compared with those exposed to RA (9 of 40) (Figure 1C). Staining of TAMs as F4/80+ cells revealed that they are highly concentrated in the periphery of the tumor, suggesting their active roles in tumor-related invasion processes (Figure 1D).
Figure 1.
Tumor growth and invasion from mice exposed to room air (RA) and intermittent hypoxia (IH). (A) Tumor volume measured every 3 days from Day 17 after tumor injection. IH induced an accelerated growth of tumors with respect to that observed in RA (n = 40). (B) After 4 weeks of tumor growth, tumors exposed to IH were 84% greater than controls (n = 40). (C) The number of cases with tumor invasion toward adjacent muscles was more than double in IH than in RA (n = 40). (D) Representative immunohistological sections of the tumor and adjacent muscle. Tumors from mice in RA have reduced invasion rates. Tumor-associated macrophages (TAMs) (brown arrows) are highly present in the connective tissue. However, tumors from IH-exposed mice presented more frequent invasion toward the muscle and included disruption of the tumor capsule and infiltration of tumor cells and TAMs in the muscle (black arrows). Scale bar, 1 mm (right image) and 200 μm (left image). Data are presented as mean ± SE. ***P < 0.001 by unpaired Student t test.
Immune Cells in Tumors during IH
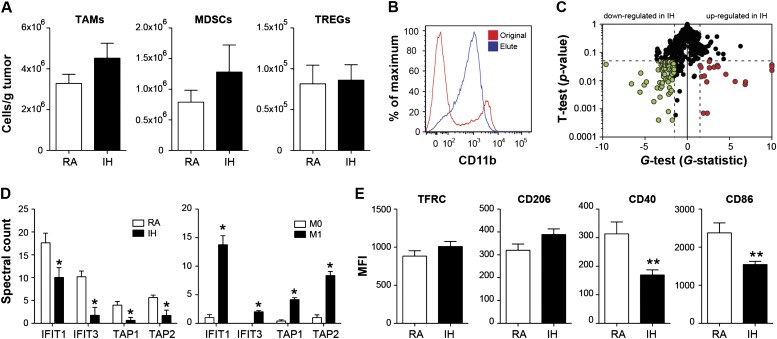
Tumors from mice exposed to IH exhibited 2.2-fold (P < 0.02), 3.7-fold (P < 0.02), and 2.6-fold (P < 0.02) increases in Tregs, MDSCs, and TAMs populations, respectively (see Figure E1 in the online supplement). However, these findings canceled out when they were normalized for tumor weight (Figure 2A), suggesting that differences in immune cell infiltration cannot explain per se the more aggressive phenotype observed.
Figure 2.
Regulatory T cell lymphocytes (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) within the tumor. (A) Immune cells, namely Tregs, MDSCs, and TAMs, showed no statistically significant differences in cell population density (cells/g) in tumors from intermittent hypoxia (IH)- or room air (RA)-exposed mice (n = 7). (B) Isolation of CD11b+ cells from tumor. (C) IH induces numerous changes to the plasma membrane proteome of TAMs (n = 4). (D) Left: IFIT1and3 and TAP1and2 proteins were markedly decreased in TAMs isolated from IH-treated mice. Right: Macrophages treated in vitro with LPS and IFN-γ induced the expression of all of these proteins. (E) Assessment of CD86 and CD40 (M1 markers) and TFRC and CD206 (M2 markers) by flow cytometry in TAMs. Down-regulation of both M1 markers was observed but no effect in either of both M2 markers (For CD86 and CD206, n = 7; for CD40 and TFRC, n = 4). Data are presented as mean ± SE. *P < 0.05 and **P < 0.01 by unpaired Student t test. MFI = mean fluorescence intensity.
To test whether TAMs exhibit a different phenotype and/or function, we purified TAMs from tumors (Figure 2B) and interrogated them using quantitative plasma membrane proteomics (22). Using stringent statistical criteria (22), we identified 66 proteins that were down-regulated and 19 proteins that were up-regulated in IH relative to control TAMs (Figure 2C, Table E1). Interestingly, IFIT1, IFIT3, TAP1, and TAP2, which are proteins involved in the IFN response and antigen processing and presentation, respectively, were markedly decreased in TAMs isolated from IH-treated mice (Figure 2D). Because treating macrophages in vitro with LPS and IFN-γ (classical activation; M1) induces expression of all of these proteins (Figure 2D), we hypothesized that IH attenuates the proinflammatory phenotype in TAMs.
To confirm this hypothesis, we measured cell surface expression of CD86 and CD40 (M1 markers) and TFRC and CD206 (M2 markers) in TAMs (22, 23). Consistent with the proteomic analyses, down-regulation of M1 markers and no effect on M2 marker expression emerged in IH-exposed tumors (Figure 2E).
Isolated TAMs from Mice Subjected to IH Increase Proliferation, Migration, Invasion, and Extravasation Properties of Tumor Cells
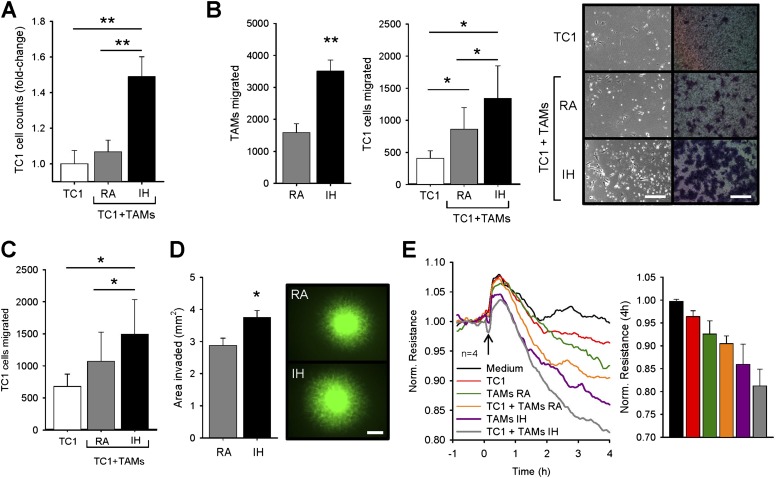
Coculture of TC1 cells with RA-exposed TAMs was associated with a trend toward increased TC1 cell proliferation versus isolated TC1 cell cultures (∼7%). However, TAMs obtained from tumors exposed to IH substantially increased TC1 proliferation compared with single culture (∼49%, P < 0.01; Figure 3A).
Figure 3.
Effects of tumor-associated macrophages (TAMs) on malignant properties of TC1 cells. (A) Proliferation of TC1 in single culture and in coculture with room air (RA) TAMs and intermittent hypoxia (IH) TAMs. TC1 cells increased their proliferation only in coculture with IH TAMs (n = 6). (B) Number of TAMs and TC1 cells in the bottom chamber after 18 hours of migration. TC1 cells were cultured either alone or in coculture with RA or IH TAMs. IH TAMs migrated faster than RA TAMs. Higher migrated TC1 cells were found in coculture with RA TAMs with respect to TC1 in single culture, but also their migration was enhanced further by IH TAMs (n = 7). Representative phase contrast images from the bottom well surface (scale bar, 200 μm) and Giemsa staining (scale bar, 150 μm) of cells attached to the bottom surface of the polycarbonate membrane for all three groups. (C) Migration of TC1 cells induced by TAMs-conditioned medium (n = 7). Tumor cells migrated faster in response to RA TAMs-conditioned medium compared with regular medium. IH TAMs promoted higher increase with respect to that obtained from RA TAMs. (D) TC1–green fluorescent protein cells presented more invasiveness in coculture with IH TAMs compared with RA TAMs (n = 8). Fluorescent images of three-dimensional spheroid cultures showing TC1–green fluorescent protein cell invasion in coculture with RA TAMs and IH TAMs. Scale bar, 500 μm. *P < 0.05 by unpaired Student t test. (E) Continuous sampling of endothelial resistance after 4 hours of adding TC1 cells alone, RA or IH TAMs alone, and TC1 with RA or IH TAMs. TC1 cells are able to disrupt the endothelial monolayer. Application of TAMs (either alone or mixed with TC1) decreased the transendothelial electrical resistance, which was much lower from IH TAMs than from RA TAMs (n = 4). Data are presented as mean ± SE. (*P < 0.05, **P < 0.01, ***P < 0.001 by one-way repeated analysis of variance unless otherwise noted.)
Compared with isolated cultures, cocultures of TAMs with TC1 tumor cells increased their migration 2.1-fold (P < 0.05) when fetal bovine serum was used as chemoattractant in the lower chamber of the Transwell system. However, the migration processes were markedly enhanced when IH-exposed TAMs were used (3.3-fold compared with single culture, P < 0.05; Figure 3B). Also, IH-exposed TAMs exhibited a 2.2-fold increase in migration compared with RA-exposed TAMs (P < 0.005). Similarly, additional migration tests revealed that normoxic TAMs are able to secrete motility factors that facilitate tumor migration (57%, P < 0.05). However, migration of TC1 cells was further increased by conditioned medium from IH-exposed TAMs (2.2-fold versus no-conditioned medium, P < 0.05; Figure 3C).
We further used a 3D-culture assay to assess invasion of TC1 cells in coculture with TAMs. In this setting, IH-exposed TAMs facilitated TC1 cell invasion, as demonstrated by higher areas of invasion (3.75 ± 0.21 mm2) compared with RA-exposed TAMs (2.87 ± 0.23 mm2, P < 0.02; Figure 3D), thereby lending support to the in vivo observations suggesting increased invasion rates.
TC1 cell addition to the endothelial layer decreased monolayer resistance by 3.4% with respect to control conditions (i.e., no cell addition). Similar approaches and magnitude of changes have been reported with human H358 lung tumor cells (24). Addition of RA-exposed TAMs alone decreased the electrical resistance by 7.2% and by 9.3% when TC1 tumor cells and RA-exposed TAMs were applied together. IH-exposed TAMs demonstrated increased ability to extravasate through the endothelial monolayer, as indicated by the largest decreases in endothelial electrical resistance values at 4 hours, either alone (13.8%, P < 0.01) or together with TC1 tumor cells (18.6%, P < 0.01; Figure 3E).
IH Increases Tumor Proliferation In Vitro but Only in Coculture with Macrophages
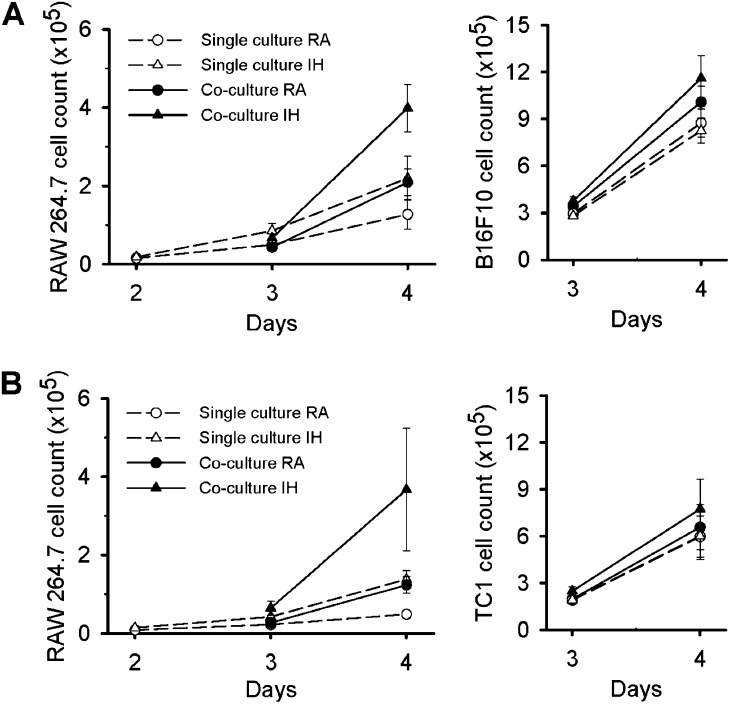
Recent studies in a mouse model of OSA consisting of environmental IH exposures have demonstrated accelerated B16F10 melanoma tumor growth (9, 25). Here we assessed proliferative rates of both B16F10 and TC1 cells in the presence or absence of a macrophage cell line (RAW 264.7). Compared with RA, IH did not alter proliferative rates of either B16F10 or TC1 cells when cultured alone. However, coculture with RAW 264.7 cells promoted increased cellular proliferation of B16F10 cells by ∼15%, (P < 0.01; Figure 4A), and a similar trend was observed in TC1 cells during RA conditions (∼10%, P = 0.13; Figure 4B).
Figure 4.
B16F10 and TC1 tumor cells in single culture and in coculture with RAW 264.7 cells. (A) Cell count of RAW 264.7 cells and B16F10 cells either in single culture (dashed line, open symbols) or in coculture (solid line, filled symbols). RAW 264.7 cells proliferate faster in coculture with B16F10 cells in room air (RA) (circles) compared with in single culture. However, intermittent hypoxia (IH) (triangles) enhanced their proliferation in single culture and in coculture. B16F10 increased their proliferation in coculture with macrophages when compared with single culture conditions, and these features were further enhanced in IH. (B) Cell count of RAW 264.7 cells and TC1 cells either in single culture or in coculture. These results show the same behavior of TC1 cells in response to RAW 264.7 and IH to that obtained from B16F10.
RAW 264.7 proliferative rates were increased in coculture with both B16F10 (∼64%, P < 0.01) and TC1 cells (2.5-fold, P < 0.01) in RA. However, proliferation of RAW 264.7 cells was markedly enhanced by IH (3.1-fold when cocultured with B16F10 cells, P < 0.01 and 7.6-fold when cocultured with TC1 cells, P < 0.01, respectively). Furthermore, IH induced phenotypic changes in RAW 264.7 cells with a lower M1/M2 ratio during coculture (P < 0.001) (Figure E3). These findings recapitulate the shifts toward the protumoral M2 macrophage subtype reported above in in vivo IH conditions. Similarly, the growth of B16F10 and TC1 cells in coculture with RAW 264.7 cells was further accelerated in the presence of IH (∼40%, P < 0.04 and ∼28%, P < 0.04, respectively) when compared with isolated conditions in IH (Figure 4).
Discussion
This study shows that murine epithelial lung tumors exposed to IH exhibit increased growth and invasiveness. The accelerated tumor growth is remarkably similar to that previously reported in melanoma cells (9, 25). The more aggressive tumor phenotype associated with IH can be explained in part by alterations in TAMs. Our findings provide a working model and biological plausibility to the adverse cancer outcomes reported in patients with OSA.
The presence of hypoxia is a common feature of human solid tumors, even if hypoxic regions are not homogenously distributed across the tumor and are usually confined to the core, where blood supply is less abundant (26). The content and location of TAMs within the tumor is positively correlated with the degree of overall hypoxia in liver metastases from breast and colorectal tumors (27). Because TAMs are able to respond to hypoxia and represent a major component of the tumor stroma (18, 28), we explored their potential role in a model of OSA to identify their potential contributory role associated with accelerated cancer growth and invasiveness during IH. However, as shown herein, the interactions and specific functions assumed by the parallel alterations in Tregs and MDSCs in the context of IH will definitely have to be examined in greater detail in future studies, particularly considering how these host-derived immune cells operate in an exquisitely coordinated fashion within the tumor microenvironment (29). Indeed, the increased numbers of Tregs and MDSCs within the tumors of mice exposed to IH and their ability to suppress the immune system and to develop a protumor environment would justify such efforts (14, 20, 30).
Although it is highly likely that a large spectrum of macrophage phenotypes exists (31–33), two major phenotypes have been recognized in tumors (17), namely the classically activated (M1) and the alternatively activated (M2) macrophage phenotypes. Although both phenotypes share many common cellular markers and functions, these cells can be readily classified based on the expression patterns of now well-characterized singular surface proteins (34). M1 macrophages primarily upregulate proinflammatory cytokines and induce increased formation of reactive oxidative species, ultimately promoting inflammation and apoptosis. Conversely, M2 macrophages enhance angiogenesis, cell proliferation, and protumoral functions (17). Here, we selected four major macrophage surface proteins (i.e., CD86, CD40 and CD206, TFRC), which have been previously used as specific M1 or M2 markers, respectively (22, 23). Heightened recruitment of TAMs within the tumor, and their shift in phenotype (mainly by a reduction in M1 markers), may be explained in part by differential hypoxic maps within the tumor elicited by IH (28). Indeed, hypoxia can activate mitogenic, proinvasive, proangiogenic, and prometastatic genes in TAMs through hypoxia-inducible transcription factors such as HIF-1α and HIF-2α (28). However, although TAMs phenotype can be affected by hypoxia, the major role of these cells is not exclusively restricted to those hypoxic areas, but, perhaps even more importantly, they play critical functions within those regions in the outer boundaries of the tumor, where not only a higher blood supply is present but also the invasion process occurs. Patients with OSA typically manifest repetitive blood oxygen desaturation events during sleep, and such relapsing swings in oxygen supply are more prominent in tissues with high perfusion and increased metabolic rates (35). Therefore, intermittent oscillations in oxygen tension in the peripheral circulation will more readily be apparent in highly perfused areas in the periphery of the tumor, and such oscillations will therefore alter macrophage phenotype and function. Chronic IH, such as in OSA, has been implicated in the generation of excessive reactive oxygen species through various sources (36), all of which could in turn participate in the signaling cascades underlying the changes in various different immune responses observed with IH. Indeed, our findings from the in vitro IH experiments clearly establish that IH per se modifies the phenotype of macrophages and that such alterations appear to be critically involved and upstream from the changes in tumor proliferation. The absolute higher content of TAMs in tumors subjected to IH, and more importantly their enhanced ability to migrate, and their role in the other malignant properties assessed in this study can be all explained by their change in phenotype. To further understand whether the recurrent cycles of hypoxia-reoxygenation are a critical component of OSA-enhanced tumorigenesis, we performed complementary experiments involving sustained hypoxia (5% O2) (Figure E3). Increases in tumor proliferation either in single or coculture with macrophages occurred in sustained hypoxia, whereas increased proliferation of tumor cells did not occur in IH (Figures 4 and E3). During IH, the presence of macrophages is essential and is the major contributor to the increased tumor growth observed during IH conditions (Figure E3A). In addition, our findings suggest that the increase in the proliferation is due to hypoxia rather than to reoxygenation events (Figure E3B). Furthermore, other less-explored stromal elements within and surrounding the tumor, such as other immune cells, adipocytes, endothelial cells, cancer-associated fibroblasts, and external angiogenic modulators (25), could all be concomitantly modified by the IH exposures and ply synergistically to induce accelerated cancer progression, invasion, and resistance (37, 38). As shown here, in-depth proteomic analysis clearly depicts a complex cascade of macrophage alterations by IH and identifies surface macrophage proinflammatory proteins (e.g., IFIT1, IFIT3, TAP1, TAP2) that may potentially represent therapeutic targets. The down-regulation of these macrophage membrane proteins may indicate decreased antigen presentation capacity, which may contribute to the altered tumor phenotype observed in IH. Cells isolated using CD11b beads also uniformly express CD45 and F4/80 (Figure E2). Such markers are extensively used to define TAMs in vivo (39). However, some dendritic cells and TAMs share common cell surface markers including F4/80, such that there are probably a small number of dendritic cells that could be included by this approach (40).
To ascertain that the IH-induced functional changes on TAMs indeed enhance tumor malignant properties, we performed a series of in vitro experiments and assessed proliferation, migration, invasion, and extravasation. Coculture of TC1 cells with RA-exposed TAMs promoted a trend toward increased TC1 cell proliferation when compared with isolated TC1 cells. Interestingly, similar responses emerged when TC1 cells were cocultured with RAW 264.7 cells in RA. Similar responses have been reported when RAW 264.7 cells were cocultured with breast tumor cells (41), thereby confirming previous findings that macrophages can respond to tumor cells and induce their proliferation (42). In addition, coculture of TAMs with TC1 tumor cells increase their migration, implying that the presence of macrophages will increase tumor cell migration toward adjacent tissues via TAMs secretion of motility factors that facilitate tumor migration. Comparable increases were reported with conditioned medium of RAW 264.7 on CT26 colon tumor cells using similar approaches (43). Globally, similar effects occurred when we tested the ability of TC1 cells to disrupt an endothelial cell monolayer and the effect of TAMs on such properties. Analogous changes have been reported with human H358 lung tumor cells (24). The higher disruption triggered by TAMs may illustrate their recruitment within the tumor during tumor development (18) and the possible role of leadership during invasion and metastasis processes (16). Taken together, the findings obtained from TAMs and RAW 264.7 cells support the now widely accepted concept that macrophages enhance tumor progression and invasion. Most importantly, however, here we show how IH can modify the function of those macrophages and increase their ability to proliferate, migrate, and extravasate compared with macrophages exposed to RA conditions. Considering that TAMs were isolated from tumors and cultured in the same conditions and with the same ratio (TAMs vs. tumor cells) in vitro, the changes in tumor cell malignant properties can only be explained by the changes in TAMs phenotype, rather than by their number. Therefore, the in vitro experimental data support the functional changes in TAMs observed in vivo. In addition, IH-exposed TAMs facilitated TC1 cell invasion, as demonstrated by a novel coculture 3D system, thereby confirming the in vivo increased invasion rates. We also presume that the effect of IH on TAMs as far as their ability to disrupt and extravasate an endothelial monolayer may explain, at least in part, the higher frequency of metastases previously reported in a melanoma mouse model of OSA (8). Therefore, these studies clearly implicate IH-induced host immune responses on the overall adverse changes in the biological properties of solid tumors.
The enhanced and accelerated tumor progression and invasion induced by IH and described herein provides biological plausibility to recent epidemiological data obtained from two separate cohorts in patients with OSA. In these studies, strong and independent severity-dependent associations emerged between OSA and cancer aggressiveness and mortality, particularly with the index of hypoxemia (i.e., the frequency of hypoxemic events per hour of sleep) (4, 5). However, such studies did not examine whether measures of sleep integrity also accounted for the variance in cancer outcomes. In the current studies, we used a well-established murine model that is restricted to IH and that mimics the oxyhemoglobin desaturations of patients with moderate to severe OSA (21). Thus, it is unclear whether milder or more severe degrees of hypoxemia and changes in the frequency of such events would yield parallel changes in the magnitude of the changes in tumor biology. Furthermore, because the current model is unavoidably accompanied by persistent alterations in sleep architecture (44, 45), the contributions of underlying sleep disruption cannot be separated from those of IH. Finally, although we have demonstrated that host immune responses are critical to hypoxia-mediated tumor responses, the molecular mechanisms involved are still unclear. Based on the wide range of alterations in mechanistic pathways induced by IH, including oxidative stress, metabolic, autonomic nervous system, and hormonal changes, the elucidation of the pathways mediating the coordinated repertoire of such responses will require an extensive effort that clearly is beyond the scope of the current study.
In summary, our findings support the notion that the more aggressive tumor phenotype observed in the context of IH conditions is dependent on IH-induced alterations in the host immune response in general and, more specifically, in a shift in macrophage polarity from M1 to M2. These findings are closely aligned with our current understanding of the role of host immunity in cancer malignancy and prognosis (46, 47) and further indicate that alteration in TAMs by IH underlie the enhanced proliferation, migration, invasion, and extravasation properties of tumor cells.
Acknowledgments
Acknowledgment
The authors thank Zhuanhong Qiao for technical assistance in the FACS analyses.
Footnotes
Supported by National Institutes of Health grants HL-65270, HL-086662, and HL-107160 (D.G.); by Beatriu de Pinós fellowship from Generalitat de Catalunya (2010 BP_A 00238) and SEPAR (I.A.); and by the Spanish Ministry of Economy and Competitiveness (SAF2011-22576) (R.F.).
Author Contributions: I.A. participated in the conceptual framework of the project, performed experiments, analyzed data, and drafted components of the manuscript. Y.W. analyzed data, drafted portions of the manuscript, and served as blinded observer. B.R.C., K.S.S., and L.B. performed all proteomic assessments of tumor-associated macrophages and analyses of the mass spectrometry findings. J.Z., F.H., F.E.L., A.C., and S.X.Z. performed experiments. R.F. provided critical insights and participated in the drafting of the manuscript. D.G. conceptualized the project, provided critical input in all phases of the experiments, analyzed data, drafted the previous versions of the manuscript, and is responsible for the financial support of the project and the manuscript content. All authors have reviewed and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201310-1830OC on January 28, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6:557–566. doi: 10.1586/ers.12.44. [DOI] [PubMed] [Google Scholar]
- 4.Campos-Rodriguez F, Martinez-Garcia MA, Martorell-Calatayud A, Perez-Gil A, Nagore E, Valero-Sanchez I, Selma-Ferrer MJ, Chiner-Vives E, Montserrat JM, Carreras C, et al. Association between markers of aggressiveness of malignant cutaneous melanoma and sleep disordered-breathing. Eur Respir J. 2013;42:639s. doi: 10.1183/09031936.00115413. [DOI] [PubMed] [Google Scholar]
- 5.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SL, Rouhi P, Dahl JL, Zhang D, Ji H, Hauptmann G, Ingham P, Cao Y. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci USA. 2009;106:19485–19490. doi: 10.1073/pnas.0909228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rofstad EK, Gaustad JV, Egeland TA, Mathiesen B, Galappathi K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int J Cancer. 2010;127:1535–1546. doi: 10.1002/ijc.25176. [DOI] [PubMed] [Google Scholar]
- 8.Almendros I, Montserrat JM, Torres M, Dalmases M, Cabanas ML, Campos-Rodriguez F, Navajas D, Farre R. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2013;186:303–307. doi: 10.1016/j.resp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Almendros I, Montserrat JM, Ramirez J, Torres M, Duran-Cantolla J, Navajas D, Farre R. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39:215–217. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res. 2012;18:1207–1213. doi: 10.1158/1078-0432.CCR-11-1591. [DOI] [PubMed] [Google Scholar]
- 13.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oleinika K, Nibbs RJ, Graham GJ, Fraser AR. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol. 2013;171:36–45. doi: 10.1111/j.1365-2249.2012.04657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obeid E, Nanda R, Fu YX, Olopade OI. The role of tumor-associated macrophages in breast cancer progression. Int J Oncol. 2013;43:5–12. doi: 10.3892/ijo.2013.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 18.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am J Physiol Regul Integr Comp Physiol. 2012;303:R700–R709. doi: 10.1152/ajpregu.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker L, Liu NC, Averill MM, Yuan W, Pamir N, Peng Y, Irwin AD, Fu X, Bornfeldt KE, Heinecke JW. Unique proteomic signatures distinguish macrophages and dendritic cells. PLoS ONE. 2012;7:e33297. doi: 10.1371/journal.pone.0033297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lennon FE, Mirzapoiazova T, Mambetsariev B, Salgia R, Moss J, Singleton PA. Overexpression of the mu-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology. 2012;116:857–867. doi: 10.1097/ALN.0b013e31824babe2. [DOI] [PubMed] [Google Scholar]
- 25.Almendros I, Montserrat JM, Torres M, Bonsignore MR, Chimenti L, Navajas D, Farre R. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012;13:1254–1260. doi: 10.1016/j.sleep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Shao Q, Morgounova E, Jiang C, Choi J, Bischof J, Ashkenazi S. In vivo photoacoustic lifetime imaging of tumor hypoxia in small animals. J Biomed Opt. 2013;18:076019. doi: 10.1117/1.JBO.18.7.076019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stessels F, Van den Eynden G, Van der Auwera I, Salgado R, Van den Heuvel E, Harris AL, Jackson DG, Colpaert CG, van Marck EA, Dirix LY, et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer. 2004;90:1429–1436. doi: 10.1038/sj.bjc.6601727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117:701–708. doi: 10.1002/ijc.21422. [DOI] [PubMed] [Google Scholar]
- 29.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng B, Zhu JM, Wang Y, Liu TT, Ding YB, Xiao WM, Lu GT, Bo P, Shen XZ. Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-beta1 in gastric cancer. PLoS ONE. 2013;8:e63777. doi: 10.1371/journal.pone.0063777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duechler M, Peczek L, Zuk K, Zalesna I, Jeziorski A, Czyz M. The heterogeneous immune microenvironment in breast cancer is affected by hypoxia-related genes. Immunobiology. 2014;219:158–165. doi: 10.1016/j.imbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Panni RZ, Linehan DC, Denardo DG. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy. 2013;5:1075–1087. doi: 10.2217/imt.13.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Almendros I, Farre R, Planas AM, Torres M, Bonsignore MR, Navajas D, Montserrat JM. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34:1127–1133. doi: 10.5665/SLEEP.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–316. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32:550–570. doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castells M, Thibault B, Delord JP, Couderc B. Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci. 2012;13:9545–9571. doi: 10.3390/ijms13089545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin CW, Shen SC, Ko CH, Lin HY, Chen YC. Reciprocal activation of macrophages and breast carcinoma cells by nitric oxide and colony-stimulating factor-1. Carcinogenesis. 2010;31:2039–2048. doi: 10.1093/carcin/bgq172. [DOI] [PubMed] [Google Scholar]
- 42.Komohara Y, Horlad H, Ohnishi K, Fujiwara Y, Bai B, Nakagawa T, Suzu S, Nakamura H, Kuratsu J, Takeya M. Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci. 2012;103:2165–2172. doi: 10.1111/cas.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green CE, Liu T, Montel V, Hsiao G, Lester RD, Subramaniam S, Gonias SL, Klemke RL. Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS ONE. 2009;4:e6713. doi: 10.1371/journal.pone.0006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7:7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol. 1985;2001:2758–2766. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 47.Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010;127:748–758. doi: 10.1002/ijc.25464. [DOI] [PubMed] [Google Scholar]