To the Editor:
Influenza causes significant morbidity and mortality (1–3). Effectiveness of the vaccine and severity of the clinical manifestations of infection are highly variable each year. The rate of influenza vaccination in the United States has increased in recent years but remains poor (4, 5). We have previously reported our experience in the intensive care unit (ICU) with influenza A, H1N1 pandemic 2009 virus (pH1N1) (6, 7). We now report our initial observations for the 2013–2014 influenza season. We observe a very high number of otherwise healthy individuals with critical illness requiring care in the ICU. Most patients who required ICU level care were not previously vaccinated.
To determine whether patients requiring ICU care have a lower rate of vaccination prior to hospitalization, we reviewed the records of all hospitalized patients who tested positive for the influenza virus by polymerase chain reaction assay at our institution between November 1, 2013, and January 8, 2014. Basic patient demographics and underlying risk factors for severe influenza are provided (Table 1). The median age in our cohort was 28.5 years (range: 2 mo to 101 yr), similar to the age distribution observed during the 2009–2010 influenza season (8).
Table 1:
Patient Demographics
| Patient Characteristics | Number (Percentage) |
|---|---|
| Age, median (range) | 28.5 (2 mo to 101 yr) |
| <18 yr old | 4 (7.3) |
| 18–49 yr old | 27 (49.1) |
| 50–64 yr old | 18 (32.7) |
| ≥65 yr old | 6 (10.9) |
| Sex | |
| Male | 33 (60.0) |
| Female | 22 (40.0) |
| Race/ethnicity | |
| White | 26 (47.3) |
| African American | 23 (41.8) |
| Hispanic | 3 (5.5) |
| Other | 2 (3.6) |
| Native American | 1 (1.8) |
| Body mass index ≥ 30 | 22 (40.0) |
| Current tobacco use | 19 (34.5) |
| Comorbidities* | |
| Pulmonary disease | 23 (41.8) |
| Diabetes mellitus | 12 (21.8) |
| Cardiovascular disease | 10 (18.2) |
| Renal disease | 8 (14.5) |
| Other immunosuppression† | 6 (10.9) |
| Neuromuscular disorder | 3 (5.5) |
| Solid organ transplant | 3 (5.5) |
| Hematologic malignancy | 3 (5.5) |
| Pregnancy | 2 (3.6) |
Subjects may have more than one underlying condition.
Immunosuppressive condition other than solid organ transplant and hematologic malignancy or immunosuppressive medications.
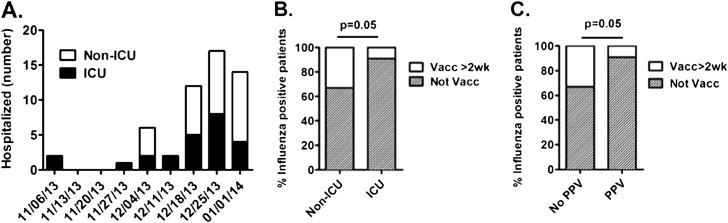
We have observed a dramatic increase in the number of hospitalizations and ICU admissions in recent weeks (Figure 1A). The majority of patients (48/55; 87.3%) were infected with pH1N1, consistent with recent Centers of Disease Control and Prevention (CDC) reporting (9). Only one case of influenza H3 and one case of influenza B were observed.
Figure 1.
Hospitalized patients with confirmed influenza and vaccination status. (A) We report the number of cases of influenza at a single academic institution by week in non–intensive care unit (ICU) and ICU settings. (B) There was a high number of individuals that had not received the flu vaccine within 2 weeks of acute illness. There was a higher frequency of unvaccinated individuals in the ICU when compared with hospitalized non-ICU patients. (C) Similarly, there was a high number of individuals that had not received the flu vaccine who required positive pressure ventilation (PPV) (Optiflow [Fisher & Paykel, Auckland, New Zealand], bilevel positive airway pressure or conventional mechanical ventilation) for respiratory insufficiency, when compared with hospitalized patients who did not require ventilator support. Two-tailed Fisher exact test was used for statistical comparisons.
Thirteen of 55 (23.6%) patients were vaccinated against influenza at least 2 weeks prior to the onset of their acute illness. The vaccination rate in hospitalized patients appears lower than the CDC-reported early-season vaccination rate of 36.5% for the 2012–2013 and 39.5% for the 2013–2014 periods (5). Furthermore, the rate of influenza vaccination in patients requiring ICU care was lower than in patients admitted to general wards: 9.1% (2/22) of patients requiring ICU admission were vaccinated, compared with 33.3% (11/33) of patients treated in non-ICU settings (P = 0.053; odds ratio = 5.0 [95% confidence interval (CI), 0.98–25.4]) (Figure 1B). Similarly, 8.7% (2/23) of patients who required either mechanical ventilation or bilevel positive airway pressure were vaccinated, compared with 34.4% (11/32) of patients who did not require positive pressure ventilation (P = 0.051; odds ratio = 5.5 [95% CI, 1.084–27.90]) (Figure 1C). Of the two patients admitted to the ICU who did receive influenza vaccination, one had chronic lymphocytic leukemia and was receiving rituximab, likely impeding an adequate immune response to vaccination; the second patient had hepatitis C and ethanol abuse and was admitted to the ICU overnight for alcohol withdrawal. Of the 11 patients who were treated in the non-ICU setting and received influenza vaccinations, 9 could be considered immunocompromised due to a variety of conditions, including hematologic malignancy, solid organ transplant, liver cirrhosis, renal failure, and use of medications known to suppress the immune system. Together, these observations support that vaccination may provide protection from severe illness requiring hospitalization and is in agreement with previous reports (10, 11).
Forty percent (22/55) of patients were admitted to the ICU, 18.2% (10/55) were treated with noninvasive ventilation, and 29.1% (16/55) required mechanical ventilation for respiratory failure. Sixteen of 22 (72.7%) ICU patients developed acute respiratory distress syndrome, and 31.3% (5/16) of those patients required support with extracorporeal membrane oxygenation. Thus far, death occurred in 5.4% (3/55) of all patients; however, 18 patients still remained hospitalized at the time of this initial report. The rate of ICU admission among hospitalized patients infected with the H1N1 virus was 39.6% (19/48), which is 50% greater than the previously reported rate of 20–25% during the 2009 pandemic influenza season (8). We are uncertain whether this high rate of ICU admission and mechanical ventilation represents a diagnosis bias or whether severity of illness being caused by the current H1N1 virus is higher. Because only the critically ill patients routinely receive bronchoscopy with bronchoalveolar lavage (BAL), it is possible that the high rate of patients who are admitted to the ICU reflects underdiagnosis of influenza in the non-ICU patient population, who may have false-negative nasopharyngeal samples.
Interestingly, of the 22 patients admitted to the ICU, 31.8% (7/22) patients had previously negative influenza tests, including 4 patients with false-negative rapid influenza antigen tests (RIDTs). Although RIDTs have the advantage of quick turn-around time, their poor performance characteristics were illustrated in a metaanalysis of 159 studies, showing pooled sensitivity of 62.3% (95% CI, 57.9–66.6%) (12). The CDC recommends that antiviral treatment should not be withheld from patients with signs and symptoms suggestive of influenza infection in spite of a negative RIDT (9). Because H1N1 is known to have a high propensity for affecting the lower respiratory tract (1–3, 6, 8), BAL specimens may be more sensitive for H1N1 diagnosis than samples obtained from the upper respiratory tract (13, 14). Antivirals should not be discontinued in patients with an influenza-consistent illness until the lower tract is sampled, even if upper respiratory tract specimens are negative.
Treatment with oseltamivir was started in 81.8% (45/55) of patients in our cohort; however, delayed treatment (i.e., ≥24 h after influenza diagnosis was considered) was observed in 33.3% (15/45) of patients. Ten hospitalized patients (non-ICU) never received antiviral treatment for various reasons. The CDC recommends that the decision regarding antiviral treatment of influenza should not await laboratory confirmation and that indications for antiviral treatment include hospitalization, severe complicated and progressive illness, and presence of risk factors for influenza complications, independent of the duration of symptoms (9).
Together, our initial observations during this influenza season support a high prevalence of the H1N1 virus affecting young adults who develop severe lung injury requiring ICU care, high false-negative rates of RIDTs, and delay in starting antiviral treatment. We also note very low vaccination rates among both hospitalized and ICU patients, as well as patients requiring positive pressure ventilation. Our observations support encouraging influenza vaccination for all individuals without a contraindication, as this may prevent severe lower respiratory tract complications requiring ICU level care.
Acknowledgments
Acknowledgment
The authors greatly appreciate the support provided by the Clinical Microbiology Laboratory at Duke University Medical Center.
Footnotes
Supported by NIH grant ES013611 (J.W.H. and L.G.Q.).
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, et al. 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 2.Tuite AR, Greer AL, Whelan M, Winter AL, Lee B, Yan P, Wu J, Moghadas S, Buckeridge D, Pourbohloul B, et al. Estimated epidemiologic parameters and morbidity associated with pandemic H1N1 influenza. CMAJ. 2010;182:131–136. doi: 10.1503/cmaj.091807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 4.Lu P-J, Singleton JA, Euler GL, Williams WW, Bridges CB. Seasonal influenza vaccination coverage among adult populations in the United States, 2005–2011. Am J Epidemiol. 2013;178:1478–1487. doi: 10.1093/aje/kwt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and PreventionNational Early Season Flu Vaccination Coverage, United States, November 2013[accessed 2014 Jan 10]. Available from: http://www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2013.htm
- 6.Norfolk SG, Hollingsworth CL, Wolfe CR, Govert JA, Que LG, Cheifetz IM, Hollingsworth JW. Rescue therapy in adult and pediatric patients with pH1N1 influenza infection: a tertiary center intensive care unit experience from April to October 2009. Crit Care Med. 2010;38:2103–2107. doi: 10.1097/CCM.0b013e3181f268f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin SS, Hollingsworth CL, Norfolk SG, Wolfe CR, Hollingsworth JW. Reversible cardiac dysfunction associated with pandemic 2009 influenza A (H1N1) Chest. 2010;137:1195–1197. doi: 10.1378/chest.10-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhung MA, Swerdlow D, Olsen SJ, Jernigan D, Biggerstaff M, Kamimoto L, Kniss K, Reed C, Fry A, Brammer L, et al. Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis. 2011;52:S13–S26. doi: 10.1093/cid/ciq008. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and PreventionHealth advisory. Notice to clinicians: early reports of pH1N1-associated illnesses for the 2013–14 influenza season. CDC Health Alert Network. December 24, 2013[accessed 2014 Jan 10]. Available from: http://emergency.cdc.gov/HAN/han00359.asp
- 10.Centers for Disease Control and Prevention. Estimated influenza illnesses and hospitalizations averted by influenza vaccination - United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep. 2013;62:997–1000. [PMC free article] [PubMed] [Google Scholar]
- 11.Castilla J, Godoy P, Domínguez A, Martínez-Baz I, Astray J, Martín V, Delgado-Rodríguez M, Baricot M, Soldevila N, Mayoral JM, et al. CIBERESP Cases and Controls in Influenza Working Group Spain. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis. 2013;57:167–175. doi: 10.1093/cid/cit194. [DOI] [PubMed] [Google Scholar]
- 12.Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 2012;156:500. doi: 10.7326/0003-4819-156-7-201204030-00403. [DOI] [PubMed] [Google Scholar]
- 13.Blyth CC, Iredell JR, Dwyer DE. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;361:2493. doi: 10.1056/NEJMc0909049. [DOI] [PubMed] [Google Scholar]
- 14.Singh K, Vasoo S, Stevens J, Schreckenberger P, Trenholme G. Pitfalls in diagnosis of pandemic (novel) A/H1N1 2009 influenza. J Clin Microbiol. 2010;48:1501–1503. doi: 10.1128/JCM.02483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]