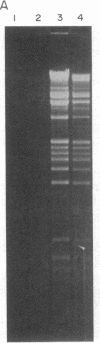
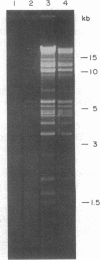
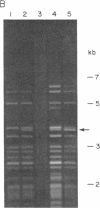
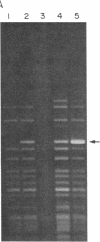
Abstract
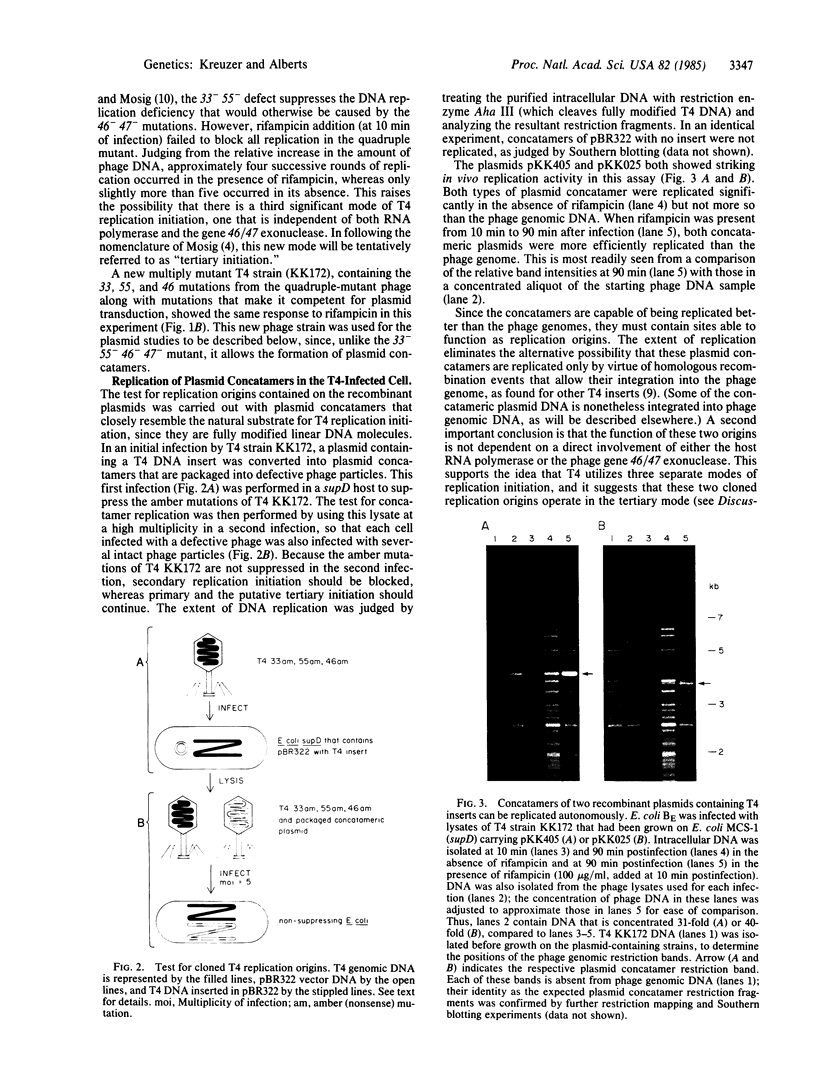
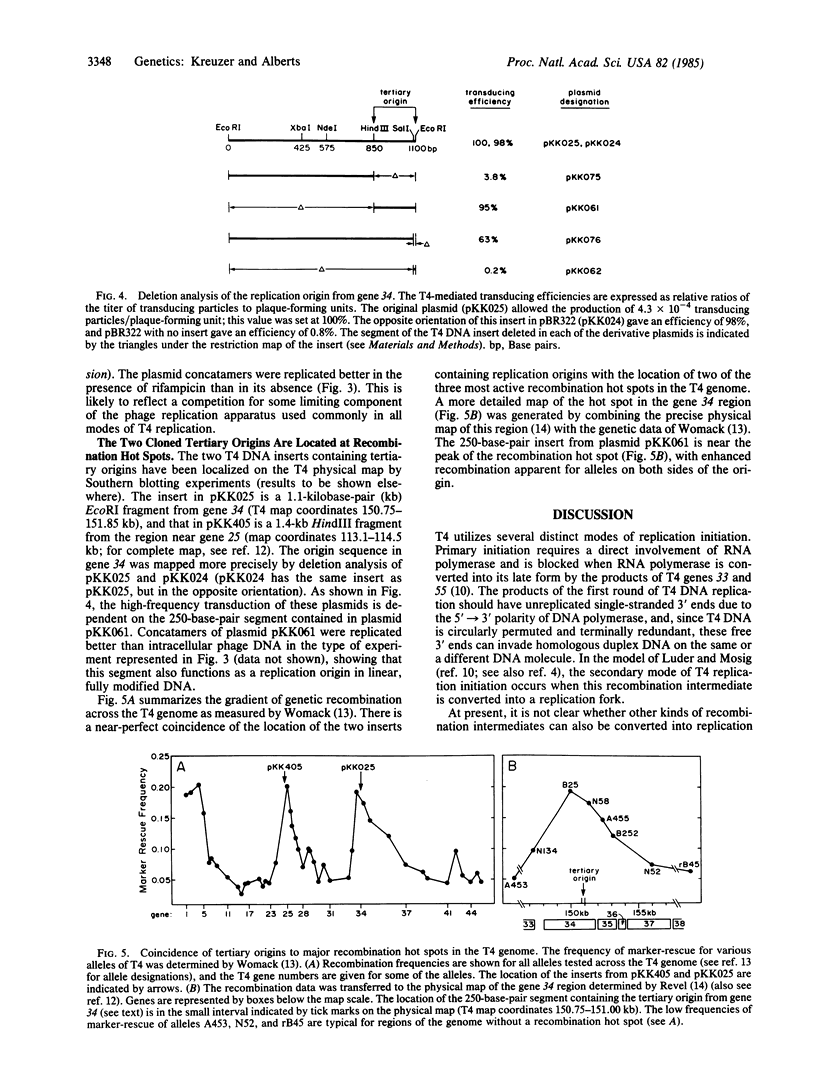
Plasmid transduction mediated by bacteriophage T4 has been used to study putative T4 DNA replication origins cloned as inserts in the Escherichia coli plasmid pBR322. Two particular inserts from the T4 genome allow high-frequency plasmid transduction, suggesting that each insert might contain a T4 replication origin. T4 infection of these plasmid-containing cells produces large numbers of defective phage particles that contain long linear concatamers of the plasmid DNA. During a second cycle of infection, these defective phage genomes can be replicated better than normal phage chromosomes present in the same infected cell; consequently, the T4 DNA inserts must be functioning as replication origins. Both of these origins appear to utilize a previously unrecognized mode of T4 replication initiation. Moreover, each origin coincides with a major recombination hot spot in the phage genome, and therefore this mode of replication initiation seems to involve a local stimulation of homologous genetic recombination. From a purely practical standpoint, additional DNA fragments can be cloned in an origin-containing plasmid, allowing isolation of large amounts of any DNA sequence with the glucosylated hydroxymethylcytosine modifications of T4 DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckendorf S. K., Wilson J. H. A recombination gradient in bacteriophage T4 gene 34. Virology. 1972 Nov;50(2):315–321. doi: 10.1016/0042-6822(72)90382-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Kornberg A. Purification and characterization of phiX174 gene A protein. A multifunctional enzyme of duplex DNA replication. J Biol Chem. 1979 Jun 25;254(12):5328–5332. [PubMed] [Google Scholar]
- Halpern M. E., Mattson T., Kozinski A. W. Origins of phage T4 DNA replication as revealed by hybridization to cloned genes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6137–6141. doi: 10.1073/pnas.76.12.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Yudelevich A., Hurwitz J. Isolation and characterization of the protein coded by gene A of bacteriophage phiX174 DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2669–2673. doi: 10.1073/pnas.73.8.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Ling S. K. Genetic specificity of DNA synthesized in the absence of T4 bacteriophage gene 44 protein. J Virol. 1982 Oct;44(1):256–262. doi: 10.1128/jvi.44.1.256-262.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. N., Goldberg E. B. Region-specific recombination in phage T4. I. A special glucosyl-dependent recombination system. Genetics. 1980 Mar;94(3):519–530. doi: 10.1093/genetics/94.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. N., Goldberg E. B. Region-specific recombination in phage T4. II. Structure of the recombinants. Genetics. 1980 Mar;94(3):531–547. doi: 10.1093/genetics/94.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luder A., Mosig G. Two alternative mechanisms for initiation of DNA replication forks in bacteriophage T4: priming by RNA polymerase and by recombination. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1101–1105. doi: 10.1073/pnas.79.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson T., Van Houwe G., Bolle A., Epstein R. Fate of cloned bacteriophage T4 DNA after phage T4 infection of clone-bearing cells. J Mol Biol. 1983 Oct 25;170(2):343–355. doi: 10.1016/s0022-2836(83)80152-1. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Sinha N. K., Alberts B. M. Reconstruction of bacteriophage T4 DNA replication apparatus from purified components: rolling circle replication following de novo chain initiation on a single-stranded circular DNA template. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4800–4804. doi: 10.1073/pnas.72.12.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R. Molecular cloning of the T4 genome: organization and expression of the tail fiber gene cluster 34--38. Mol Gen Genet. 1981;182(3):445–455. doi: 10.1007/BF00293934. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Saito H. High-frequency transduction of pBR322 by cytosine-substituted T4 bacteriophage: evidence for encapsulation and transfer of head-to-tail plasmid concatemers. Plasmid. 1982 Jul;8(1):29–35. doi: 10.1016/0147-619x(82)90038-5. [DOI] [PubMed] [Google Scholar]
- Wilson G. G., Young K. Y., Edlin G. J., Konigsberg W. High-frequency generalised transduction by bacteriophage T4. Nature. 1979 Jul 5;280(5717):80–82. doi: 10.1038/280080a0. [DOI] [PubMed] [Google Scholar]
- Womack F. C. Cross-reactivation differences in bacteriophage T4D. Virology. 1965 Aug;26(4):758–760. [PubMed] [Google Scholar]